The Antidepressants Amitriptyline and Paroxetine Induce Changes in the Structure and Functional Traits of Marine Nematodes
Abstract
1. Introduction
2. Material and Methods
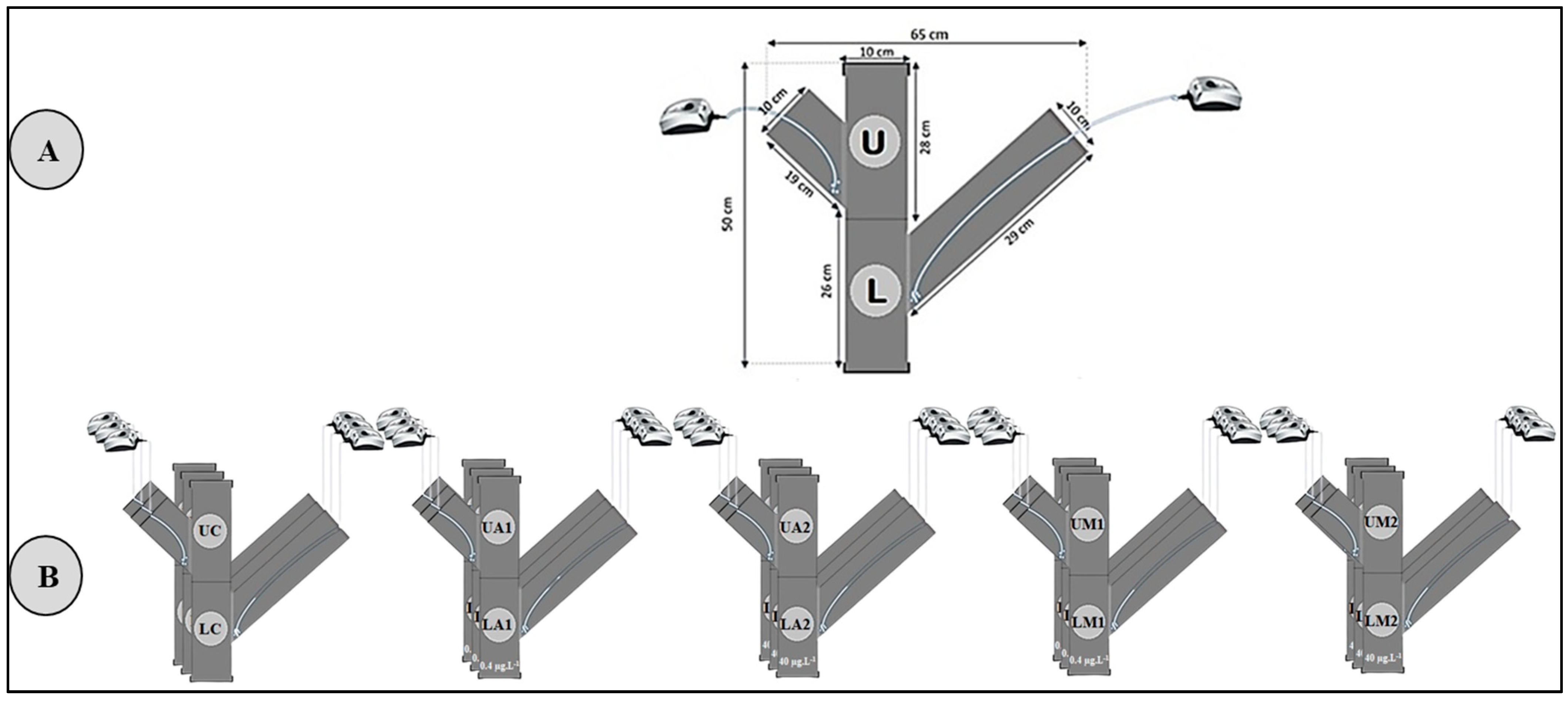
