Toxic and Trace Elements in Seaweeds from a North Atlantic Ocean Region (Tenerife, Canary Islands)
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples and Sampling Zone
2.2. Treatment of the Samples
2.3. Analytical Method
2.4. Statistical Analysis
3. Results and Discussion
3.1. Trace Element Concentrations in the Genres Analysed
3.2. Study of Correlations between Elements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-Palacios, J.M.; Martín Esquivel, J.L. Naturaleza de las Islas Canarias: Ecología y Conservación, 2nd ed.; Turquesa: Santa Cruz de Tenerife, Spain, 2001; p. 171. [Google Scholar]
- Pérez, O.M.; Telfer, T.C.; Ross, L.G. Geographical information systems-based models for offshore floating marine fish cage aquaculture site selection in Tenerife, Canary Islands. Aquacult. Res. 2005, 36, 946–961. [Google Scholar] [CrossRef]
- Córdoba-Jabonero, C.; Sorribas, M.; Guerrero-Rascado, J.; Adame, J.A.; Hernández, Y.; Lyamani, H.; Cachorro, V.; Gil, M.; Alados-Arboledas, L.; Cuevas, E.; et al. Synergetic monitoring of Saharan dust plumes and potential impact on surface: A case study of dust transport from Canary Islands to Iberian Peninsula. Atmos. Chem. Phys. 2011, 11, 3067–3091. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Pérez, S.; Cuevas, E.; Pérez García-Pando, C.; Querol, X.; Baldasano, J.; Draxler, R.; Bustos, J. Trend changes of African airmass intrusions in the marine boundary layer over the subtropical Eastern North Atlantic region in winter. Tellus B 2011, 63, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Baldasano, J.M.; Massagué, J. Trends and patterns of air quality in Santa Cruz de Tenerife (Canary Islands) in the period 2011–2015. Air Qual. Atmos. Health 2017, 10, 939–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín Esquivel, J. Atlas de Biodiversidad de Canarias; Gobierno de Canarias: Canary Islands, Spain, 2010. [Google Scholar]
- Al-Enazi, N.M.; Awaad, A.S.; Alqasoumi, S.I.; Alwethairi, M.F. Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharm. J. 2018, 26, 25–32. Available online: https://www.sciencedirect.com/science/article/pii/S1319016417301986 (accessed on 20 April 2022). [CrossRef] [PubMed]
- Tabraue, H.; Haroun, R.; Gil-Rodríguez, M.; Candelaria, M.; Prud’homme van Reine, W.F.; Castro, D.; Jesús; Mar, F.; Biología; Conservación, B. A Checklist of the Marine Plants from the Canary Islands (Central Eastern Atlantic Ocean). Bot. Mar. 2002, 45, 139–169. [Google Scholar] [CrossRef] [Green Version]
