Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge
Abstract
1. Introduction
2. Materials and Methods
2.1. Cocoa Shell Preparation
2.2. Chocolate Production
2.3. Rheological Properties
2.4. Particle Size Distribution
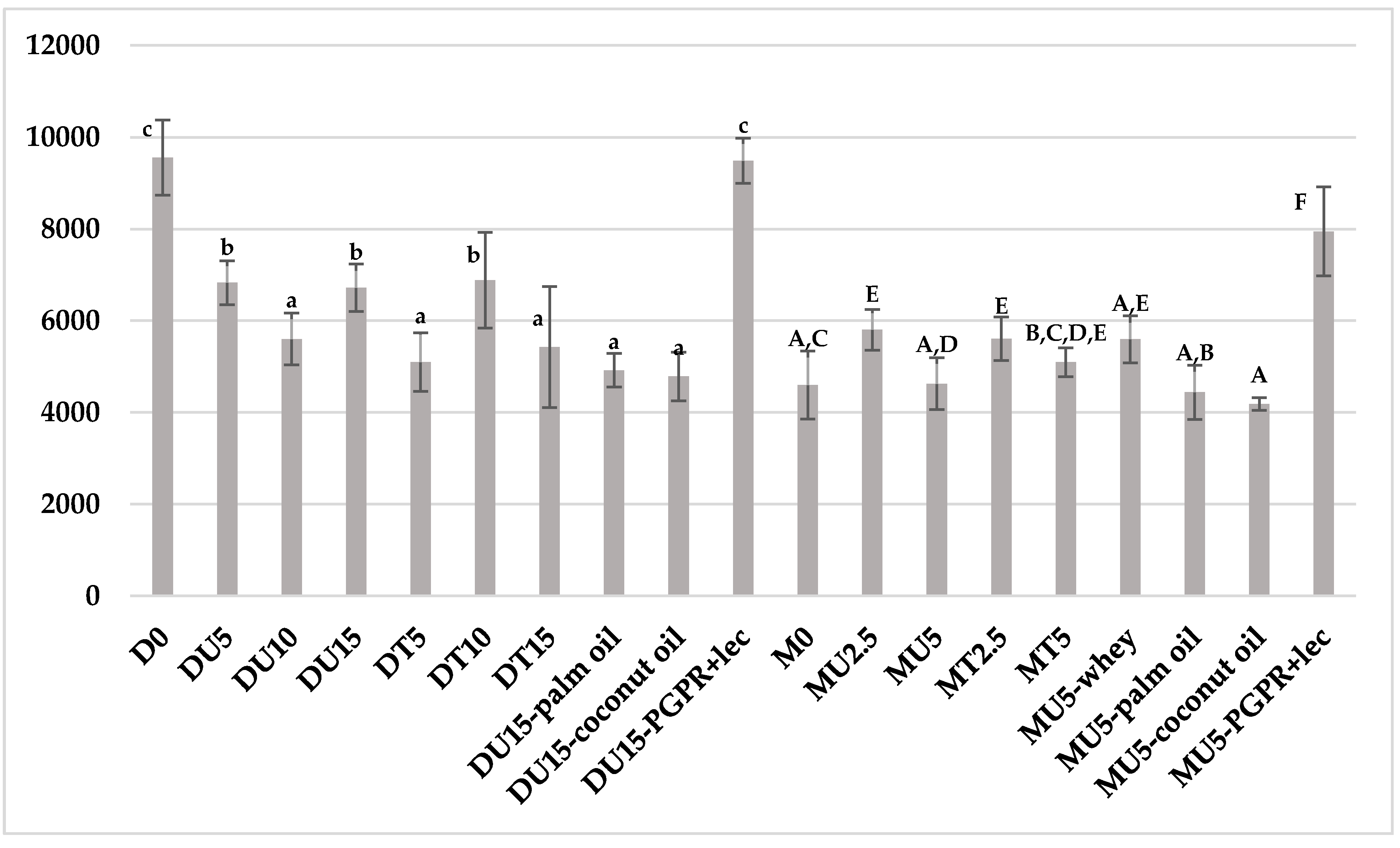
2.5. Hardness
2.6. Color
2.7. Statistical Analysis
3. Results and Discussion
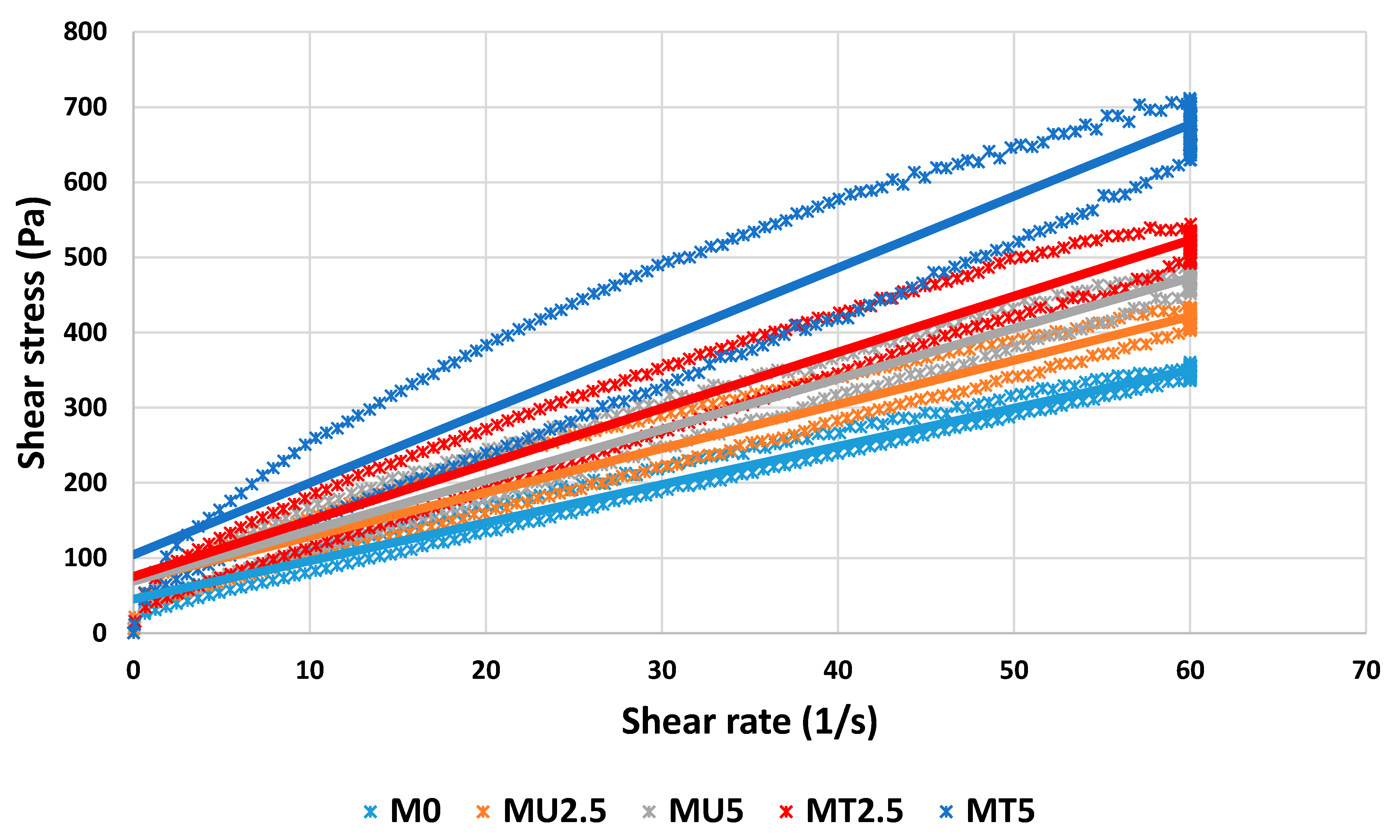
3.1. Rheological Properties
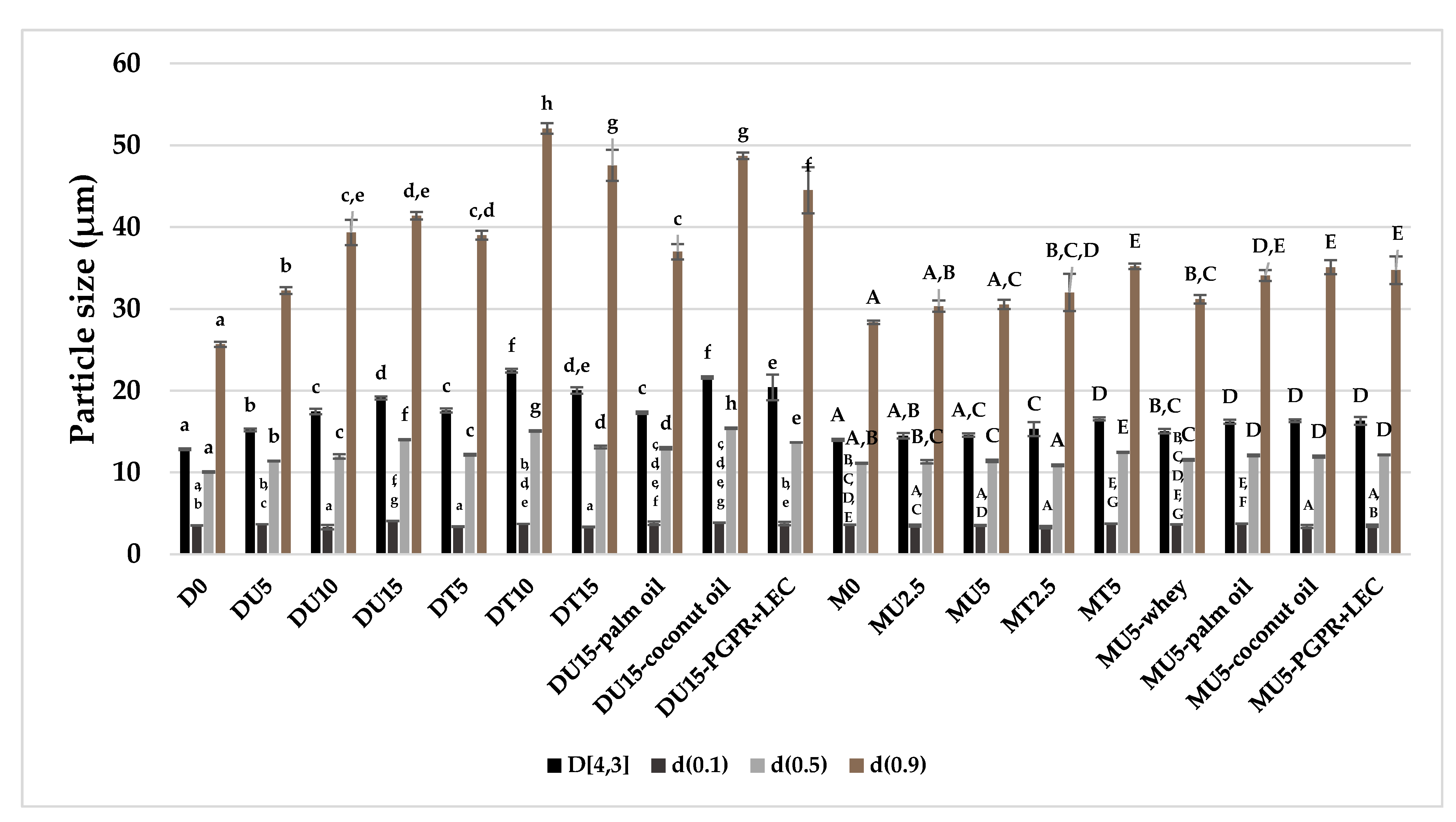
3.2. Particle Size Distribution
3.3. Hardness
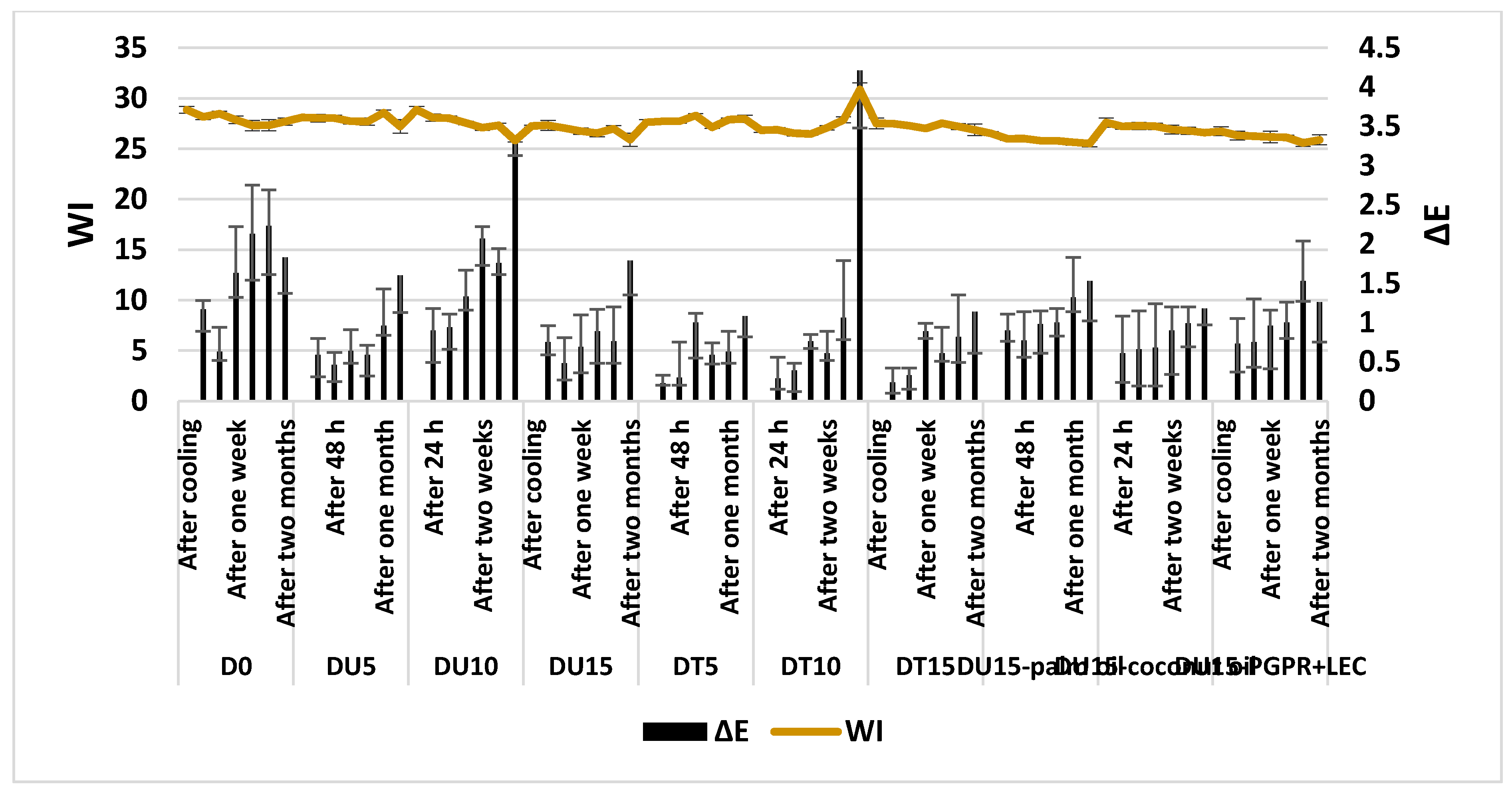
3.4. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Jokić, S.; Grgić, I.; Ačkar, Đ. Effect of Addition of Fibres and Polyphenols on Properties of Chocolate–A Review. Food Rev. Int. 2019, 1–19. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Rheological properties, melting behaviours and physical quality characteristics of sugar-free chocolates processed using inulin/polydextrose bulking mixtures sweetened with stevia and thaumatin extracts. LWT 2015, 62, 592–597. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Zeppa, G.; Stévigny, C. Cocoa Bean Shell—A By-Product with Nutritional Properties and Biofunctional Potential. Nutrients 2020, 12, 1123. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Bruna, C.; Eichholz, I.; Rohn, S.; Kroh, W.L.; Huyskens-Keil, S. Bioactive compounds and antioxidant activity of cocoa hulls (Theobroma cacao L.) from different origins. J. Appl. Bot. Food Qual. 2009, 83, 9–13. [Google Scholar]
- Bernaert, H.; Ruysscher, I.D. Process of producing cocoa shell powder. US Patent 9,375,024B2, 28 June 2016. [Google Scholar]
