Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
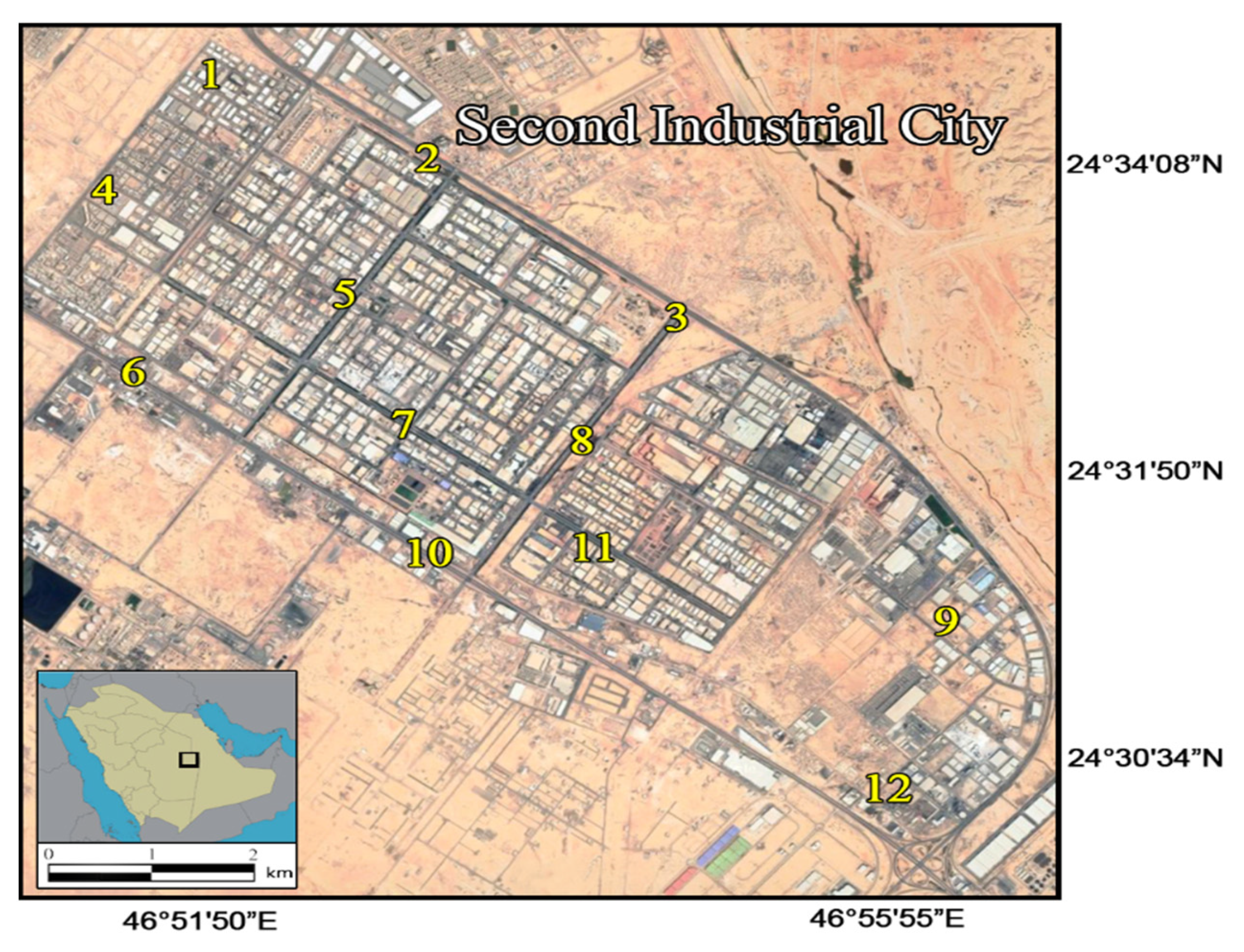
2.1. Study Area
2.2. Soil Sampling and Analyses
2.3. Plant Sampling and Analyses
2.4. Data Precision and Accuracy
2.5. Quantification of Soil Contamination of the Study Area
2.5.1. Enrichment Factor (EF)
2.5.2. Contamination Factor (CF)
2.5.3. Pollution Load Index (PLI)
2.5.4. Bioaccumulation Factor for Plants (BF)
2.6. Statistical Analyses
3. Results and Discussion
3.1. Soil
3.1.1. Physicochemical Parameters
3.1.2. Heavy Metal Concentration in Soils
3.2. Indices of Pollution
3.2.1. Enrichment Factor (EF)
3.2.2. Contamination Factor (CF) and Pollution Load Index (PLI)
3.2.3. Heavy Metal Concentration in Plants
3.2.4. Bioaccumulation Factor (BF)
4. Practical Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alloway, J.B. Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-4469-1. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A Critical Review on the Bio-Removal of Hazardous Heavy Metals from Contaminated Soils: Issues, Progress, Eco-Environmental Concerns and Opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.; Santanen, A.; Mäkelä, P. Recycling Sludge on Cropland as Fertilizer—Advantages and Risks. Resour. Conserv. Recycl. 2019, 155, 104647. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace Elements in Agroecosystems and Impacts on the Environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Tang, J.; Chai, L.; Li, H.; Yang, Z.; Yang, W. A 10-Year Statistical Analysis of Heavy Metals in River and Sediment in Hengyang Segment, Xiangjiang River Basin, China. Sustainability 2018, 10, 1057. [Google Scholar] [CrossRef] [Green Version]
- Aloud, S.S. Impact of Municipal and Industrial Waste on the Distribution and Accumulation of Some Heavy Metals in Sandy Soils of Al-Qassim Region at Central of Saudi Arabia. J. Environ. Sci. Technol. 2008, 1, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments—A Review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Wang, J. Distribution of Urban Soil Heavy Metal and Pollution Evaluation in Different Functional Zones of Yinchuan City. Huan Jing Ke Xue 2016, 37, 710–716. [Google Scholar]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-Environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. Rev. Environ. Contam. Toxicol. 2020, 249, 71–131. [Google Scholar] [CrossRef] [Green Version]