- Haroun Tabrure, R.; Gil Rodríguez, M.C.; de la Torre, W.W. Plantas Marinas de las Islas Canarias, 1st ed.; Canseco: Talavera de la Reina, Spain, 2003; p. 319. [Google Scholar]
- Serfor-Armah, Y.; Carboo, D.; Akuamoah, R.K.; Chatt, A. Micelle-mediated extraction and neutron activation determination of nanogram levels of vanadium in seaweeds. J. Radioanal. Nucl. 2018, 318, 2039–2047. [Google Scholar] [CrossRef]
- Domínguez-González, R.; Romarís-Hortas, V.; García-Sartal, C.; Moreda-Piñeiro, A.; Barciela-Alonso Mdel, C.; Bermejo-Barrera, P. Evaluation of an in vitro method to estimate trace elements bioavailability in edible seaweeds. Talanta 2010, 82, 1668–1673. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. Available online: https://www.mdpi.com/2073-4360/14/7/1375 (accessed on 20 April 2022). [CrossRef]
- Khan, S.; Naushad, M.; Al-Gheethi, A.; Iqbal, J. Engineered nanoparticles for removal of pollutants from wastewater: Current status and future prospects of nanotechnology for remediation strategies. J. Environ. Chem. Eng. 2021, 9, 106160. Available online: https://www.sciencedirect.com/science/article/pii/S2213343721011374 (accessed on 18 April 2022). [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. Available online: https://www.sciencedirect.com/science/article/pii/S0013935121019101 (accessed on 16 April 2022). [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. Available online: https://www.sciencedirect.com/science/article/pii/S0016236121024121 (accessed on 14 April 2022). [CrossRef]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of Commercial Seaweed Liquid Extract (TAM®) Biostimulant and Its Bioactive Molecules on Growth and Antioxidant Activities of Hot Pepper (Capsicum annuum). Plants 2021, 10, 1045. Available online: https://www.mdpi.com/2223-7747/10/6/1045 (accessed on 20 April 2022). [CrossRef] [PubMed]
- Ashour, M.; Mabrouk, M.M.; Abo-Taleb, H.A.; Sharawy, Z.Z.; Ayoub, H.F.; Van Doan, H.; Davies, S.J.; El-Haroun, E.; Goda, M.S.-A.A. A liquid seaweed extract (TAM®) improves aqueous rearing environment, diversity of zooplankton community, whilst enhancing growth and immune response of Nile tilapia, Oreochromis niloticus, challenged by Aeromonas hydrophila. Aquaculture 2021, 543, 736915. Available online: https://www.sciencedirect.com/science/article/pii/S0044848621005780 (accessed on 20 April 2022). [CrossRef]
- Rizzolo, J.A.; Barbosa, C.G.G.; Borillo, G.C.; Godoi, A.F.L.; Souza, R.A.F.; Andreoli, R.V.; Manzi, A.O.; Sá, M.O.; Alves, E.G.; Pöhlker, C.; et al. Soluble iron nutrients in Saharan dust over the central Amazon rainforest. Atmos. Chem. Phys. 2017, 17, 2673–2687. Available online: https://acp.copernicus.org/articles/17/2673/2017/; https://acp.copernicus.org/articles/17/2673/2017/acp-17-2673-2017.pdf (accessed on 18 April 2022). [CrossRef] [Green Version]
