High Voltage Electric Discharge for Recovery of Chlorogenic Acid from Tobacco Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Chemicals
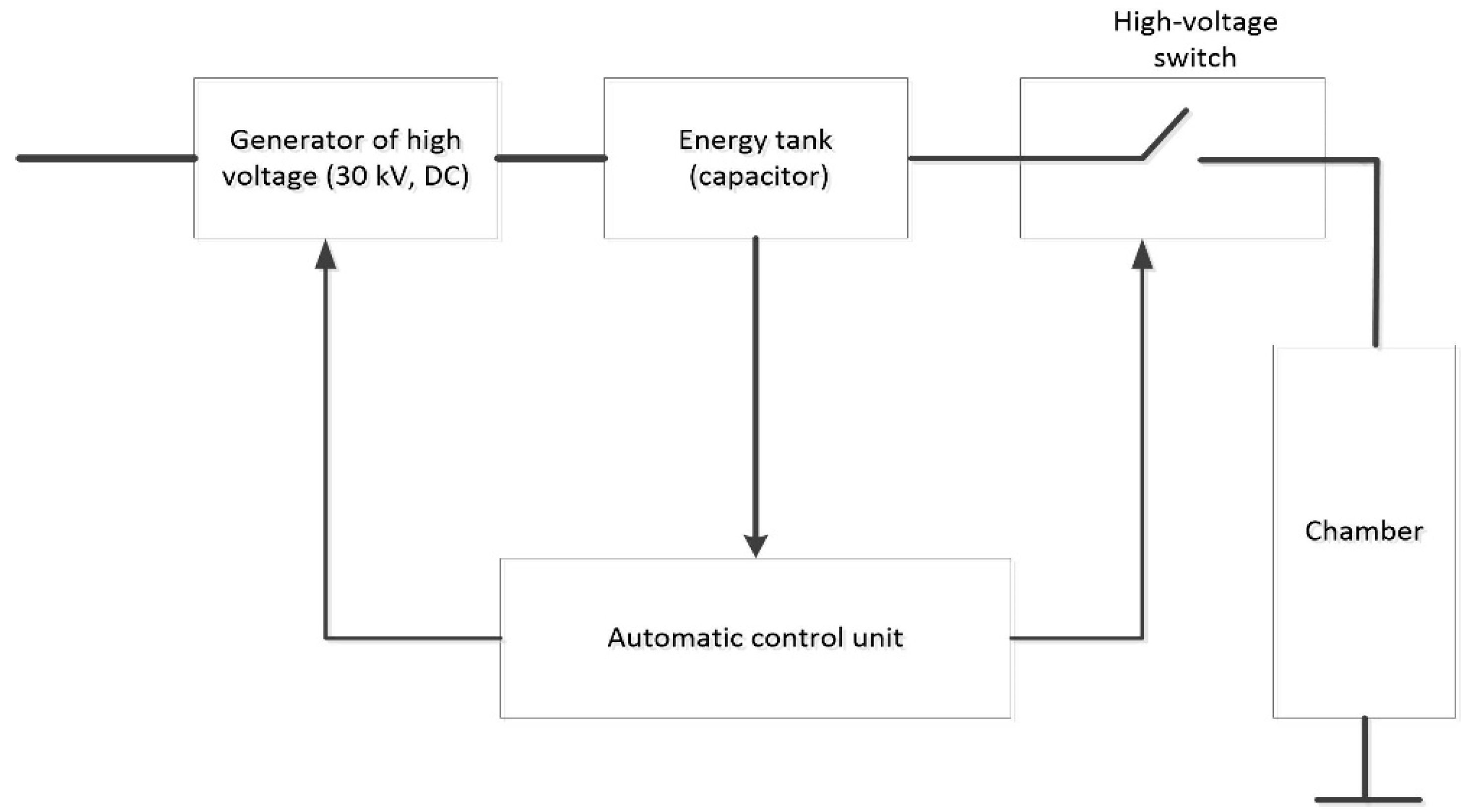
2.3. High Voltage Electric Discharge-Assisted Extraction
2.4. Reference Extraction Procedure
2.5. Determination of the Physical Properties of Extracts (pH, Conductivity and Temperature)
2.6. Spectrophotometric Analysis
2.7. High Performance Liquid Chromatography (HPLC)
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Influence of HVED Treatment on Physical Properties of Tobacco Waste Extracts
3.2. Influence of HVED Treatment on Total Phenol Content and Radical Scavenging Activity of HVED Extracts
3.3. Influence of HVED Treatment on Extraction of CA from Tobacco Waste
3.4. Comparison of HVED Assisted Extraction Efficiency with Conventional Extraction Technique
3.5. Optimization of HVED Parameters and Validation of Proposed Models
3.6. Role of Novel Extraction Techniques in Tobacco Sustainability Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability 2021, 13, 233. [Google Scholar] [CrossRef]
- Hendlin, Y.H.; Bialous, S.A. The environmental externalities of tobacco manufacturing: A review of tobacco industry reporting. Ambio 2020, 49, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Sifola, M.I.; Carrino, L.; Cozzolino, E.; del Piano, L.; Graziani, G.; Ritieni, A. Potential of Pre-Harvest Wastes of Tobacco (Nicotiana tabacum L.) Crops, Grown for Smoke Products, as Source of Bioactive Compounds (Phenols and Flavonoids). Sustainability 2021, 13, 2087. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind. Crop. Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Banožić, M.; Aladić, K.; Jerković, I.; Jokić, S. Volatile organic compounds of tobacco leaves versus waste (scrap, dust, and midrib): Extraction and optimization. J. Sci. Food Agric. 2021, 101, 1822–1832. [Google Scholar] [CrossRef]
- Chen, Y.; Jimmy Yu, Q.; Li, X.; Luo, Y.; Liu, H. Extraction and HPLC Characterization of Chlorogenic acid from Tobacco Residuals. Sep. Sci. Technol. 2007, 42, 3481–3492. [Google Scholar] [CrossRef] [Green Version]
