Response of Potential Indicators of Soil Quality to Land-Use and Land-Cover Change under a Mediterranean Climate in the Region of Al-Jabal Al-Akhdar, Libya
Abstract
:1. Introduction
2. Materials and Methods
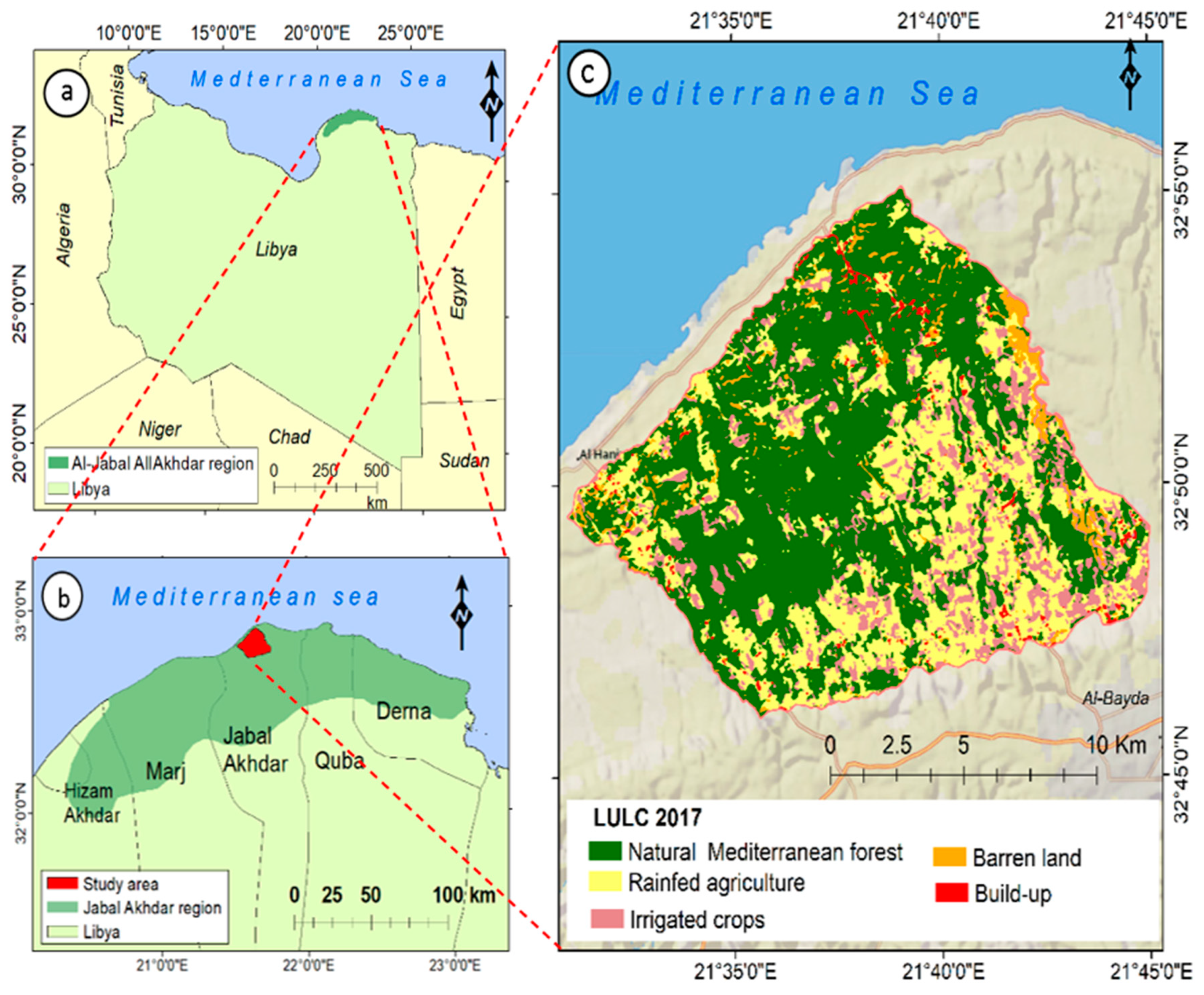
2.1. Study Area
2.2. Soil Sampling and Preparation
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Response of Chemical Soil Quality Indicators to Land-Use/Land-Cover (LULC) Change
3.1.1. Soil Organic Matter (SOM)
3.1.2. Soil Reaction (pH)
3.1.3. Electrical Conductivity (EC)
3.1.4. Calcium Carbonate Content (CaCO3)
3.1.5. Cation Exchange Capacity (CEC)
3.1.6. Available Potassium (Kav)
3.1.7. Available Phosphorus (Pav)
3.1.8. Nitrate Nitrogen (NO3-N)
3.2. Response of Physical Soil Quality Indicators to LULC Change
3.2.1. Soil Particle Size Distribution
3.2.2. Bulk Density (BD)
3.2.3. Soil Porosity
3.2.4. Available Water Holding Capacity (AWHC)
3.2.5. Saturated Hydraulic Conductivity (Ksat)
3.3. Response of Biological Soil Quality Indicator to LULC Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laytimi, A. Market and Trade Policies for Mediterranean Agriculture: The Case of Fruit/Vegetable and Olive Oil. Agricultural Situation Report—LIBYA; The EU Research Framework Programme: Chania, Greece, 2006. [Google Scholar]
- FAO. Country Profile—Libya; Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Zurqani, H.A.; Ben Mahmoud, K.R. Land Cover, Land Use, and Vegetation Distribution. In The Soils of Libya; Zurqani, H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 77–89. [Google Scholar] [CrossRef]
- OMU. Studying and Evaluating the Natural Vegetation in Al Jabal Al Akhdar Area; Omar Al-Mukhtar University: Al Bayda, Libya, 2005. (In Arabic) [Google Scholar]
- Mahmoud, A.; Hasmadi, I.M.; Alias, M.S.; Azani, A.M. Rangeland degradation assessment in the south slope of the Al-Jabal Al-Akhdar, northeast Libya using remote sensing technology. J. Rangel. Sci. 2016, 6, 73–81. [Google Scholar]
- Aburas, M.; Abdelrahman, Y. Soil depth in relation to soil properties at Lussaita area, Al-Jabal al Akhdar, Libya. Al-Mukhtar J. Sci. 2016, 1, 144–160. (In Arabic) [Google Scholar]
- Aburas, M.M. Assessment of Soil Erodibility in Relation to Soil Degradation and Land Use in Mediterranean Libya. Ph.D. Thesis, University of Newcastle, Newcastle, UK, 2009. [Google Scholar]
- Alawamy, J.S.; Balasundram, S.K.; Hanif, A.H.M.; Boon Sung, C.T. Detecting and analyzing land use and land cover changes in the region of Al-Jabal Al-Akhdar, Libya using time-series landsat data from 1985 to 2017. Sustainability 2020, 12, 4490. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Hossieni, M.; Zeraatpisheh, M.; Francaviglia, R. Land use change effects on soil quality and biological fertility: A case study in northern Iran. Eur. J. Soil Biol. 2019, 95, 103119. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Bakhshandeh, E.; Hosseini, M.; Alavi, S.M. Assessing the effects of deforestation and intensive agriculture on the soil quality through digital soil mapping. Geoderma 2020, 363, 114139. [Google Scholar] [CrossRef]
- Abdalrahman, Y.F.; Aburas, M.M.; Mnsur, S.; Abushnaf, F.F. Assessment of the Vegetation Role in Soil Resistance to Water Erosion at Al-Jabal Alkhdar Region, Libya. GMSET 2020, 6, A22–A38. (In Arabic) [Google Scholar]