- Saleh, B. Algae Efficacy as a Potent Tool for Heavy Metals Removal: An Overview. J. Stress Physiol. Biochem. 2019, 15, 53–67. [Google Scholar]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef] [Green Version]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of chromium with microorganisms and plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Pattnaik, M.M.; Mishra, A.K.; Patra, H.K. Bio-concentration of chromium—An in situ phytoremediation study at South Kaliapani chromite mining area of Orissa, India. Environ. Monit. Assess. 2012, 184, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef] [Green Version]
- Hanaa, M.S.; Eweida, A.E.; Azza, F. Heavy Metals in Drinking Water and Their Environmental Impact on Human Health. In Proceedings of the International Conference on the Environ Hazards Mitigation, Cairo, Egypt, 9–12 September 2000; pp. 542–546. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. Available online: https://www.sciencedirect.com/science/article/pii/S0160412003001867 (accessed on 20 April 2022). [CrossRef]
- Hardisson, A.; Revert, C.; González-Weller, D.; Gutiérrez, A.; Paz, S.; Rubio, C. Aluminium Exposure Through the Diet. HSOA J. Food Sci. Nutr. 2017, 3, 19. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Kumar, D. Prospects and Challenges in Algal Biotechnology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Álvarez-Hernández, C.; Cairós, C.; López-Darias, J.; Mazzetti, E.; Hernández-Sánchez, C.; González-Sálamo, J.; Hernández-Borges, J. Microplastic debris in beaches of Tenerife (Canary Islands, Spain). Mar. Pollut. Bull. 2019, 146, 26–32. Available online: https://www.sciencedirect.com/science/article/pii/S0025326X19304321 (accessed on 20 April 2022). [CrossRef]
- González-Hernández, M.; Hernández-Sánchez, C.; González-Sálamo, J.; López-Darias, J.; Hernández-Borges, J. Monitoring of meso and microplastic debris in Playa Grande beach (Tenerife, Canary Islands, Spain) during a moon cycle. Mar. Pollut. Bull. 2020, 150, 110757. Available online: https://www.sciencedirect.com/science/article/pii/S0025326X19309130 (accessed on 14 April 2022). [CrossRef]
- Reinold, S.; Herrera, A.; Hernández-González, C.; Gómez, M. Plastic pollution on eight beaches of Tenerife (Canary Islands, Spain): An annual study. Mar. Pollut. Bull. 2020, 151, 110847. Available online: https://www.sciencedirect.com/science/article/pii/S0025326X19310033 (accessed on 14 April 2022). [CrossRef]