- Banožić, M.; Banjari, I.; Jakovljević, M.; Šubarić, D.; Tomas, S.; Babić, J.; Jokić, S. Optimization of Ultrasound-Assisted Extraction of Some Bioactive Compounds from Tobacco Waste. Molecules 2019, 24, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lu, D.; Zhao, H.; Jiang, B.; Wang, J.; Ling, X.; Chai, H.; Ouyang, P. Discrimination and classification of tobacco wastes by identification and quantification of polyphenols with LC–MS/MS. J. Serb. Chem. Soc. 2010, 75, 875–891. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Banožić, M.; Škerget, M. Separation of active compounds from tobacco waste using subcritical water extraction. J. Supercrit. Fluids 2019, 153, 104593. [Google Scholar] [CrossRef]
- Rincón, J.; De Lucas, A.; García, M.A. Preliminary study on the supercritical carbon dioxide extraction of nicotine from tobacco wastes. Sep. Sci. Technol. 1998, 33, 411–423. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W. Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J. Supercrit. Fluids 2018, 138, 228–237. [Google Scholar] [CrossRef]
- Banožić, M.; Matić, M.; Šafranko, S.; Cikoš, A.; Jakovljević, M.; Molnar, M.; Jokić, S. Ekstrakcija bioaktivnih spojeva iz duhanskog otpada primjenom eutektičkih otapala. Kemija u Industriji 2020, 69, 1–10. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52. [Google Scholar] [CrossRef]
- Boussetta, N.; Reess, T.; Vorobiev, E.; Lanoisellé, J.L. Pulsed electrical discharges: Principles and application to extraction of biocompounds. In Enhancing Extraction Processes in the Food Industry; Lebovka, N., Vorobiev, E., Chemat, F., Eds.; Taylor & Francis: Abingdon, UK; CRC Press: Abingdon, UK, 2012; pp. 145–172. [Google Scholar]
- Barskaya, A.V.; Kuretz, B.I.; Lobanova, G.L. Extraction of water soluble matters from vegetative raw material by electrical pulsed discharges. In Proceedings of the 1st International Congress on Radiation Physics, High Current Electronics, and Modification of Materials, Tomsk, Russia, 14–29 September 2000; pp. 533–535. [Google Scholar]
- Jokić, S.; Pavlović, N.; Jozinović, A.; Ačkar, Đ.; Babić, J.; Šubarić, D. High-voltage electric discharge extraction of bioactive compounds from the cocoa bean shell. Chem. Biochem. Eng. Q. 2019, 33, 271–280. [Google Scholar] [CrossRef]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chim. 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Križić, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Ačkar, Đ. Impact of high-voltage electric discharge treatment on cocoa shell phenolic components and methylxanthines. J. Food Process Eng. 2019, 43, e13057. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Deng, Y.; Ju, T.; Xi, J. Circulating Polyphenols Extraction System with High Voltage Electrical Discharge: Design and Performance Evaluation. Sustain. Chem. Eng. 2018, 6, 15402–15410. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Vorobiev, E.; Lebovka, N. Electrotechnologies for Extraction from Food Plants and Biomaterials; Springer: New York, NY, USA, 2008; pp. 39–81. [Google Scholar]
- Vorobiev, E.; Lebovka, N. Enhanced extraction from solid foods and biosuspensions by pulsed electrical energy. Food Eng. Rev. 2010, 2, 95–108. [Google Scholar] [CrossRef]
- Molnar, M.; Jerković, I.; Suknović, D.; Rajs, B.B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules 2017, 22, 348. [Google Scholar] [CrossRef] [Green Version]
- Bas, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Locke, B.R. Environmental applications of electrical discharge plasma with liquid water—A mini review. Curr. Res. Nutr. Food Sci. J. 2012, 6, 194–203. [Google Scholar]
- Poojitha, P.; Athmaselvi, K.A. Changes in Physicochemical and Bioactive Profile of Garlic Pulp by High Voltage Electric Field. Curr. Res. Nutr. Food Sci. J. 2020, 8, 131–143. [Google Scholar] [CrossRef]
- Nutrizio, M.; Kljusurić, J.G.; Marijanović, Z.; Dubrović, I.; Viskić, M.; Mikolaj, E.; Chemat, F.; Jambrak, A.R. The Potential of High Voltage Discharges for Green Solvent Extraction of Bioactive Compounds and Aromas from Rosemary (Rosmarinus officinalis L.)—Computational Simulation and Experimental Methods. Molecules 2020, 25, 3711. [Google Scholar] [CrossRef] [PubMed]
- Briški, F.; Kopčić, N.; Ćosić, I.; Kučić, D.; Vuković, M. Biodegradation of tobacco waste by composting: Genetic identification of nicotine-degrading bacteria and kinetic analysis of transformations in leachate. Chem. Pap. 2012, 66, 1103–1110. [Google Scholar] [CrossRef]
- Tangkoonboribun, R.; Sassanarakkit, S. Molluscicide from Tobacco Waste. J. Agric. Sci. 2009, 1, 76. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Bogomaz, A.A.; Goryachev, V.L.; Remmenui, A.S.; Rutberg, P.G. Efficiency of pulsed electric discharges in water disinfection. Lett. J. Theor. Phys. 1991, 17, 65–68. [Google Scholar]
- Chen, Y.-S.; Zhang, X.-S.; Dai, Y.-C.; Yuan, W.-K. Pulsed high-voltage discharge plasma for degradation of phenol in aqueous solution. Sep. Purif. Technol. 2004, 34, 5–12. [Google Scholar] [CrossRef]