- Stulić, V.; Vukušić, T.; Jambrak, A.R.; Bačun-Družina, V.; Popović, D.; Mrvčić, J.; Herceg, Z. Quantitative microbial assessment for Escherichia coli after treatment by high voltage gas phase plasma. Innov. Food Sci. Emerg. Technol. 2019, 53, 26–35. [Google Scholar] [CrossRef]
- Grinevich, V.I.; Kvitkova, E.Y.; Plastinina, N.A.; Rybkin, V.V. Application of Dielectric Barrier Discharge forWaste Water Purification. Plasma Chem. Plasma Process. 2011, 201131, 573–583. [Google Scholar] [CrossRef]
- IOCCC (International Office of Cocoa, Chocolate, and Sugar Confectionery). Viscosity of Cocoa and Chocolate Products (Analytical Method: 46); CAOBISCO: Belgium, Brussels, 2000. [Google Scholar]
- Bonarius, G.A.; Vieira, J.B.; van der Goot, A.J.; Bodnár, I. Rheological behaviour of fibre-rich plant materials in fat-based food systems. Food Hydrocoll. 2014, 40, 254–261. [Google Scholar] [CrossRef]
- Barišić, V.; Flanjak, I.; Kopjar, M.; Benšić, M.; Jozinović, A.; Babić, J.; Šubarić, D.; Miličević, B.; Doko, K.; Jašić, M.; et al. Does High Voltage Electrical Discharge Treatment Induce Changes in Tannin and Fiber Properties of Cocoa Shell? Foods 2020, 9, 810. [Google Scholar] [CrossRef]
- Bolenz, S.; Amtsberg, K.; Schape, R. The broader usage of sugars and fillers in milk chocolate made possible by the new EC cocoa directive. Int. J. Food Sci. Technol. 2006, 41, 45–55. [Google Scholar] [CrossRef]
- Chaloeichitratham, N.; Mawilai, P.; Pongsuttiyakorn, T.; Pornchalermpong, P. Effect of drying methods on properties of green curry powder. MATEC Web Conf. 2018, 192, 03023. [Google Scholar] [CrossRef]