- Aburas, M.; Yousef, M.S.; Alferjani, A.S. Land degradation at the southern slopes of Al-Jabal al Akhdar, Libya. Al-Mukhtar J. Sci. 2015, 30, 67–79. (In Arabic) [Google Scholar]
- Abdalrahman, Y.F.; Mossa, A.A. Reduction in sensitivity of land desertification through land management and land use in Libya Aljabal Alakhdar region. Glob. J. Agric. Food Saf. Sci. 2015, 2, 188–214. [Google Scholar]
- Panday, D.; Maharjan, B.; Chalise, D.; Shrestha, R.K.; Twanabasu, B. Digital soil mapping in the Bara district of Nepal using kriging tool in ArcGIS. PLoS ONE 2018, 13, e0206350. [Google Scholar] [CrossRef]
- Ashry, M. Manifestations and Combating Methods of Agricultural Lands Desertification in the Northern Part of Al-Jabal Al-Akhdar. Master’s Thesis, Garyounis University, Benghazi, Libya, 2005. (In Arabic). [Google Scholar]
- Selkhoz Prom Export. Soil Studies in the Eastern Zone of the Socialist People’s Libyan Arab Jamahiria; Secretariat for Agricuttural Reclamation and Land Development: Tripoli, Libya, 1980. [Google Scholar]
- Bin-Mahmoud, K.M. The Libyan Soils; The National Council of Scientific Research: Tripoli, Libya, 1995. (In Arabic) [Google Scholar]
- GEFLI. Jebel Lakhdar Soil and Water Conservation Project; Groupement d’Etudes Francais en Libye: Paris, France, 1975. [Google Scholar]
- Gebril, M.A. Water Erosion on the Northern Slope of Al-Jabal Al-Akhdar of Libya. Ph.D. Thesis, Durham University, Durham, UK, 1995. [Google Scholar]
- Altbeil, K.M. The Basics of Soil Fertility and Fertilization, 1st ed.; Al Fateh Complex for Universities: Tripoli, Libya, 1989. (In Arabic) [Google Scholar]
- Allafi, K. The Impact of Changing Agricultural Policies on Libyan Agricultural Performance. Ph.D. Thesis, Sheffield Hallam University, Sheffield, UK, 2014. [Google Scholar]
- EL-Barasi, Y.M.; Ahmaida, N.A.; Barrani, M.W.; EL-amrouni, A.O.; Omran, A. Pollution of Agricultural Lands by Fertilizers and Pesticides on El-Gubba and El-Abraq Area in Libya. Int. J. Eng. 2010, 8, 97–102. [Google Scholar]
- Faris, A.M.; Omran, A.S.; Shalloof, F.M. The wrong utilization of chemical compoands and it’s Effect on agricultural production and environment in the region of Jebel AlAkhdar-Libya. Al-Mukhtar J. Sci. 2004, 2, 1–17. (In Arabic) [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Black, C.A.; Evans, D.D.; Ensminger, L.E.; White, J.L.; Clark, F.E. Methods of Soil Analysis. Part II. In Chemical and Microbiological Properties; No. 9 in Series Agronomy; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture Circular Number 939; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Yang, J.E.; Kim, J.J.; Skogley, E.O.; Schaff, B.E. A simple spectrophotometric determination of nitrate in water, resin, and soil extracts. Soil Sci. Soc. Am. J. 1998, 62, 1108–1115. [Google Scholar] [CrossRef]
- Arshad, M.; Lowery, B. Physical tests for monitoring soil quality. In Methods Assess Soil Quality; Soil Science Society of America: Madison, WI, USA, 1996; pp. 123–141. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. Methods of Soil Analysis: Part 1. Agron. Monogr. 1986, 5, 687–734. [Google Scholar]
- Flint, L.E.; Alan, L. Flint. Porosity. In Methods of Soil Analysis; Part 4, Physical Methods; Dane, J.H., Topp, G., Campbell, G.S., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; pp. 241–254. [Google Scholar]