- Rubio, C.; Napoleone, G.; Luis-González, G.; Gutiérrez, A.J.; González-Weller, D.; Hardisson, A.; Revert, C. Metals in edible seaweed. Chemosphere 2017, 173, 572–579. Available online: https://www.sciencedirect.com/science/article/pii/S0045653517300747 (accessed on 14 April 2022). [CrossRef]
- Rubio, C.; Paz, S.; Tius, E.; Hardisson, A.; Gutierrez, A.J.; Gonzalez-Weller, D.; Caballero, J.M.; Revert, C. Metal Contents in the Most Widely Consumed Commercial Preparations of Four Different Medicinal Plants (Aloe, Senna, Ginseng, and Ginkgo) from Europe. Biol. Trace Elem. Res. 2018, 186, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.A. Nomenclature in evaluation of analytical methods including detection and quantification capabilities (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Nordstokke, D.; Zumbo, B. A New Nonparametric Levene Test for Equal Variances. Psicológica 2010, 31, 401–430. [Google Scholar]
- Razali, N.M.; Wah, Y.B. Power Comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling Tests. J. Stat. Modeling Anal. 2011, 2, 21–33. [Google Scholar]
- Wadgeri, Y.; Sudeep, P.; Sabu, M.K.; Rajan, J. Non-Local Means Image Denoising Using Shapiro-Wilk Similarity Measure. IEEE Access 2018, 6, 66914–66922. [Google Scholar] [CrossRef]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. Corsini Encycl. Psychol. 2022, 4, 1. [Google Scholar] [CrossRef]
- Flores, S.R.L.; Dobbs, J.; Dunn, M.A. Mineral nutrient content and iron bioavailability in common and Hawaiian seaweeds assessed by an in vitro digestion/Caco-2 cell model. J. Food Compos. Anal. 2015, 43, 185–193. Available online: https://www.sciencedirect.com/science/article/pii/S0889157515001507 (accessed on 14 April 2022). [CrossRef]
- Huerta-Diaz, M.A.; de León-Chavira, F.; Lares, M.L.; Chee-Barragán, A.; Siqueiros-Valencia, A. Iron, manganese and trace metal concentrations in seaweeds from the central west coast of the Gulf of California. Appl. Geochem. 2007, 22, 1380–1392. Available online: https://www.sciencedirect.com/science/article/pii/S0883292707001254 (accessed on 10 April 2022). [CrossRef]
- Sánchez-Quiles, D.; Marbà, N.; Tovar-Sánchez, A. Trace metal accumulation in marine macrophytes: Hotspots of coastal contamination worldwide. Sci. Total Environ. 2017, 576, 520–527. Available online: https://www.sciencedirect.com/science/article/pii/S0048969716323336 (accessed on 13 April 2022). [CrossRef] [Green Version]