- Badawy, S.H.; Helal, M.I.D.; Chaudri, A.M.; Lawlor, K.; McGrath, S.P. Soil Solid-Phase Controls Lead Activity in Soil Solution. J. Environ. Qual. 2002, 31, 162–167. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic Metal Compounds: Recent Insight into Molecular and Cellular Mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, V.; Adeniran, A. Determination of Heavy Metal Fallout on the Surrounding Flora and Aquifer: Case Study of A Scrap Metal Smelting Factory in Odogunyan Area, Ikorodu, Lagos-State, Nigeria. Int. Res. J. Environ. Sci. 2014, 3, 93–100. [Google Scholar]
- Alyemeni, M.; Sher, H.; El-Sheikh, M.; Eid, E. Bioaccumulation of Nutrient and Heavy Metals by Calotropis Procera and Citrullus Colocynthis and Their Potential Use as Contamination Indicators. Sci. Res. Essays 2011, 6, 966–976. [Google Scholar]
- Kaur, J.; Bhat, S.A.; Singh, N.; Bhatti, S.S.; Kaur, V.; Katnoria, J.K. Assessment of the Heavy Metal Contamination of Roadside Soils Alongside Buddha Nullah, Ludhiana, (Punjab) India. Int. J. Environ. Res. Public Health 2022, 19, 1596. [Google Scholar] [CrossRef]
- Deng, J.; Li, B.; Zhang, S.; Li, Z.; Zu, Y.; He, Y.; Chen, J.; Li, T. Plant Species Diversity of Plant Communities and Heavy Metal Accumulation in Buffer Zone of Momianhe Stream Along a Long-Term Mine Wastes Area, China. Bull. Environ. Contam. Toxicol. 2021, 107, 1136–1142. [Google Scholar] [CrossRef]
- Lenart-Boroń, A.; Wolny-Koładka, K. Heavy Metal Concentration and the Occurrence of Selected Microorganisms in Soils of a Steelworks Area in Poland. Plant Soil Environ. 2015, 61, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Crnković, D.; Ristić, M.; Antonović, D. Distribution of Heavy Metals and Arsenic in Soils of Belgrade (Serbia and Montenegro). Soil Sediment Contam. Int. J. 2006, 15, 581–589. [Google Scholar] [CrossRef]
- Aloud, S.S. Extraction of Heavy Metals by Phosphate and Oxalate from a Contaminated Soil. In Proceedings of the 1st IASTED International Conference on Unconventional Oil, Calgary, AB, Canada, 4–6 July 2011. [Google Scholar] [CrossRef]
- Aloud, S.S. Mutual Effect of EDTA and Some Plant Species on Phytoremediation of Arsenic and Cadmium Contaminated Soil. Egypt. J. Appl. Sci. 2007, 8A, 264–275. [Google Scholar]
- Aloud, S.S. Enhancement of Heavy Metals Movement by Capillary Forces by Addition of Chelate Solution in Polluted Soil. In Proceedings of the 6th International Conference on Environmental Science and Technology (ICEST), Houston, TX, USA, 25–29 June 2012; pp. 339–340. [Google Scholar]