- Hudson, B.D. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Alef, K. Estimating of soil respiration. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press Ltd.: Cambridge, MA, USA, 1995; pp. 214–219. [Google Scholar]
- Lilliefors, H.W. On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. J. Am. Stat. Assoc. 1967, 62, 399–402. [Google Scholar] [CrossRef]
- Pallant, J.; Manual, S.S. A Step by Step Guide to Data Analysis Using SPSS, 4th ed.; Allen & Unwin Book Publishers: Crows Nest, Australia, 2013. [Google Scholar]
- Raiesi, F. A minimum data set and soil quality index to quantify the effect of land use conversion on soil quality and degradation in native rangelands of upland arid and semiarid regions. Ecol. Indic. 2017, 75, 307–320. [Google Scholar] [CrossRef]
- Lizaga, I.; Quijano, L.; Gaspar, L.; Ramos, M.C.; Navas, A. Linking land use changes to variation in soil properties in a Mediterranean mountain agroecosystem. Catena 2019, 172, 516–527. [Google Scholar] [CrossRef]
- Willy, D.K.; Muyanga, M.; Mbuvi, J.; Jayne, T. The effect of land use change on soil fertility parameters in densely populated areas of Kenya. Geoderma 2019, 343, 254–262. [Google Scholar] [CrossRef]
- Vasylieva, N. Improvement of agricultural management: Functional comparative approach. Montenegrin J. Econ. 2019, 15, 227–238. [Google Scholar] [CrossRef]
- Irshad, M.; Faridullah, J.A.; Eneji, E.E. Chemical properties of soil and runoff water under different land uses in Abbottabad, Pakistan. Environ. Earth Sci. 2015, 74, 3501–3506. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Bizuhoraho, T.; Kayiranga, A.; Manirakiza, N.; Mourad, K.A. The effect of land use systems on soil properties; A case study from Rwanda. Sustain. Agric. Res. 2018, 7, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Zaher, H.; Sabir, M.; Benjelloun, H.; Paul-Igor, H. Effect of forest land use change on carbohydrates, physical soil quality and carbon stocks in Moroccan cedar area. J. Environ. Manag. 2020, 254, 109544. [Google Scholar] [CrossRef]
- Catania, P.; Badalucco, L.; Laudicina, V.A.; Vallone, M. Effects of tilling methods on soil penetration resistance, organic carbon and water stable aggregates in a vineyard of semiarid Mediterranean environment. Environ. Earth Sci. 2018, 77, 348. [Google Scholar] [CrossRef]
- Olson, K.R.; Al-Kaisi, M.M. The importance of soil sampling depth for accurate account of soil organic carbon sequestration, storage, retention and loss. Catena 2015, 125, 33–37. [Google Scholar] [CrossRef]
- Ding, F.; Hu, Y.-L.; Li, L.-J.; Li, A.; Shi, S.; Lian, P.Y.; Zeng, D.H. Changes in soil organic carbon and total nitrogen stocks after conversion of meadow to cropland in Northeast China. Plant Soil 2013, 373, 659–672. [Google Scholar] [CrossRef]
- Lal, R. Land use and soil management effects on soil organic matter dynamics on Alfisols in Western Nigeria. In Soil Processes and the Carbon Cycle; Lal, R., Kimble, J.M., Follett, R.F., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 109–126. [Google Scholar]
- FAO. Plant Nutrition for Food Security—A Guide for Integrated Nutrient Management; Fertilizer and Plant Nutrition Bulletin No. 16; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Abad, J.R.S.; Khosravi, H.; Alamdarlou, E.H. Assessment the effects of land use changes on soil physicochemical properties in Jafarabad of Golestan province, Iran. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 296–300. [Google Scholar]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. Clim. Chang. Agric. 2019, 13, 197–222. [Google Scholar] [CrossRef] [Green Version]