- Nasr, S.B.; Aazza, S.; Mnif, W.; Miguel, M. Phenol content and antioxidant activity of different young and adult plant parts of tobacco from Tunisia, dried at 40 and 70 °C. J. Appl. Pharm. Sci. 2014, 4, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Huang, D.; Tang, Z.; Deng, C.; Zhanga, X. Fast determination of chlorogenic acid in tobacco residues using microwave-assisted extraction and capillary zone electrophoresis technique. Talanta 2010, 82, 1181–1185. [Google Scholar] [CrossRef]
- Wenwen, Z.; Yuru, C.; Shaofeng, L.; Kewei, D.; Yan, C.; Shijie, Y. Separation and purification of CA from tobacco by-products by polyamide and silicagel column chromatography. Afr. J. Biotechnol. 2015, 14, 1731–1736. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci. Rep. 2015, 5, srep14119. [Google Scholar] [CrossRef] [PubMed]
- Ayomi, M.W. Tobacco industry and sustainability: A case of Indonesia Cigaretes Company. Asia Pac. Manag. Bus. Appl. 2015, 2, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Abay, C.; Miran, B.; Günden, C. An analysis of input use efficiency in tobacco production with respect to sustainability: The case study of Turkey. J. Sustain. Agric. 2004, 24, 123–143. [Google Scholar] [CrossRef]
- Adesanya, A.; Yang, B.; Iqdara, F.W.B.; Yang, Y. Improving sustainability performance through supplier relationship management in the tobacco industry. Supply Chain Manag. 2020, 25, 413–426. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]



| Run | Solvent:Solid Ratio (mL/g) | Frequency (Hz) | Time (min) |
|---|---|---|---|
| 1 | 700 | 40 | 30 |
| 2 | 300 | 40 | 30 |
| 3 | 300 | 100 | 30 |
| 4 | 500 | 40 | 45 |
| 5 | 700 | 70 | 45 |
| 6 | 500 | 100 | 45 |
| 7 | 500 | 70 | 30 |
| 8 | 500 | 100 | 15 |
| 9 | 500 | 70 | 30 |
| 10 | 500 | 70 | 30 |
| 11 | 500 | 40 | 15 |
| 12 | 500 | 70 | 30 |
| 13 | 700 | 100 | 30 |
| 14 | 300 | 70 | 45 |
| 15 | 500 | 70 | 30 |
| 16 | 700 | 70 | 15 |
| 17 | 300 | 70 | 15 |
| Tobacco Waste | Run | Starting Temperature (°C) | Final Temperature (°C) | pH-Value before Treatment | pH-Value after Treatment | Conductivity (μS/cm) before Treatment | Conductivity (μS/cm) after Treatment | Z Value | |
|---|---|---|---|---|---|---|---|---|---|
| Scrap | |||||||||
| 1 | 22.5 | 32.1 | 5.96 | 6.46 | 298 | 310 | 0.14 | ||
| 2 | 22.8 | 33.0 | 6.17 | 6.15 | 307 | 329 | 0.28 | ||