- Baker, A.; Brooks, R. Terrestrial Higher Plants Which Hyperaccumulate Metallic Elements, A Review of Their Distribution, Ecology and Phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Küpper, H.; Leitenmaier, B. Cadmium-Accumulating Plants. Met. Ions Life Sci. 2013, 11, 373–393. [Google Scholar] [CrossRef]
- Rodríguez-Bocanegra, J.; Roca, N.; Febrero, A.; Bort, J. Assessment of Heavy Metal Tolerance in Two Plant Species Growing in Experimental Disturbed Polluted Urban Soil. J. Soils Sediments 2018, 18, 2305–2317. [Google Scholar] [CrossRef]
- Holliday, V.T. Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods (2nd Edition), A. Klute, Ed., 1986, American Society of Agronomy, Agronomy Monographs 9(1), Madison, Wisconsin, 1188 pp., \$60.00. Geoarchaeol. Int. J. 1990, 5, 87–89. [Google Scholar] [CrossRef]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of Soil Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 437–474. ISBN 9780891188667. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 9780891189770. [Google Scholar]
- Thomas, G.W. Soil PH and Soil Acidity. In Methods of Soil Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 475–490. ISBN 9780891188667. [Google Scholar]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region, 3rd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- Ho, H.H.; Rudy, S.; Damme, A. Distribution and Contamination Status of Heavy Metals in Estuarine Sediments near CauOng Harbor, Ha Long Bay, Vietnam. Geology Belgica. Geol. Belgica 2010, 13, 37–47. [Google Scholar]
- Lizárraga-Mendiola, L.; Durán-Domínguez-de-Bazúa, M.D.C.; González-Sandoval, M.-R. Environmental Assessment of an Active Tailings Pile in the State of Mexico (Central Mexico). Res. J. Environ. Sci. 2008, 2, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Demková, L.; Jezný, T.; Bobulska, L. Assessment of Soil Heavy Metal Pollution in a Former Mining Area—Before and after the End of Mining Activities. Soil Water Res. 2017, 12, 2017. [Google Scholar] [CrossRef] [Green Version]
- Sutherlad, R. Bed Sediment-Associated Trace Metals in an Urban Stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Usero, J.G.; Amarillo, J.F. Evaluación de La Calidad de Las Aguas y Sedimentos Del Litoral de Andalucía; Consejería de Medio Ambiente: Sevilla, Spain, 2000; 164p. [Google Scholar]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed Wetlands for Advanced Treatment of Wastewater with a Complex Matrix from a Metal-Processing Plant: Bioconcentration and Translocation Factors of Various Metals in Acorus Gramineus and Cyperus Alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Rai, U.; Upadhyay, A.; Singh, N.; Dwivedi, S.; Tripathi, R. Seasonal Applicability of Horizontal Sub-Surface Flow Constructed Wetland for Trace Elements and Nutrient Removal from Urban Wastes to Conserve Ganga River Water Quality at Haridwar, India. Ecol. Eng. 2015, 81, 115–122. [Google Scholar] [CrossRef]