- García-Casal, M.N.; Pereira, A.C.; Leets, I.; Ramírez, J.; Quiroga, M.F. High Iron Content and Bioavailability in Humans from Four Species of Marine Algae. J. Nutr. 2007, 137, 2691–2695. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Romarís-Hortas, V.; Domínguez-González, R.; Alonso-Rodríguez, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. Trace metals in marine foodstuff: Bioavailability estimation and effect of major food constituents. Food Chem. 2012, 134, 339–345. Available online: https://www.sciencedirect.com/science/article/pii/S0308814612003809 (accessed on 12 April 2022). [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, A.J.; González-Weller, D.; Revert, C.; Hardisson, A. Metal Concentrations in Wild-Harvested Phaeophyta Seaweed from the Atlantic Ocean (Canary Islands, Spain). J. Food Prot. 2018, 81, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Muse, J.O.; Tudino, M.B.; d’Huicque, L.; Troccoli, O.E.; Carducci, C.N. A survey of some trace elements in seaweeds from Patagonia, Argentina. Environ. Pollut. 1995, 87, 249–253. Available online: https://www.sciencedirect.com/science/article/pii/0269749194P2613E (accessed on 18 April 2022). [CrossRef]
- Cabrita, A.R.J.; Maia, M.R.G.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J.M. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- Sawidis, T.; Brown, M.T.; Zachariadis, G.; Sratis, I. Trace metal concentrations in marine macroalgae from different biotopes in the Aegean Sea. Environ. Int. 2001, 27, 43–47. Available online: https://www.sciencedirect.com/science/article/pii/S0160412001000526 (accessed on 14 April 2022). [CrossRef]
- Vera-Galván, M.A.; Samarín-Bello, C.; Delgado-Castro, G.; Viera-Ruíz, G. Natura 2000 en Macaronesia. Azores, Madeira, Salvajes y Canarias. 2010. Available online: https://www.gobiernodecanarias.org/medioambiente/publicaciones/material-publicado/libros/natura-2000-macaronesia/ (accessed on 20 April 2022).
- Gonzalez-Calvo, D.; Aguilar, R.M.; Criado-Hernandez, C.; Gonzalez-Mendoza, L. Multivariate influence through neural networks ensemble: Study of Saharan dust intrusion in the Canary Islands. Appl. Soft Comput. 2021, 107, 107497. Available online: https://www.sciencedirect.com/science/article/pii/S1568494621004208 (accessed on 14 April 2022). [CrossRef]
- Kleiven, W.; Johnsen, G.; Van Ardelan, M. Sea surface microlayer and elemental composition in phaeo-, chloro-, and rhodophytes in winter and spring. J. Phycol. 2022, 55, 762–774. [Google Scholar] [CrossRef]
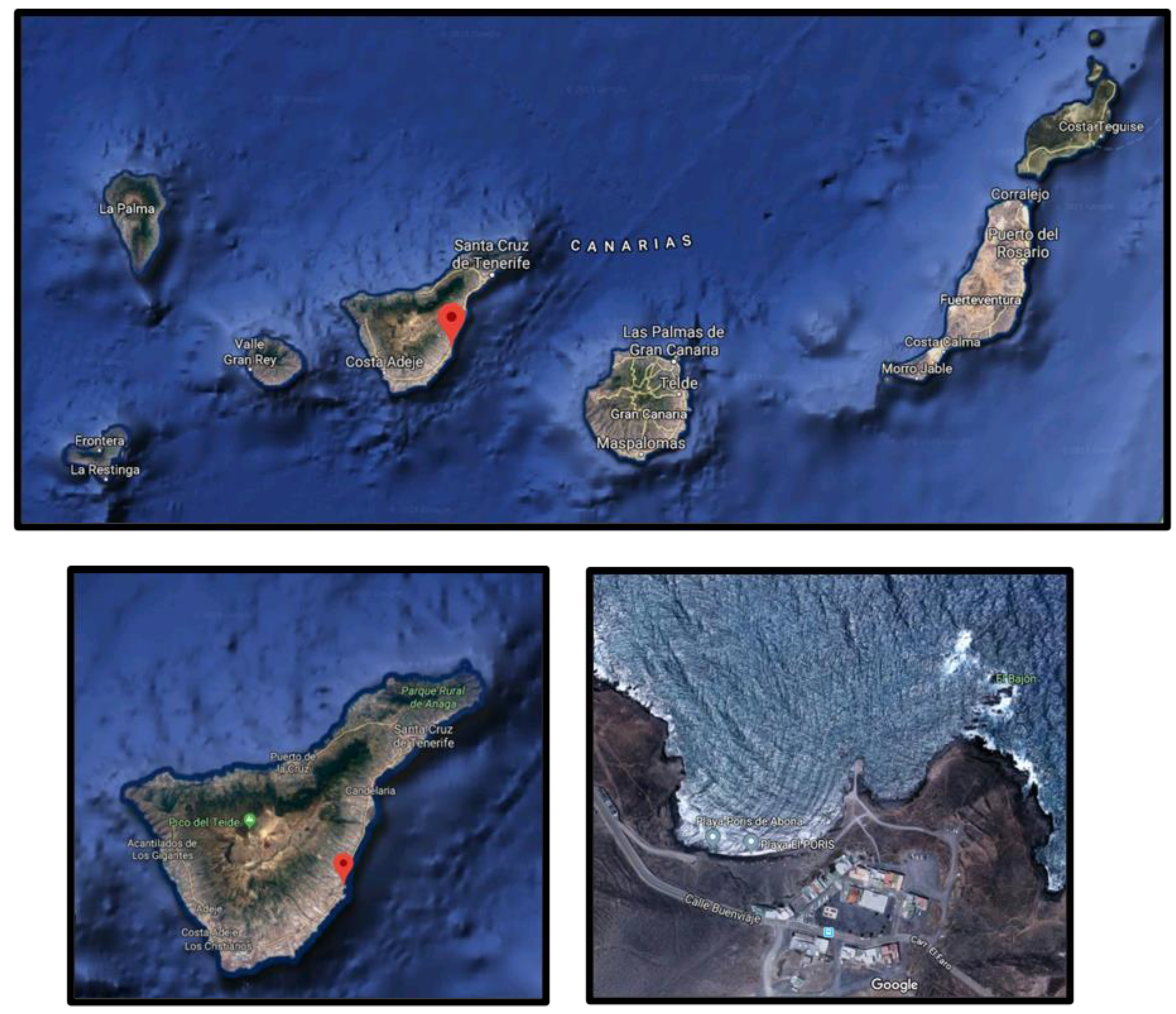

| Element | Wavelength (nm) | Detection Limit (mg/kg) | Quantification Limit (mg/kg) |