- Boussetta, N.; Turk, M.; De Taeye, C.; Larondelle, Y.; Lanoisellé, J.L.; Vorobiev, E. Effect of high voltage electrical discharges, heating and ethanol concentration on the extraction of total polyphenols and lignans from flaxseed cake. Ind. Crops Prod. 2013, 49, 690–696. [Google Scholar] [CrossRef]
- Rezende, N.V.; Benassi, M.T.; Vissotto, F.Z.; Augusto, P.P.C.; Grossmann, M.V.E. Mixture design applied for the partial replacement of fat with fibre in sucrose-free chocolates. LWT 2015, 62, 598–604. [Google Scholar] [CrossRef]
- Abdul Halim, S.H.; Selamat, J.; Hamed Mirhosseini, S.; Hussain, N. Sensory Preferences and Bloom Stability of Chocolate Containing Cocoa Butter Substitute from Coconut Oil. J. Saudi Soc. Agric. Sci. 2018, 18, 443–448. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Particle size distribution and compositional effects on textural properties and appearance of dark chocolates. J. Food Eng. 2008, 87, 181–190. [Google Scholar] [CrossRef]
- Ziegler, G.R.; Mongia, G.; Hollender, R. Role of particle size distribution of suspended solids in defining the sensory properties of milk chocolate. Int. J. Food Prop. 2001, 4, 175–192. [Google Scholar] [CrossRef]
- Feichtinger, A.; Scholten, E.; Sala, G. Effect of particle size distribution on rheological properties of chocolate. Food Funct. 2020, 11, 9547–9559. [Google Scholar] [CrossRef] [PubMed]
- Konar, N.; Özhan, B.; Artık, N.; Dalabasmaz, S.; Poyrazoglu, E.S. Rheological and physical properties of Inulin-containing milk chocolate prepared at different process conditions. CYTA J. Food 2013, 12, 55–64. [Google Scholar] [CrossRef]