- Wilson, S.C.; Tighe, M.; Paterson, E.; Ashley, P.M. Food Crop Accumulation and Bioavailability Assessment for Antimony (Sb) Compared with Arsenic (As) in Contaminated Soils. Environ. Sci. Pollut. Res. Int. 2014, 21, 11671–11681. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Al-Tayib, M.; Hag-Elsafi, S.; Al-Duwarij, N. Assessment of Pollution Sources in the Southeastern of the Riyadh and Its Impact on the Population/Saudi Arabia. Arab. J. Geosci. 2016, 9, 328. [Google Scholar] [CrossRef]
- Dragović, S.; Mihailović, N.; Gajić, B. Heavy Metals in Soils: Distribution, Relationship with Soil Characteristics and Radionuclides and Multivariate Assessment of Contamination Sources. Chemosphere 2008, 72, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Rajamani, V. Air Quality and Trace Metal Chemistry of Different Size Fractions of Aerosols in N-NW India—Implications for Source Diversity. Atmos. Environ. 2006, 40, 698–712. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination Features and Health Risk of Soil Heavy Metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, V.; Gulan, L.; Milenković, B.; Valjarević, A.; Zeremski, T.; Penjišević, I. Environmental Risk Assessment of Radioactivity and Heavy Metals in Soil of Toplica Region, South Serbia. Environ. Geochem. Health 2018, 40, 2101–2118. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shen, D.; Wu, S.; Han, Y.; Li, S.; Tang, F.; Lin, N.; Mo, R.; Liu, Y. Uptake Effects of Toxic Heavy Metals from Growth Soils into Jujube and Persimmon of China. Environ. Sci. Pollut. Res. 2018, 25, 31593–31602. [Google Scholar] [CrossRef]
- Cardwell, A.J.; Hawker, D.W.; Greenway, M. Metal Accumulation in Aquatic Macrophytes from Southeast Queensland, Australia. Chemosphere 2002, 48, 653–663. [Google Scholar] [CrossRef]
- Chaney, R.L. Toxic Element Accumulation in Soils and Crops: Protecting Soil Fertility and Agricultural Food-Chains. In Inorganic Contaminants in the Vadose Zone; Bar-Yosef, B., Barrow, N.J., Goldshmid, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Bonanno, G.; Lo Giudice, R. Heavy Metal Bioaccumulation by the Organs of Phragmites Australis (Common Reed) and Their Potential Use as Contamination Indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Hassan, Z.; Aarts, M. Opportunities and Feasibilities for Biotechnological Improvement of Zn, Cd Or Ni Tolerance and Accumulation in Plants. Environ. Exp. Bot. 2011, 72, 53–63. [Google Scholar] [CrossRef]