- Deriak, J.S. Equilibrium between potassium forms in some soils of El-Jabel Al-Akhdar, Libya. J. Pure Appl. Sci. 2009, 2, 18–26. (In Arabic) [Google Scholar]
- Eldiabani, G. The Effect of Irrigation Water Quality on Some Physical and Chemical Soiil Properties of a Number of Farms in Al-Jabal Al-Akhdar Region. Master’s Thesis, Omar Almokhtar Univesity, Elbaida, Libya, 2001. (In Arabic). [Google Scholar]
- Rahmanipour, F.; Marzaioli, R.; Bahrami, H.A.; Fereidouni, Z.; Bandarabadi, S.R. Assessment of soil quality indices in agricultural lands of Qazvin Province, Iran. Ecol. Indic. 2014, 40, 19–26. [Google Scholar] [CrossRef]
- Sidi, N.; Aris, A.Z.; Talib, S.N.; Johan, S.; Yusoff, T.S.; Ismail, M.Z. Influential factors on the cation exchange capacity in sediment of Merambong Shoal, Johor. Proc. Environ. Sci. 2015, 30, 186–189. [Google Scholar] [CrossRef] [Green Version]
- Caravaca, F.; Lax, A.; Albaladejo, J. Organic matter, nutrient contents and cation exchange capacity in fine fractions from semiarid calcareous soils. Geoderma 1999, 93, 161–176. [Google Scholar] [CrossRef]
- Novara, A.; Pisciotta, A.; Minacapilli, M.; Maltese, A.; Capodici, F.; Cerdà, A.; Gristina, L. The impact of soil erosion on soil fertility and vine vigor. A multidisciplinary approach based on field, laboratory and remote sensing approaches. Sci. Total Environ. 2018, 622, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Abo El-Defan, T.A.; Alrayes, S.A. Guidelines for Interpretation and Indication of Soil and Water Analysis Measurements; Soil, Water and Environment Research Institute: Cairo, Egypt, 2018. (In Arabic) [Google Scholar]
- Mendes, W.C.; Alves, J.; Da Cunha, P.C.R.; Silva, A.R.; Evangelista, A.W.P.; Casaroli, D. Potassium leaching in different soils as a function of irrigation depths. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 972–977. [Google Scholar] [CrossRef] [Green Version]
- Ddamulira, G.; Idd, R.; Namazzi, S.; Kalali, F.; Mundingotto, J.; Maphosa, M. Nitrogen and potassium fertilizers increase cherry tomato height and yield. J. Agric. Sci. 2019, 11, 1916–9760. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Peng, S.; Chen, Y.; Cao, Y. The coupling of leaf, litter, and soil nutrients in warm temperate forests in northwestern China. Sci. Rep. 2017, 7, 11754. [Google Scholar] [CrossRef]
- Rehman, O.; Mehdi, S.M.; Ranjha, A.M.; Sarfraz, M. Phosphorus requirement of cereal crops and fertility buildup factor in a Typic Camborthid soil. J. Biol. Sci. 2007, 7, 1072–1081. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; Xu, Y.; Hao, F.; Wang, X.; Siyang, C.; Lin, C. Effect of long-term agricultural cultivation and land use conversion on soil nutrient contents in the Sanjiang Plain. Catena 2013, 104, 243–250. [Google Scholar] [CrossRef]
- Harris, R.F.; Karlen, D.L.; Mulla, D.J. A conceptual framework for assessment and management of soil quality and health. In Methods for Assessing Soil Quality; Doran, J., Jones, A., Eds.; SSSA, Inc.: Madison, WI, USA, 1996; pp. 61–82. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=73305 (accessed on 13 March 2019).