- Do, T.A.L.; Hargreveas, J.M.; Wolf, B.; Hort, J.; Mitchell, J.R. Impact of particle size distribution on rheological and textural properties of chocolate models with reduced fat content. J. Food Sci. 2007, 72, E541–E552. [Google Scholar] [CrossRef]
- Lee, S.; Biresaw, G.; Kinney, M.P.; Inglett, G.E. Effect of cocoa butter replacement with a β-glucan-rich hydrocolloid (C-trim30) on the rheological and tribological properties of chocolates. J. Sci. Food Agric. 2009, 89, 163–167. [Google Scholar] [CrossRef]
- Barišić, V.; Lončarević, I.; Petrović, J.; Flanjak, I.; Jozinović, A.; Šubarić, D.; Babić, J.; Miličević, B.; Ačkar, Đ. Effect of coconut and palm oil on chocolate produced in ball mill. In 7th International Conference “Vallis Aurea” Focus on: Research & Innovation; Katalinić, B., Ed.; Polytechnic in Požega, Croatia & DAAAM International Vienna: Požega Croatia; Vienna, Austria, 2020; pp. 33–41. [Google Scholar]
- Verstringe, S.; De Clercq, N.; Nguyen, M.; Kadivar, S.; Dewettinck, K. Enzymatic and other modification techniques to produce cocoa butter alternatives. In Cocoa Butter and Related Compounds; Garti, N., Widlak, N.R., Eds.; AOAC Press: Urbana, IL, USA, 2012; pp. 443–474. [Google Scholar]
- Sözeri Atik, D.; Bölük, E.; Toker, O.S.; Palabiyik, I.; Konar, N. Investigating the effects of Lecithin-PGPR mixture on physical properties of milk chocolate. LWT 2020, 109548. [Google Scholar] [CrossRef]
- Erdem, Ö.; Gültekin-Özgüven, M.; Berktaş, I.; Erşan, S.; Tuna, H.E.; Karadağ, A.; Özçelik, B.; Güneşa, G.; Cutting, S.M. Development of a novel synbiotic dark chocolate enriched with Bacillus indicus HU36, maltodextrin and lemon fiber: Optimization by response surface methodology. LWT 2014, 56, 187–193. [Google Scholar] [CrossRef]