- Muhammad, S.; Shah, M.T.; Khan, S.; Saddique, U.; Gul, N.; Khan, M.U.; Malik, R.N.; Farooq, M.; Naz, A. Wild Plant Assessment for Heavy Metal Phytoremediation Potential along the Mafic and Ultramafic Terrain in Northern Pakistan. Biomed. Res. Int. 2013, 2013, 194765. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Lee, J.-S.; Chon, H.-T.; Sager, M. Heavy Metal Contamination and Health Risk Assessment in the Vicinity of the Abandoned Songcheon Au-Ag Mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Beef Cattle, 7th ed.; Update 2000; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Florida Site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Fitz, W.J.; Wenzel, W.W. Arsenic Transformations in the Soil-Rhizosphere-Plant System: Fundamentals and Potential Application to Phytoremediation. J. Biotechnol. 2002, 99, 259–278. [Google Scholar] [CrossRef]



| Parameters | Minimum | Maximum | Mean (SD a) |
|---|---|---|---|
| Sand% | 55 | 60 | 58.1 (1.4) |
| Silt% | 18 | 26 | 22.4 (2.6) |
| Clay% | 16 | 23 | 19.4 (2.1) |
| pH (1:1) | 7.4 | 8.7 | 8.2 (0.4) |
| EC b dsm−1 | 6.6 | 12.8 | 9.19 (2.0) |
| % O.M c | 0.43 | 0.98 | 0.74 (0.2) |
| CaCO3% | 28.5 | 42.2 | 35 (5.1) |
| Family | Species | Classification |
|---|---|---|
| Apocynaceae | Calotropis procera | Perennial |
| Cucurbitaceae | Citrullus colocynthis | Annual |
| Fabaceae | Cassia italica | Perennial |
| Cyperaceae | Cyperus laevigatus | Perennial |
| Papaveraceae | Argemone mexicana | Annual |
| Apocynaceae | Rhazya stricta | Perennial |
| Combretaceae | Conocarpus lancifolius | Perennial |
| Zygophyllaceae | Zygophyllum simplex | Annual |
| Amaranthaceae | Salsola imbricata | Annual or perennial |
| Malvaceae | Malva parviflora | Annual or perennial |
| Brassicaceae | Sisymbrium irio | Annual or winter-annual |
| Poaceae | Phrgmites australis | Annual or perennial |
| Sites No. | Mean (SD a) | ||||||
|---|---|---|---|---|---|---|---|
| Heavy Metal | |||||||
| Cd | Cr | Cu | Fe | Pb | Ni | Zn | |
| S1 | 1.7 (0.34) | 15.85 (0.32) | 14.6 (1.87) | 4035.5 (207.7) | 16.7 (1.12) | 26.65 (1.08) | 33.95 (0.11) |
| S2 | 22 (1.41) | 20.55 (2.38) | 11.1 (0.88) | 5218 (163.8) | 25.5 (5.12) | 21.7 (2.22) | 32.5 (0.40) |