|---|---|---|---|
| Al | 167.0 | 0.033 | 0.100 |
| B | 249.7 | 0.025 | 0.100 |
| Ba | 455.4 | 0.008 | 0.042 |
| Cd | 226.5 | 0.003 | 0.008 |
| Co | 228.6 | 0.005 | 0.017 |
| Cr | 267.7 | 0.025 | 0.067 |
| Cu | 327.3 | 0.033 | 0.100 |
| Fe | 259.9 | 0.017 | 0.042 |
| Li | 670.8 | 0.010 | 0.026 |
| Mn | 257.6 | 0.017 | 0.067 |
| Mo | 202.0 | 0.006 | 0.017 |
| Ni | 231.6 | 0.006 | 0.025 |
| Pb | 220.3 | 0.003 | 0.008 |
| Sr | 407.7 | 0.006 | 0.025 |
| V | 310.2 | 0.008 | 0.042 |
| Zn | 206.2 | 0.017 | 0.058 |
| Material | Element | C. Certified (mg/kg) | C. Obtained (mg/kg) | Recovery (%) |
|---|---|---|---|---|
| SRM 1570a Spinach leaves | Sr | 55.6 ± 0.8 | 54.2 ± 0.30 | 97.5 |
| V | 0.57 ± 0.03 | 0.55 ± 0.01 | 96.5 | |
| SRM 1515 Apple leaves | B | 27 ± 2 | 26.2 ± 0.03 | 97 |
| Ba | 49 | 48.4 ± 0.02 | 98.8 | |
| Cr | 0.3 | 0.29 ± 0.02 | 96.7 | |
| Co | 0.09 | 0.09 ± 0.02 | 100 | |
| Mn | 54.0 ± 0.3 | 53.8 ± 0.30 | 99.6 | |
| Mo | 0.09 | 0.09 ± 0.01 | 100 | |
| Fe | 80 ± 0.0 | 79.3 ± 0.02 | 99.1 | |
| Ni | 0.91 ± 0.12 | 0.89 ± 0.05 | 97.8 | |
| Al | 286 | 284 ± 0.50 | 99.3 | |
| BCR 279 Sea lettuce | Cu | 13.1 ± 0.4 | 12.7 ± 0.30 | 96.9 |
| Zn | 51.3 ± 1.2 | 51.0 ± 0.50 | 99.4 | |
| Cd | 0.27 ± 0.02 | 0.26 ± 0.01 | 96.3 | |
| Pb | 13.1 ± 0.4 | 12.8 ± 0.20 | 97.7 | |
| Standard Addition Method | Li | 0.2 ± 0.02 | 0.19 ± 0.03 | 95.0 |
| Asparagopsis spp. (n = 20) | ||
|---|---|---|
| Element | Concentration ± SD (mg/kg Dw) | Max–Min |
| Co | 0.37 ± 0.20 | 0.87–0.10 |
| Cr | 1.10 ± 0.41 | 2.01–0.38 |
| Cu | 4.90 ± 1.73 | 8.36–2.21 |
| Fe | 734 ± 521 | 1630–27.1 |
| Mn | 22.4 ± 11.4 | 48.5–5.96 |
| Mo | 0.22 ± 0.19 | 0.83–0.02 |
| Zn | 7.80 ± 4.09 | 19.2–1.83 |
| B | 77.9 ± 39 | 187–31.8 |
| Ba | 7.20 ± 2.8 | 13.6–2.36 |
| Li | 1.28 ± 0.11 | 6.28–1.11 |
| Ni | 1.54 ± 0.40 | 5.12–0.83 |
| Sr * | <0.003 | <0.003 |
| V | 5.63 ± 6.48 | 24.8–0.02 |
| Al | 288 ± 157 | 647–100 |
| Cd | 0.13 ± 0.08 | 0.31–0.02 |
| Pb | 4.63 ± 7.28 | 34.5–0.64 |
| Liagora spp. (n = 10) | ||
|---|---|---|
| Element | Concentration ± SD (mg/kg Dw) | Max–Min |
| Co | 0.18 ± 0.14 | 0.43–0.04 |
| Cr | 0.70 ± 0.16 | 0.92–0.43 |
| Cu | 6.60 ± 4.71 | 13.8–1.10 |
| Fe | 1190 ± 1545 | 1093–3.64 |
| Mn | 14.9 ± 9.92 | 29.8–1.35 |
| Mo | 0.20 ± 0.07 | 0.32–0.12 |
| Zn | 4.30 ± 3.18 | 8.48–1.03 |
| B | 80.2 ± 34.2 | 185–23.0 |
| Ba | 23.7 ± 12.3 | 11.5–3.62 |
| Li | 4.30 ± 2.67 | 7.33–0.57 |
| Ni | 3.70 ± 3.02 | 2.10–0.54 |
| Sr * | <0.003 | <0.003 |
| V | 5.41 ± 5.37 | 7.83–0.21 |
| Al | 256 ± 179 | 401–38.2 |
| Cd | 0.20 ± 0.21 | 0.20–0.01 |
| Pb | 3.92 ± 3.71 | 4.11–0.14 |