| 3 | 22.8 | 39.5 | 6.02 | 6.08 | 336 | 355 | 0.38 | ||
| 4 | 23.1 | 38.2 | 6.46 | 6.60 | 216 | 236 | 0.12 | ||
| 5 | 22.5 | 40.7 | 6.52 | 6.75 | 181 | 201 | 0.10 | ||
| 6 | 23.0 | 45.0 | 6.61 | 6.55 | 220 | 243 | 0.14 | ||
| 7 | 22.4 | 36.8 | 6.60 | 6.80 | 231 | 251 | 0.17 | ||
| 8 | 23.3 | 32.3 | 6.66 | 6.62 | 220 | 237 | 0.10 | ||
| 9 | 22.4 | 37.4 | 6.72 | 6.76 | 220 | 230 | 0.12 | ||
| 10 | 22.3 | 37.7 | 6.61 | 6.55 | 219 | 234 | 0.09 | ||
| 11 | 22.8 | 28.8 | 6.5 | 6.59 | 242 | 258 | 0.11 | ||
| 12 | 22.7 | 38.7 | 6.51 | 6.58 | 216 | 234 | 0.11 | ||
| 13 | 22.7 | 37.0 | 6.89 | 6.00 | 159 | 187 | 0.12 | ||
| 14 | 22.6 | 43.4 | 6.43 | 6.49 | 339 | 361 | 0.47 | ||
| 15 | 22.5 | 38.0 | 6.60 | 6.55 | 216 | 235 | 0.11 | ||
| 16 | 23.4 | 31.0 | 6.00 | 6.22 | 172 | 188 | 0.07 | ||
| 17 | 22.8 | 31.0 | 6.61 | 6.45 | 371 | 386 | 1.00 | ||
| Dust | |||||||||
| 1 | 22.9 | 34.4 | 6.27 | 6.72 | 187 | 207 | 0.05 | ||
| 2 | 22.9 | 34.4 | 6.39 | 6.80 | 292 | 316 | 0.09 | ||
| 3 | 21.1 | 38.9 | 6.47 | 6.77 | 305 | 325 | 0.08 | ||
| 4 | 22.4 | 38.6 | 6.21 | 6.91 | 186 | 209 | 0.06 | ||
| 5 | 22.8 | 41.5 | 6.31 | 6.81 | 146 | 170 | 0.06 | ||
| 6 | 22.4 | 41.7 | 6.23 | 6.88 | 332 | 370 | 0.17 | ||
| 7 | 22.4 | 38.3 | 6.38 | 6.89 | 347 | 382 | 0.16 | ||
| 8 | 22.7 | 33.2 | 6.34 | 6.55 | 188 | 213 | 0.07 | ||
| 9 | 22.5 | 37.4 | 6.46 | 6.87 | 335 | 395 | 0.27 | ||
| 10 | 22.8 | 38.4 | 6.57 | 6.79 | 389 | 421 | 0.19 | ||
| 11 | 22.8 | 29.8 | 6.31 | 6.52 | 189 | 206 | 0.05 | ||
| 12 | 22.8 | 37.3 | 6.31 | 6.75 | 190 | 215 | 0.17 | ||
| 13 | 23.1 | 37.0 | 6.34 | 6.64 | 256 | 301 | 0.05 | ||
| 14 | 22.9 | 43.0 | 6.29 | 6.76 | 507 | 560 | 1.00 | ||
| 15 | 22.9 | 40.1 | 6.27 | 6.83 | 162 | 171 | 0.21 | ||
| 16 | 22.5 | 22.7 | 6.19 | 6.31 | 150 | 184 | 0.08 | ||
| 17 | 22.4 | 30.7 | 6.59 | 6.56 | 312 | 343 | 0.13 | ||
| Midrib | |||||||||
| 1 | 22.3 | 36.8 | 5.84 | 5.85 | 458 | 607 | 0.34 | ||
| 2 | 22.6 | 36.5 | 5.71 | 5.71 | 748 | 845 | 0.67 | ||
| 3 | 22.6 | 38.1 | 5.55 | 5.79 | 755 | 819 | 0.47 | ||
| 4 | 22.7 | 38.3 | 5.48 | 5.71 | 851 | 892 | 1.00 | ||
| 5 | 23.1 | 42.4 | 5.63 | 5.87 | 396 | 451 | 0.11 | ||
| 6 | 22.3 | 41.6 | 5.71 | 5.93 | 368 | 441 | 0.14 | ||
| 7 | 23.7 | 39.2 | 5.44 | 5.70 | 564 | 651 | 0.27 | ||
| 8 | 22.4 | 30.5 | 5.71 | 5.70 | 397 | 490 | 0.19 | ||
| 9 | 22.6 | 38.5 | 5.65 | 5.63 | 476 | 515 | 0.09 | ||