- Pitt, J.L.; Provin, T. Phosphorus-Too Much and Plants May Suffer. In Texas Farmer Collection; Texas Coop Ext Serv: Laredo, TX, USA, 2008; Available online: http://hdl.handle.net/1969.1/86793 (accessed on 14 May 2021).
- Nosengo, N. Fertilized to death. Nature 2003, 245, 894–895. [Google Scholar] [CrossRef]
- Fan, J.; Hao, M.; Malhi, S.S. Accumulation of nitrate N in the soil profile and its implications for the environment under dryland agriculture in northern China: A review. Can. J. Soil Sci. 2010, 90, 429–440. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Gregorich, E. Quality. In Encyclopedia of Soil Science, 2nd ed.; Lal, R., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; pp. 1388–1391. [Google Scholar]
- Negasa, T.; Ketema, H.; Legesse, A.; Sisay, M.; Temesgen, H. Variation in soil properties under different land use types managed by smallholder farmers along the toposequence in southern Ethiopia. Geoderma 2017, 290, 40–50. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R.; Sitaula, B.K.; Lal, R.; Bajracharya, R.M. Soil aggregate-and particle-associated organic carbon under different land uses in Nepal. Soil Sci. Soc. Am. J. 2007, 71, 1194–1203. [Google Scholar] [CrossRef]
- Teferi, E.; Bewket, W.; Simane, B. Effects of land use and land cover on selected soil quality indicators in the headwater area of the Blue Nile basin of Ethiopia. Environ. Monit. Assess. 2016, 188, 83. [Google Scholar] [CrossRef]
- Biro, K.; Pradhan, B.; Buchroithner, M. Land use/land cover change analysis and its impact on soil properties in the northern part of Gadarif region, Sudan. Land Degrad. Dev. 2013, 24, 90–102. [Google Scholar] [CrossRef]
- USDA-NRCS. Soil Quality Kit—Guide for Educators. Natural Resources Conservation Service United States Department of Agriculture. 2014. Available online: https://cutt.us/aRBmO (accessed on 20 April 2021).
- Reynolds, W.D.; Drury, C.F.; Tan, C.S.; Fox, C.A.; Yang, X.M. Use of indicators and pore volume-function characteristics to quantify soil physical quality. Geoderma 2009, 152, 252–263. [Google Scholar] [CrossRef]
- Kargas, G.; Kerkides, P.; Sotirakoglou, K.; Poulovassilis, A. Temporal variability of surface soil hydraulic properties under various tillage systems. Soil Tillage Res. 2016, 158, 22–31. [Google Scholar] [CrossRef]
- Mandal, K.G.; Kundu, D.K.; Singh, R.; Kumar, A.; Rout, R.; Padhi, J.; Majhi, P.; Sahoo, D.K. Cropping practices, soil properties, pedotransfer functions and organic carbon storage at Kuanria canal command area in India. Springerplus 2013, 2, 631. [Google Scholar] [CrossRef] [Green Version]
- Nascente, A.S.; Li, Y.; Crusciol, C.A.C. Soil aggregation, organic carbon concentration, and soil bulk density as affected by cover crop species in a no-tillage system. Rev. Bras. Ciência Solo 2015, 39, 871. [Google Scholar] [CrossRef]
- Panday, D.; Ojha, R.B.; Chalise, D.; Das, S.; Twanabasu, B. Spatial variability of soil properties under different land use in the Dang district of Nepal. Cogent Food Agric. 2019, 5, 1600460. [Google Scholar] [CrossRef]
- Pandey, S.; Bhatta, N.P.; Paudel, P.; Pariyar, R.; Maskey, K.H.; Khadka, J.; Thapa, T.B.; Rijal, B.; Panday, D. Improving fertilizer recommendations for Nepalese farmers with the help of soil-testing mobile van. J. Crop. Improv. 2018, 32, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Kabir, E.B.; Bashari, H.; Bassiri, M.; Mosaddeghi, M.R. Effects of land-use/cover change on soil hydraulic properties and pore characteristics in a semi-arid region of central Iran. Soil Tillage Res. 2020, 197, 104478. [Google Scholar] [CrossRef]