| S3 | 3.1 (1.43) | 15.55 (15.55) | 18.7 (2.31) | 4848 (183.5) | 18.05(1.65) | 60.1 (5.94) | 20.1 (2.52) |
| S4 | 16 (0.62) | 1.25 (0.36) | 12.55 (2.67) | 655.5 (19.7) | 5.1 (1.28) | 72.8 (5.32) | 22.8 (4.05) |
| S5 | 12.9 (1.79) | 34.2 (3.37) | 16.95 (1.12) | 6478.5 (162.8) | 10.9 (1.53) | 45.0 (8.64) | 42.6 (2.13) |
| S6 | 5.5 (0.51) | 16.75 (1.15) | 22.4 (1.64) | 1660.8 (76.9) | 10.75 (1.54) | 48.8 (1.51) | 20.1 (2.56) |
| S7 | 15.1 (0.67) | 11.7 (0.57) | 12.4 (0.36) | 1303.5 (30.3) | 12.55 (1.52) | 66.5 91.16) | 21.15 (0.94) |
| S8 | 5.9 (0.57) | 4.5 (0.59) | 17.9 (0.23) | 6262 (186.6) | 22.55 (2.19) | 38.75 (0.32) | 14.35 (0.10) |
| S9 | 7.7 (0.96) | 18.1 (0.36) | 14.9 (0.23) | 4860 (60.8) | 3.35 (0.26) | 29.3 (0.93) | 21.7 (0.17) |
| S10 | 1.1 (0.08) | 5.7 (0.37) | 22.35 (0.32) | 2003.7 (7.57) | 16.25 (0.24) | 34.5 (1.08) | 11.55 (0.66) |
| S11 | 8.3 (90.36) | 2.75 (0.10) | 8.2 (0.29) | 1829.2 (117.1) | 22.55 (0.88) | 32.5 (0.50) | 15.25 (0.07) |
| S12 | 3.6 (0.22) | 1.6 (0.15) | 9.25 (0.07) | 3117.5 (181.0) | 25.5 (0.45) | 64.93 (2.12) | 16.65 (0.11) |
| Zn | Pb | Ni | Fe | Cu | Cr | Cd | |
|---|---|---|---|---|---|---|---|
| Sand% | 0.486 | −0.07 | −0.03 | 0.362 | −0.627 * | 0.244 | 0.195 |
| Silt% | −0.544 | 0.611 * | −0.127 | −0.234 | 0.103 | −0.373 | −0.194 |
| Clay% | 0.326 | −0.753 ** | 0.193 | 0.016 | 0.373 | 0.296 | 0.099 |
| pH | 0.474 | −0.527 | −0.086 | −0.095 | 0.447 | 0.462 | 0.071 |
| EC | −0.486 | 0.475 | 0.528 | −0.457 | −0.452 | −0.549 | −0.035 |
| O.M% | 0.05 | −0.119 | −0.449 | −0.241 | 0.157 | 0.307 | 0.188 |
| CaCO3% | 0.126 | −0.03 | 0.357 | 0.067 | −0.266 | −0.188 | 0.02 |
| Enrichment Factor (EF) | Cd | Cr | Cu | Pb | Zn | Ni |
|---|---|---|---|---|---|---|
| Deficiency to minimal enrichment | 0 | 66.6 | 0 | 0 | 8.33 | 8.33 |
| Moderate enrichment | 0 | 33.4 | 58.33 | 8.33 | 50 | 33.33 |
| Significant enrichment | 0 | 0 | 33.34 | 41.66 | 33.33 | 33.33 |
| Very high enrichment | 0 | 0 | 8.33 | 33.3 | 8.33 | 8.33 |
| Extremely high enrichment | 100 | 0 | 0 | 8.33 | 0 | 16.66 |
| Enrichment Factor (EF) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Sites | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 |
| Cd | 266 | 2670 | 405 | 15,457 | 1261 | 2097 | 7336 | 596 | 1003 | 347 | 2873 | 731 |
| Cr | 1.5 | 1.5 | 1.2 | 0.7 | 2 | 3.8 | 3.4 | 0.3 | 1.4 | 1.1 | 0.6 | 0.2 |
| Cu | 4.6 | 2.7 | 4.9 | 24.2 | 3.3 | 17.1 | 12 | 3.6 | 3.9 | 14.1 | 5.7 | 3.8 |
| Pb | 15.7 | 18.6 | 14.1 | 29.6 | 6.4 | 24.6 | 36.6 | 13.7 | 2.6 | 30.8 | 46.8 | 31.1 |