- Shourideh, M.; Taslimi, A.; Azizi, M.H.; Mohammadifar, M.A. Effects of D-Tagatose and Inulin on Some Physicochemical, Rheological and Sensory Properties of Dark Chocolate. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 314–319. [Google Scholar] [CrossRef][Green Version]
- Shah, A.B.; Jones, G.P.; Vasiljevic, T. Sucrose-free chocolate sweetened with Stevia rebaudiana extract and containing different bulking agents-effects on physicochemical and sensory properties. Int. J. Food Sci. Technol. 2010, 45, 1426–1435. [Google Scholar] [CrossRef]
- Toker, O.S.; Palabiyik, I.; Pirouzian, H.R.; Aktar, T.; Konar, N. Chocolate aroma: Factors, importance and analysis. Trends Food Sci. Technol. 2020, 99, 580–592. [Google Scholar] [CrossRef]
- Lohman, M.H.; Hartel, R.W. Effect of milk fat fractions on fat bloom in dark chocolate. J. Am. Oil Chem. Soc. 1994, 71, 267–276. [Google Scholar] [CrossRef]





| Sample * | Cocoa Mass (%) | Cocoa Butter (%) | Milk Powder (%) | Untreated Cocoa Shells (%) | Treated Cocoa Shells (%) | Lecithin (%) |
|---|---|---|---|---|---|---|
| D0 | 36.00 | 21.57 | 0 | 0 | 0 | 0.40 |
| DU5 | 31.00 | 21.57 | 0 | 5.00 | 0 | 0.40 |
| DU10 | 26.00 | 21.57 | 0 | 10.00 | 0 | 0.40 |
| DU15 | 21.00 | 21.57 | 0 | 15.00 | 0 | 0.40 |
| DT5 | 31.00 | 21.57 | 0 | 0 | 5.00 | 0.40 |
| DT10 | 26.00 | 21.57 | 0 | 0 | 10.00 | 0.40 |
| DT15 | 21.00 | 21.57 | 0 | 0 | 15.00 | 0.40 |
| M0 | 14.74 | 24.83 | 15.00 | 0 | 0 | 0.40 |
| MU2.5 | 12.24 | 24.83 | 15.00 | 2.50 | 0 | 0.40 |