- Adnan, M.S.; Anuar, M.A.M.; Nda, M.; Rahmat, S.N. Investigation of Infiltration Rate on Soft Clay that Affecting the Ponding Time. MATEC Web Conf. 2017, 103, 4021. [Google Scholar] [CrossRef] [Green Version]
- Tesfahunegn, G.B. Soil Quality Indicators Response to Land Use and Soil Management Systems in Northern Ethiopia’s Catchment. Land Degrad. Dev. 2016, 27, 438–448. [Google Scholar] [CrossRef]
- Huntington, T. Available water capacity and soil organic matter. In Encyclopedia of Soil Science, 2nd ed.; Lal, R., Ed.; Taylor & Francis Group: New York, NY, USA, 2006; Volume 1, pp. 139–143. [Google Scholar] [CrossRef]
- Emerson, W.W. Water-retention, organic-C and soil texture. Soil Res. 1995, 33, 241–251. [Google Scholar] [CrossRef]
- Haynes, R.J.; Beare, M.H. Aggregation and organic carbon storage in mesothermal, humid soils. In Structure and Organic Carbon Storage in Agricultural Soils: Advances in Soil Science; Carter, M.R., Stewart, M.A., Eds.; Lewis Publications: New York, NY, USA, 1996; pp. 213–263. [Google Scholar]
- Monteza, R. Effects of Land-Use Change on the Quality of Upland Tropical Soil. Master’s Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Azuka, C.V.; Igué, A.M. Spatial variability of soil properties under different land use in Koupendri catchment, Benin. Span. J. Soil Sci. 2020, 10, 2604. [Google Scholar] [CrossRef]
- Green, T.R.; Ahuja, L.R.; Benjamin, J.G. Advances and challenges in predicting agricultural management effects on soil hydraulic properties. Geoderma 2003, 116, 3–27. [Google Scholar] [CrossRef]
- Osunbitan, J.A.; Oyedele, D.J.; Adekalu, K.O. Tillage effects on bulk density, hydraulic conductivity and strength of a loamy sand soil in southwestern Nigeria. Soil Tillage Res. 2005, 82, 57–64. [Google Scholar] [CrossRef]
- Sheridan, G.J.; So, H.B.; Loch, R.J.; Walker, C.M. Estimation of erosion model erodibility parameters from media properties. Soil Res. 2000, 38, 265–284. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Francaviglia, R.; Renzi, G. A cost and time-effective method to evaluate soil microbial respiration for soil quality assessment. Appl. Soil Ecol. 2019, 140, 121–125. [Google Scholar] [CrossRef]
- Mehmandoost, F.; Owliaie, H.R.; Adhami, E.; Naghiha, R. Effect of land use change on some physicochemical and biological properties of the soils of Servak plain, Yasouj region. J. Water Soil 2018, 32, 587–599. [Google Scholar] [CrossRef]
- Desalegn, T. Soil Acidity-Induced Land Use/Cover Change and Management Systems on Soil Quality Parameters in the Central Highlands of Ethiopia. Ph.D. Thesis, University of Valladolid, Valladolid, Spain, 2015. [Google Scholar]
- Mallik, A.U.; Hu, D. Soil respiration following site preparation treatments in boreal mixedwood forest. For. Ecol. Manage. 1997, 97, 265–275. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Forest Management and Environmental Ethics in Forestry, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Mavi, M.S.; Marschner, P.; Chittleborough, D.J.; Cox, J.W.; Sanderman, J. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol. Biochem. 2012, 45, 8–13. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Sarikhani, M.R.; Sinegani, A.A.S.; Ahmadi, A.; Keesstra, S. Estimating the soil respiration under different land uses using artificial neural network and linear regression models. Catena 2019, 174, 371–382. [Google Scholar] [CrossRef]