| 10 | 22 | 37.7 | 5.66 | 5.70 | 435 | 490 | 0.12 | ||
| 11 | 22.4 | 28.1 | 5.35 | 5.68 | 460 | 514 | 0.13 | ||
| 12 | 20.9 | 36.2 | 5.84 | 5.75 | 403 | 479 | 0.16 | ||
| 13 | 21.1 | 36.1 | 5.72 | 5.7 | 348 | 410 | 0.11 | ||
| 14 | 21.4 | 42.4 | 5.42 | 5.71 | 760 | 842 | 0.62 | ||
| 15 | 21.7 | 38.2 | 5.47 | 5.75 | 490 | 532 | 0.10 | ||
| 16 | 21.5 | 28.6 | 5.64 | 5.75 | 346 | 371 | 0.05 | ||
| 17 | 21.9 | 29.5 | 5.4 | 5.54 | 675 | 761 | 0.40 | ||
| Tobacco Waste | ||||||
|---|---|---|---|---|---|---|
| RUN | Scrap | Dust | Midrib | |||
| TPC (mg GAE/100 g) | DPPH (%) | TPC (mg GAE/100 g) | DPPH (%) | TPC (mg GAE/100 g) | DPPH (%) | |
| 1 | 36.49 | 53.54 | 48.16 | 48.83 | 28.86 | 49.39 |
| 2 | 17.56 | 49.28 | 28.95 | 44.75 | 8.64 | 45.29 |
| 3 | 84.64 | 59.41 | 21.02 | 46.19 | 12.64 | 45.67 |
| 4 | 43.63 | 58.62 | 42.99 | 53.00 | 27.09 | 48.71 |
| 5 | 40.98 | 50.68 | 50.49 | 48.32 | 26.62 | 47.19 |
| 6 | 38.24 | 53.68 | 52.09 | 39.40 | 12.99 | 47.58 |
| 7 | 34.14 | 50.27 | 32.09 | 49.38 | 19.40 | 49.20 |
| 8 | 33.50 | 52.49 | 65.04 | 49.90 | 15.68 | 65.24 |
| 9 | 27.73 | 49.90 | 43.76 | 50.38 | 16.58 | 49.77 |
| 10 | 25.17 | 50.82 | 46.19 | 56.10 | 18.24 | 47.15 |
| 11 | 29.40 | 54.84 | 41.32 | 50.02 | 14.53 | 47.72 |
| 12 | 30.04 | 52.15 | 45.55 | 51.71 | 17.22 | 50.00 |
| 13 | 49.41 | 52.83 | 75.62 | 48.35 | 20.34 | 47.38 |
| 14 | 22.25 | 52.59 | 28.33 | 46.43 | 10.18 | 51.40 |
| 15 | 23.37 | 49.86 | 52.47 | 41.05 | 16.45 | 50.27 |
| 16 | 41.87 | 50.58 | 78.85 | 47.91 | 19.44 | 49.89 |
| 17 | 23.87 | 51.53 | 31.10 | 47.29 | 7.25 | 48.25 |
| RUN | Tobacco Waste | ||
|---|---|---|---|
| Scrap | Dust | Midrib | |
| CA (mg/100 g) | CA (mg/100 g) | CA (mg/100 g) | |
| 1 | 3.66 | 2.36 | 3.14 |
| 2 | 2.69 | 2.38 | 2.48 |
| 3 | 1.54 | 2.43 | 2.51 |
| 4 | 3.33 | 2.14 | 3.38 |
| 5 | 2.93 | 2.45 | 2.65 |
| 6 | 2.23 | 1.97 | 2.30 |
| 7 | 2.81 | 2.10 | 2.77 |
| 8 | 2.28 | 2.31 | 2.48 |
| 9 | 2.63 | 2.06 | 2.53 |
| 10 | 2.32 | 2.08 | 2.37 |
| 11 | 3.38 | 2.38 | 2.44 |
| 12 | 2.54 | 2.11 | 2.39 |
| 13 | 3.28 | 2.43 | 2.55 |
| 14 | 2.88 | 2.41 | 2.53 |
| 15 | 2.45 | 1.90 | 2.60 |
| 16 | 3.41 | 2.96 | 2.53 |
| 17 | 2.84 | 2.90 | 2.35 |
| Tobacco Waste | Scrap | Dust | Midrib | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Parameters | TPC (mgGAE/100 g) | DPPH (%) | CA (mg/100 g) | TPC (mgGAE/100 g) | DPPH (%) | CA (mg/100 g) | TPC (mgGAE/100 g) | DPPH (%) | CA (mg/100 g) | |