| MU5 | 9.74 | 24.83 | 15.00 | 5.00 | 0 | 0.40 |
| MT2.5 | 12.24 | 24.83 | 15.00 | 0 | 2.50 | 0.40 |
| MT5 | 9.74 | 24.83 | 15.00 | 0 | 5.00 | 0.40 |
| Sample * | Cocoa Mass (%) | Cocoa Butter (%) | Milk Powder (%) | Untreated Cocoa Shells (%) | Palm Oil (%) | Coconut Oil (%) | Whey (%) | Lecithin (%) | PGPR (%) |
|---|---|---|---|---|---|---|---|---|---|
| DU15—palm oil | 21.00 | 16.57 | 0 | 15.00 | 5.00 | 0 | 0 | 0.40 | 0 |
| DU15—coconut oil | 21.00 | 16.57 | 0 | 15.00 | 0 | 5.00 | 0 | 0.40 | 0 |
| DU15—PGPR + LEC | 21.00 | 21.57 | 0 | 15.00 | 0 | 0 | 0 | 0.20 | 0.20 |
| MU5—whey | 9.74 | 24.83 | 10.00 | 5.00 | 0 | 0 | 5.00 | 0.40 | 0 |
| MU5—palm oil | 9.74 | 19.83 | 15.00 | 5.00 | 5.00 | 0 | 0 | 0.40 | 0 |
| MU5—coconut oil | 9.74 | 19.83 | 15.00 | 5.00 | 0 | 5.00 | 0 | 0.40 | 0 |
| MU5—PGPR + LEC | 9.74 | 24.83 | 15.00 | 5.00 | 0 | 0 | 0 | 0.20 | 0.20 |
| Depending Variable | Grouping Variable | p-Value * | Depending Variable | Grouping Variable | p-Value * |
|---|---|---|---|---|---|
| Hardness | Chocolate type | 0.000039 | SSA | Chocolate type | 0.269958 |
| Cocoa shell type | 0.128834 | Cocoa shell type | 0.226690 | ||
| Content of cocoa shells | 0.180568 | Content of cocoa shells | 0.009543 | ||
| WI after cooling | Chocolate type | <0.000001 | d(0.1) | Chocolate type | 0.709513 |
| Cocoa shell type | 0.003174 | Cocoa shell type | 0.410736 | ||
| Content of cocoa shells | 0.000001 | Content of cocoa shells | 0.328927 | ||
| ΔE after 2 months | Chocolate type | 0.068028 | d(0.5) | Chocolate type | 0.016237 |
| Cocoa shell type | 0.326695 | Cocoa shell type | 0.002778 | ||