- Dube, F.; Zagal, E.; Stolpe, N.; Espinosa, M. The influence of land-use change on the organic carbon distribution and microbial respiration in a volcanic soil of the Chilean Patagonia. For. Ecol. Manag. 2009, 257, 1695–1704. [Google Scholar] [CrossRef]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson Education Limited: Harlow, UK, 2017. [Google Scholar]
- Lin, X.G.; Yin, R.; Zhang, H.Y.; Huang, J.F.; Chen, R.R.; Cao, Z.H. Changes of soil microbiological properties caused by land use changing from rice–wheat rotation to vegetable cultivation. Environ. Geochem. Health 2004, 26, 119–128. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]




| SOM | pH | EC | CaCO3 | CEC | Kav | Pav | NO3−N | Clay | Silt | Sand | BD | TP | MP | AWHC | Ksat | |
| pH | −0.09 ns | |||||||||||||||
| EC | −0.40 ** | −0.24 ** | ||||||||||||||
| CaCO3 | −0.01 ns | 0.37 ** | −0.02 ns | |||||||||||||
| CEC | 0.44 ** | 0.37 ** | −0.27 ** | 0.44 ** | ||||||||||||
| Kav | 0.53 ** | −0.12 ns | −0.35 ** | −0.11 ns | 0.21 ** | |||||||||||
| Pav | 0.14 ns | −0.22 ** | 0.52 ** | 0.08 ns | −0.04 ns | −0.08 ns | ||||||||||
| NO3−N | −0.20 ** | −0.12 ns | 0.54 ** | 0.14 ns | −0.01 ns | −0.19 * | 0.67 ** | |||||||||
| Clay | −0.62 ** | 0.14 ns | 0.31 ** | 0.21 ** | −0.21 ** | −0.49 ** | 0.15 * | 0.20 ** | ||||||||
| Silt | 0.53 ** | −0.05 ns | −0.29 ** | −0.20 ** | 0.20 ** | 0.50 ** | −0.17 * | −0.24 ** | −0.82 ** | |||||||
| Sand | 0.21 ** | −0.15 * | −0.06 ** | −0.03 ns | 0.03 ns | 0.04 ns | 0.01 ns | 0.03 ns | −0.41 ** | −0.20 ** | ||||||
| BD | −0.65 ** | 0.14 ns | 0.35 ** | 0.08 ns | −0.32 ** | −0.48 ** | 0.18 * | 0.17 * | 0.48 ** | −0.46 ** | −0.09 ns | |||||
| TP | 0.64 ** | 0.14 ns | −0.33 ** | −0.08 ns | 0.32 ** | 0.47 ** | −0.16 * | −0.16 * | −0.48 ** | 0.45 ** | −0.09 ns | −0.98 ** | ||||
| MP | 0.58 ** | −0.21 ** | −0.11 ns | −0.11 ns | 0.05 ns | 0.24 ** | −0.08 ns | −0.10 ns | −0.26 ** | 0.23 ** | 0.08 ns | −0.81 ** | 0.82 ** | |||
| AWHC | 0.62 ** | −0.10 ns | −0.46 ** | −0.06 ns | 0.23 ** | 0.51 ** | −0.29 ** | −0.27 ** | −0.54 ** | 0.48 ** | 0.15 * | −0.61 ** | 0.60 ** | 0.38 ** | ||
| Ksat | 0.81 ** | −0.03 ns | −0.46 ** | −0.02 ns | 0.42 ** | 0.58 ** | −0.15 * | −0.29 ** | −0.63 ** | 0.58 ** | 0.15 * | −0.64 ** | 0.63 ** | 0.35 ** | 0.63 ** | |
| BSR | 0.74 ** | −0.07 ns | −0.24 ** | 0.01 ns | 0.25 ** | 0.23 ** | −0.10 ns | −0.10 ns | −0.30 ** | 0.27 * | 0.22 ** | −0.39 ** | 0.38 ** | 0.26 ** | 0.30 ** | 0.40 ** |
| Soil Property | Land Use/Cover | Mean | Standard Deviation | Standard Errors | Min. | Max. | p-Value |
|---|---|---|---|---|---|---|---|
| SOM (%) | NMF | 4.98 a | 0.734 | 0.095 | 3.80 | 6.37 | <0.01 ** |
| RA | 3.20 b | 0.627 | 0.080 | 1.79 | 4.23 | ||
| IC | 2.96 c | 0.412 | 0.053 | 2.18 | 3.89 | ||
| pH | NMF | 7.93 a | 0.193 | 0.025 | 7.50 | 8.25 | 0.105 ns |
| RA | 7.94 a | 0.240 | 0.031 | 7.51 | 8.32 | ||
| IC | 8.01 a | 0.210 | 0.027 | 7.52 | 8.41 | ||
| EC (dS m−1) | NMF | 0.24 a | 0.044 | 0.006 | 0.15 | 0.35 | <0.01 ** |
| RA | 0.26 a | 0.062 | 0.008 | 0.16 | 0.47 | ||
| IC | 0.54 b | 0.138 | 0.018 | 0.31 | 0.98 | ||
| CaCO3 (%) | NMF | 0.84 a | 0.877 | 0.113 | 0.09 | 4.04 | 0.349 ns |
| RA | 0.98 a | 1.134 | 0.146 | 0.18 | 4.46 | ||
| IC | 1.10 a | 1.128 | 0.146 | 0.12 | 3.88 | ||
| CEC (meq 100 g−1) | NMF | 19.93 a | 2.598 | 0.335 | 13.70 | 26.09 | <0.01 ** |