| Zn | 6.4 | 4.7 | 3.2 | 26.4 | 5 | 9.2 | 12.3 | 1.7 | 3.4 | 4.4 | 6.3 | 4.1 |
| Ni | 6.3 | 4 | 11.8 | 105.5 | 6.6 | 27.9 | 48.5 | 5.9 | 5.7 | 16.4 | 16.9 | 19.8 |
| Site ID | Contamination Factor (CF) | Pollution Load Index (PLI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Pb | Zn | Ni | ||
| S1 | 28.33 | 0.16 | 0.49 | 0.11 | 1.67 | 0.68 | 0.67 | 0.78 |
| S2 | 366.67 | 0.21 | 0.37 | 0.14 | 2.55 | 0.65 | 0.54 | 1.19 |
| S3 | 51.67 | 0.16 | 0.62 | 0.13 | 1.81 | 0.4 | 1.5 | 0.95 |
| S4 | 266.67 | 0.01 | 0.42 | 0.02 | 0.51 | 0.46 | 1.82 | 0.52 |
| S5 | 215 | 0.34 | 0.57 | 0.17 | 1.09 | 0.85 | 1.13 | 1.33 |
| S6 | 91.67 | 0.17 | 0.75 | 0.04 | 1.08 | 0.4 | 1.22 | 0.83 |
| S7 | 251.67 | 0.12 | 0.41 | 0.03 | 1.26 | 0.42 | 1.66 | 0.87 |
| S8 | 98.33 | 0.05 | 0.6 | 0.16 | 2.26 | 0.29 | 0.97 | 0.83 |
| S9 | 128.33 | 0.18 | 0.5 | 0.13 | 0.34 | 0.43 | 0.73 | 0.77 |
| S10 | 18.33 | 0.06 | 0.75 | 0.05 | 1.63 | 0.23 | 0.86 | 0.54 |
| S11 | 138.33 | 0.03 | 0.27 | 0.05 | 2.26 | 0.31 | 0.81 | 0.60 |
| S12 | 60 | 0.02 | 0.31 | 0.08 | 2.55 | 0.33 | 1.62 | 0.62 |
| Range | 18.3–366.7 | 0.01–0.34 | 0.27–0.75 | 0.02–0.17 | 0.34–2.55 | 0.23–0.85 | 0.54–1.82 | 0.52–1.33 |
| Heavy Metals | Minimum (mg·kg−1) | Maximum (mg·kg−1) | Mean (mg·kg−1) |
|---|---|---|---|
| Zn | 6.6 | 22.1 | 12.75 |
| Pb | 0.7 | 22.1 | 5.4 |
| Ni | 7.5 | 28 | 12.9 |
| Fe | 125.9 | 2308 | 544.8 |
| Cu | 4.0 | 63 | 27.6 |
| Cr | 1.2 | 7.8 | 4.271 |
| Cd | 0.2 | 2.4 | 1.12 |
| Plant Species | Mean (SD a) | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Ni | Pb | Zn | |
| Calotropis procera | 1.2 (0.80) | 5 (1.41) | n.d. | 445.98 (43.17) | 15.1 (0.22) | 5.5 (0.62) | 17.6 (1.61) |
| Citrullus colocynthis | 1.9 (0.22) | 4.9 (0.45) | 13 (1.41) | 629.73 (86.93) | 9.7 (1.84) | 9.6 (1.81) | 10.7 (1.37) |
| Cassia italica | 1.7 (0.22) | 7.8 (1.23) | n.d. | 578.07 (31.96) | 11.8 (2.65) | 1.1 (0.65) | 6.9 (0.92) |
| Phragmite australis | 0.97 (0.16) | 1.2 (0.22) | 9.4 (0.78) | 379.47 (27.69) | 8.7 (1.44) | 0.7 (0.33) | 12.8 (2.57) |
| Cyperus laevigatus | 2.4 (0.22) | 3.2 (0.75) | 10.5 (1.22) | 171.25 (10.15) | 14.5 (1.31) | 3 (0.82) | 22.1 (1.56) |
| Argemone mexicana | 0.7 (0.14) | 5.8 (0.67) | 5.8 (2.19) | 156.47 (4.94) | 13.3 (1.63) | 2.3 (0.29) | 15.2 (2.62) |
| Rhazya stricta | 1.3 (0.29) | 5.3 (0.16) | 7.2 (0.11) | 125.98 (4.02) | 28 (1.08) | 22.1 (0.43) | 18.9 (0.29) |
| Conocarpus lancifolius | 1.1 (0.22) | 2.41 (0.13) | 14.13 (0.29) | 242.21 (917.18) | 11.40 (0.45) | 7.51 (1.63) | 8.9 (0.29) |