| Content of cocoa shells | <0.000001 | Content of cocoa shells | <0.000001 | ||
| WI after 2 months | Chocolate type | <0.000001 | d(0.9) | Chocolate type | 0.000828 |
| Cocoa shell type | 0.01627 | Cocoa shell type | 0.000107 | ||
| Content of cocoa shells | 0.000001 | Content of cocoa shells | <0.000001 | ||
| D [4,3] | Chocolate type | 0.001408 | |||
| Cocoa shell type | 0.000124 | ||||
| Content of cocoa shells | <0.000001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barišić, V.; Petrović, J.; Lončarević, I.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Blažić, M.; Ačkar, Đ. Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge. Sustainability 2021, 13, 2620. https://doi.org/10.3390/su13052620
Barišić V, Petrović J, Lončarević I, Flanjak I, Šubarić D, Babić J, Miličević B, Doko K, Blažić M, Ačkar Đ. Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge. Sustainability. 2021; 13(5):2620. https://doi.org/10.3390/su13052620
Chicago/Turabian StyleBarišić, Veronika, Jovana Petrović, Ivana Lončarević, Ivana Flanjak, Drago Šubarić, Jurislav Babić, Borislav Miličević, Kristina Doko, Marijana Blažić, and Đurđica Ačkar. 2021. "Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge" Sustainability 13, no. 5: 2620. https://doi.org/10.3390/su13052620
APA StyleBarišić, V., Petrović, J., Lončarević, I., Flanjak, I., Šubarić, D., Babić, J., Miličević, B., Doko, K., Blažić, M., & Ačkar, Đ. (2021). Physical Properties of Chocolates Enriched with Untreated Cocoa Bean Shells and Cocoa Bean Shells Treated with High-Voltage Electrical Discharge. Sustainability, 13(5), 2620. https://doi.org/10.3390/su13052620