| RA | 16.05 b | 4.112 | 0.531 | 9.80 | 23.75 | ||
| IC | 17.03 b | 3.422 | 0.442 | 10.45 | 24.15 | ||
| Kav (meq 100 g−1) | NMF | 1.78 a | 0.326 | 0.042 | 0.89 | 2.78 | <0.01 ** |
| RA | 1.27 b | 0.345 | 0.044 | 0.59 | 2.43 | ||
| IC | 1.14 b | 0.279 | 0.036 | 0.73 | 2.00 | ||
| Pav (mg L−1) | NMF | 8.74 a | 3.720 | 0.480 | 2.85 | 18.87 | <0.01 ** |
| RA | 5.27 b | 3.533 | 0.456 | 1.23 | 15.23 | ||
| IC | 16.53 c | 6.156 | 0.795 | 9.01 | 33.45 | ||
| NO3-N (mg L−1) | NMF | 12.67 a | 3.369 | 0.435 | 7.02 | 23.60 | <0.01 ** |
| RA | 12.21 a | 3.599 | 0.465 | 7.26 | 21.60 | ||
| IC | 18.33 b | 5.021 | 0.648 | 9.96 | 27.43 |
| Soil Property | Land Use/Cover | Mean | Standard Deviation | Standard Errors | Min. | Max. | p-Value |
|---|---|---|---|---|---|---|---|
| Clay (%) | NMF | 46.36 a | 3.360 | 0.434 | 38.38 | 51.73 | <0.01 ** |
| RA | 53.94 b | 3.633 | 0.469 | 44.98 | 61.28 | ||
| IC | 54.79 b | 3.317 | 0.428 | 46.91 | 62.43 | ||
| Silt (%) | NMF | 33.45 a | 3.457 | 0.446 | 27.66 | 40.88 | <0.01 ** |
| RA | 26.80 b | 3.242 | 0.419 | 19.58 | 34.92 | ||
| IC | 26.11 b | 3.608 | 0.466 | 16.76 | 34.04 | ||
| Sand (%) | NMF | 20.19 a | 1.722 | 0.222 | 16.96 | 25.61 | 0.097 ns |
| RA | 19.26 a | 3.605 | 0.465 | 12.35 | 30.12 | ||
| IC | 19.09 a | 3.293 | 0.425 | 11.50 | 25.52 | ||
| BD (g cm−3) | NMF | 1.19 a | 0.070 | 0.009 | 1.09 | 1.35 | <0.01 ** |
| RA | 1.33 b | 0.086 | 0.011 | 1.12 | 1.44 | ||
| IC | 1.36 b | 0.081 | 0.010 | 1.18 | 1.50 | ||
| TP (%) | NMF | 54.91 a | 2.621 | 0.338 | 49.01 | 58.79 | <0.01 ** |
| RA | 49.54 b | 3.299 | 0.426 | 45.17 | 57.55 | ||
| IC | 48.68 b | 3.043 | 0.393 | 43.89 | 55.25 | ||
| MP (%) | NMF | 19.74 a | 3.088 | 0.399 | 12.71 | 25.61 | <0.01 ** |
| RA | 17.50 b | 3.424 | 0.442 | 10.76 | 24.72 | ||
| IC | 16.94 b | 3.390 | 0.438 | 10.17 | 23.21 | ||
| AWHC (%) | NMF | 17.08 a | 2.546 | 0.329 | 10.97 | 23.29 | <0.01 ** |
| RA | 14.24 b | 1.891 | 0.244 | 10.97 | 19.01 | ||
| IC | 12.31 c | 1.170 | 0.151 | 9.19 | 17.02 | ||
| Ksat (cm hr−1) | NMF | 6.87 a | 0.989 | 0.128 | 4.95 | 8.32 | <0.01 ** |
| RA | 3.66 b | 0.905 | 0.117 | 2.49 | 5.88 | ||
| IC | 3.34 b | 0.818 | 0.106 | 1.75 | 4.77 |
| Land Use/Cover | Mean BSR | Standard Deviation | Standard Errors | Min. | Max. | p-Value |
|---|---|---|---|---|---|---|
| NMF | 0.20 a | 0.051 | 0.007 | 0.10 | 0.34 | <0.01 ** |
| RA | 0.17 b | 0.055 | 0.007 | 0.08 | 0.28 | |
| IC | 0.15 c | 0.036 | 0.005 | 0.05 | 0.26 | |
| Study area | 0.17 | 0.052 | 0.004 | 0.05 | 0.34 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alawamy, J.S.; Balasundram, S.K.; Mohd. Hanif, A.H.; Teh Boon Sung, C. Response of Potential Indicators of Soil Quality to Land-Use and Land-Cover Change under a Mediterranean Climate in the Region of Al-Jabal Al-Akhdar, Libya. Sustainability 2022, 14, 162. https://doi.org/10.3390/su14010162
Alawamy JS, Balasundram SK, Mohd. Hanif AH, Teh Boon Sung C. Response of Potential Indicators of Soil Quality to Land-Use and Land-Cover Change under a Mediterranean Climate in the Region of Al-Jabal Al-Akhdar, Libya. Sustainability. 2022; 14(1):162. https://doi.org/10.3390/su14010162
Chicago/Turabian StyleAlawamy, Jamal Suliman, Siva K. Balasundram, Ahmad Husni Mohd. Hanif, and Christopher Teh Boon Sung. 2022. "Response of Potential Indicators of Soil Quality to Land-Use and Land-Cover Change under a Mediterranean Climate in the Region of Al-Jabal Al-Akhdar, Libya" Sustainability 14, no. 1: 162. https://doi.org/10.3390/su14010162