| C1 | 1/300 mL/g, 15 min | 17.21 | 41.01 | 2.09 | 15.85 | 39.54 | 2.20 | 5.01 | 31.05 | 1.71 |
| C2 | 1/300 mL/g, 30 min | 18.13 | 42.05 | 2.11 | 16.01 | 40.01 | 1.89 | 5.66 | 37.15 | 1.79 |
| C3 | 1/300 mL/g, 45 min | 19.21 | 49.97 | 2.48 | 16.55 | 42.55 | 2.12 | 5.91 | 44.07 | 2.02 |
| C4 | 1/500 mL/g, 15 min | 19.38 | 33.80 | 2.18 | 11.13 | 21.22 | 1.81 | 4.15 | 17.71 | 1.78 |
| C5 | 1/500 mL/g, 30 min | 22.58 | 42.21 | 2.31 | 14.72 | 29.87 | 1.98 | 8.01 | 25.96 | 1.81 |
| C6 | 1/500 mL/g, 45 min | 32.22 | 48.78 | 2.98 | 29.87 | 37.11 | 2.02 | 9.58 | 34.21 | 1.85 |
| C7 | 1/700 mL/g, 15 min | 27.15 | 31.78 | 1.81 | 25.87 | 26.87 | 1.74 | 10.87 | 29.52 | 1.51 |
| C8 | 1/700 mL/g, 30 min | 37.42 | 39.56 | 2.01 | 31.47 | 21.78 | 1.80 | 11.01 | 31.45 | 1.89 |
| C9 | 1/700 mL/g, 45 min | 38.47 | 41.74 | 2.62 | 37.95 | 39.99 | 2.22 | 25.65 | 38.03 | 2.13 |
| Optimal Parameters and Results | Tobacco Waste | ||
|---|---|---|---|
| Scrap | Dust | Midrib | |
| Solvent:solid ratio (mL/g) | 695.24 | 700.00 | 700.00 |
| Frequency (Hz) | 47.16 | 58.5 | 40.01 |
| Time (min) | 15.06 | 15.0 | 15.00 |
| Predicted CA content (mg/mL) | 3.82 | 2.88 | 2.73 |
| Obtained CA content (mg/mL) | 3.91 | 2.79 | 2.68 |
| Predicted temperature changes (°C) | 5.97 | 1.99 | 6.21 |
| Obtained temperature changes (°C) | 6.02 | 2.01 | 2.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banožić, M.; Jozinović, A.; Grgić, J.; Miličević, B.; Jokić, S. High Voltage Electric Discharge for Recovery of Chlorogenic Acid from Tobacco Waste. Sustainability 2021, 13, 4481. https://doi.org/10.3390/su13084481
Banožić M, Jozinović A, Grgić J, Miličević B, Jokić S. High Voltage Electric Discharge for Recovery of Chlorogenic Acid from Tobacco Waste. Sustainability. 2021; 13(8):4481. https://doi.org/10.3390/su13084481
Chicago/Turabian StyleBanožić, Marija, Antun Jozinović, Jovana Grgić, Borislav Miličević, and Stela Jokić. 2021. "High Voltage Electric Discharge for Recovery of Chlorogenic Acid from Tobacco Waste" Sustainability 13, no. 8: 4481. https://doi.org/10.3390/su13084481
APA StyleBanožić, M., Jozinović, A., Grgić, J., Miličević, B., & Jokić, S. (2021). High Voltage Electric Discharge for Recovery of Chlorogenic Acid from Tobacco Waste. Sustainability, 13(8), 4481. https://doi.org/10.3390/su13084481