| Concentration (mg/kg Dw) | ||||
|---|---|---|---|---|
| Element | Porís de Abona (Present Study, 2022) | Porís de Abona [47] | La Punta del Hidalgo [47] | El Socorro [47] |
| Fe | 962 | 629 | 49.1 | 59.3 |
| B | 79.05 | 74 | 103 | 133 |
| Ba | 15.45 | 13.4 | 7.04 | 5.66 |
| Li | 2.79 | 2.54 | 2.46 | 4.72 |
| Ni | 2.62 | 2.3 | 1.2 | 0.88 |
| V | 5.52 | 3.12 | 0.36 | 1.31 |
| Al | 272 | 212 | 40.2 | 57.7 |
| Cd | 0.165 | 0.13 | 0.22 | 0.16 |
| Pb | 4.275 | 2.7 | 0.34 | 0.43 |
| Spearman’s Rho | ||||
| Cd | Zn | |||
| Cd | Correlation coefficient | 1.000 | 0.309 ** | |
| Sig. (bilateral) | 0.000 | |||
| N | 158 | 158 | ||
| Zn | Correlation coefficient | 0.309 ** | 1.000 | |
| Sig. (bilateral) | 0.000 | |||
| N | 158 | 158 | ||
| Spearman’s Rho | ||||
| Al | Pb | Cd | ||
| Al | Correlation coefficient | 1.000 | 0.863 ** | 0.310 ** |
| Sig. (bilateral) | . | 0.000 | 0.000 | |
| N | 158 | 158 | 158 | |
| Pb | Correlation coefficient | 0.863 ** | 1.000 | 0.303 ** |
| Sig. (bilateral) | 0.000 | . | 0.000 | |
| N | 158 | 158 | 158 | |
| Cd | Correlation coefficient | 0.310 ** | 0.303 ** | 1.000 |
| Sig. (bilateral) | 0.000 | 0.000 | ||
| N | 158 | 158 | 158 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, S.; Rubio-Armendáriz, C.; Frías, I.; Guillén-Pino, F.; Niebla-Canelo, D.; Alejandro-Vega, S.; Gutiérrez, Á.J.; Hardisson, A.; González-Weller, D. Toxic and Trace Elements in Seaweeds from a North Atlantic Ocean Region (Tenerife, Canary Islands). Sustainability 2022, 14, 5967. https://doi.org/10.3390/su14105967
Paz S, Rubio-Armendáriz C, Frías I, Guillén-Pino F, Niebla-Canelo D, Alejandro-Vega S, Gutiérrez ÁJ, Hardisson A, González-Weller D. Toxic and Trace Elements in Seaweeds from a North Atlantic Ocean Region (Tenerife, Canary Islands). Sustainability. 2022; 14(10):5967. https://doi.org/10.3390/su14105967
Chicago/Turabian StylePaz, Soraya, Carmen Rubio-Armendáriz, Inmaculada Frías, Fernando Guillén-Pino, Daniel Niebla-Canelo, Samuel Alejandro-Vega, Ángel J. Gutiérrez, Arturo Hardisson, and Dailos González-Weller. 2022. "Toxic and Trace Elements in Seaweeds from a North Atlantic Ocean Region (Tenerife, Canary Islands)" Sustainability 14, no. 10: 5967. https://doi.org/10.3390/su14105967
APA StylePaz, S., Rubio-Armendáriz, C., Frías, I., Guillén-Pino, F., Niebla-Canelo, D., Alejandro-Vega, S., Gutiérrez, Á. J., Hardisson, A., & González-Weller, D. (2022). Toxic and Trace Elements in Seaweeds from a North Atlantic Ocean Region (Tenerife, Canary Islands). Sustainability, 14(10), 5967. https://doi.org/10.3390/su14105967