| Zygophyllum simplex | 0.3 (0.11) | 3.0 (0.36) | 4.76 (0.20) | 412.46 (23.17) | 7.46 (0.09) | 2.71 (0.19) | 12.1 (0.22) |
| Salsola imbricata | 0.2 (0.01) | 4.1 (0.16) | 17.25 (0.33) | 543.3 (16.7) | 8.8 (0.26) | 6.3 (0.28) | 6.6 (0.15) |
| Malva parviflora | 1.25 (0.20) | 3.2 (0.21) | 22.6 (0.49) | 2308 (221.5) | 12.92 (0.07) | 3.3 (0.24) | 11.1 (0.62) |
| Sisymbrium irio | 0.46 (0.05) | 1.4 (0.14) | 14.2 (0.11) | 936.8 (18.26) | 6.7 (0.12) | 5.2 (0.03) | 10.1 (0.15) |
| Plant Species | Sites | Bioconcentration Factor (BF) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Pb | Zn | Ni | ||
| Calotropis procera | S1 | 0.71 | 0.32 | n.d. | 0.11 | 0.33 | 0.52 | 0.57 |
| Citrullus colocynthis | S2 | 0.09 | 0.24 | 1.17 | 0.12 | 0.38 | 0.33 | 0.45 |
| Cassia italica | S3 | 0.55 | 0.5 | n.d. | 0.12 | 0.06 | 0.34 | 0.20 |
| Phragmite australis | S4 | 0.06 | 0.96 | 0.75 | 0.58 | 0.14 | 0.56 | 0.12 |
| Cyperus laevigatus | S5 | 0.19 | 0.09 | 0.62 | 0.03 | 0.28 | 0.52 | 0.32 |
| Argemone mexicana | S6 | 0.13 | 0.35 | 0.26 | 0.09 | 0.21 | 0.76 | 0.27 |
| Rhazya stricta | S7 | 0.09 | 0.45 | 0.58 | 0.10 | 0.60 | 0.89 | 0.42 |
| Conocarpus lancifolius | S8 | 0.19 | 0.54 | 0.79 | 0.04 | 0.98 | 0.62 | 0.29 |
| Zygophyllum simplex | S9 | 0.04 | 0.17 | 0.32 | 0.08 | 0.81 | 0.56 | 0.25 |
| Salsola imbricata | S10 | 0.18 | 0.72 | 0.77 | 0.27 | 0.39 | 0.57 | 0.26 |
| Malva parviflora | S11 | 0.15 | 1.16 | 2.76 | 1.26 | 0.15 | 0.73 | 0.40 |
| Sisymbrium irio | S12 | 0.13 | 0.88 | 1.54 | 0.30 | 0.20 | 0.61 | 0.10 |
| Range | 0.04–0.71 | 0.09–1.16 | 0.26–2.76 | 0.03–1.26 | 0.06–0.98 | 0.33–0.89 | 0.1–0.57 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloud, S.S.; Alotaibi, K.D.; Almutairi, K.F.; Albarakah, F.N. Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia. Sustainability 2022, 14, 5993. https://doi.org/10.3390/su14105993
Aloud SS, Alotaibi KD, Almutairi KF, Albarakah FN. Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia. Sustainability. 2022; 14(10):5993. https://doi.org/10.3390/su14105993
Chicago/Turabian StyleAloud, Saud S., Khaled D. Alotaibi, Khalid F. Almutairi, and Fahad N. Albarakah. 2022. "Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia" Sustainability 14, no. 10: 5993. https://doi.org/10.3390/su14105993
APA StyleAloud, S. S., Alotaibi, K. D., Almutairi, K. F., & Albarakah, F. N. (2022). Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia. Sustainability, 14(10), 5993. https://doi.org/10.3390/su14105993