2.1. Sampling Site and Acclimatization
2.2. Experiment Set-Up
2.3. Sediment Contamination
2.4. Meiofauna Study
2.5. Statistical Analyses
3. Results
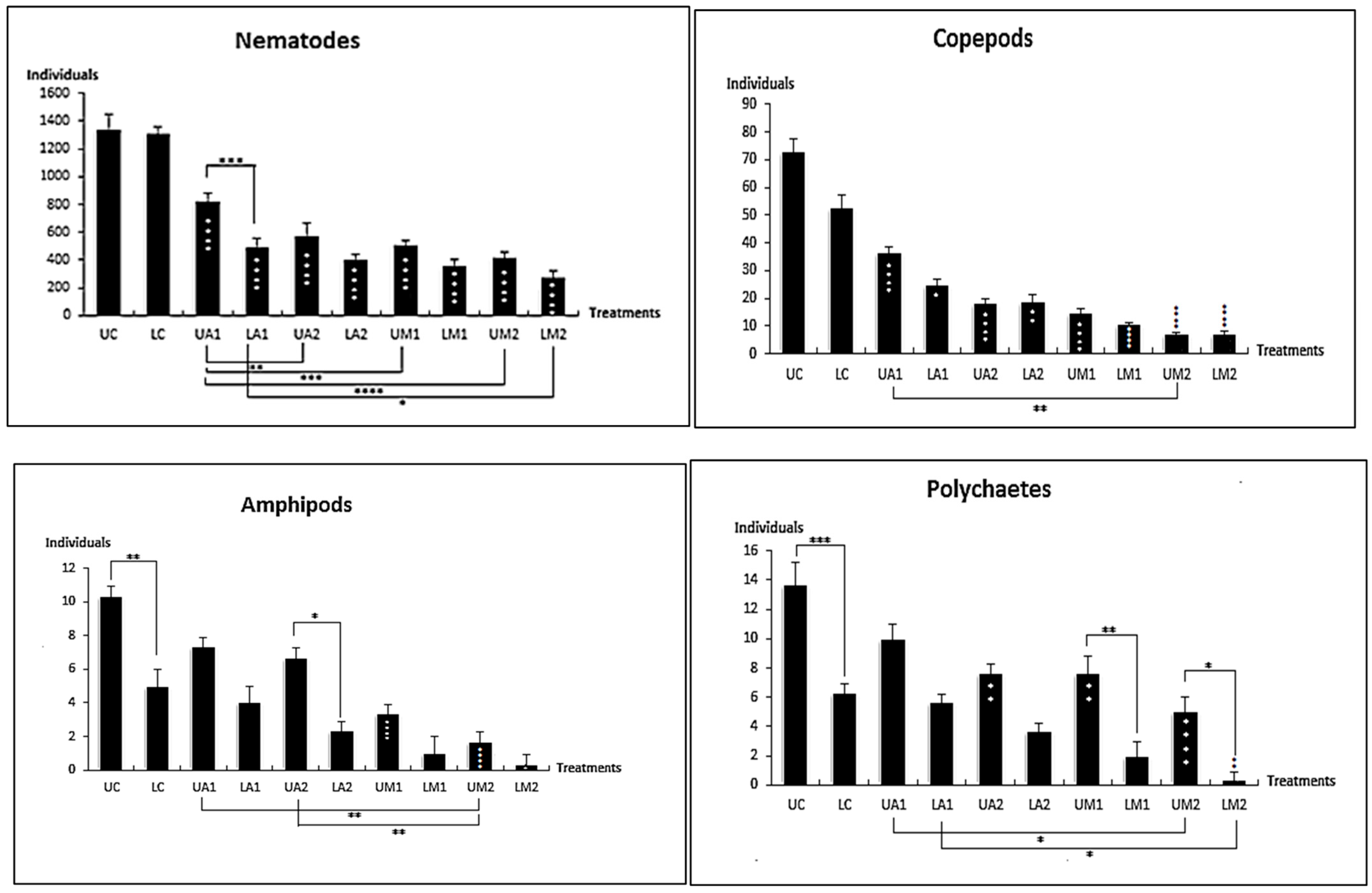
3.1. Taxonomic Composition
3.2. Univarites Indices
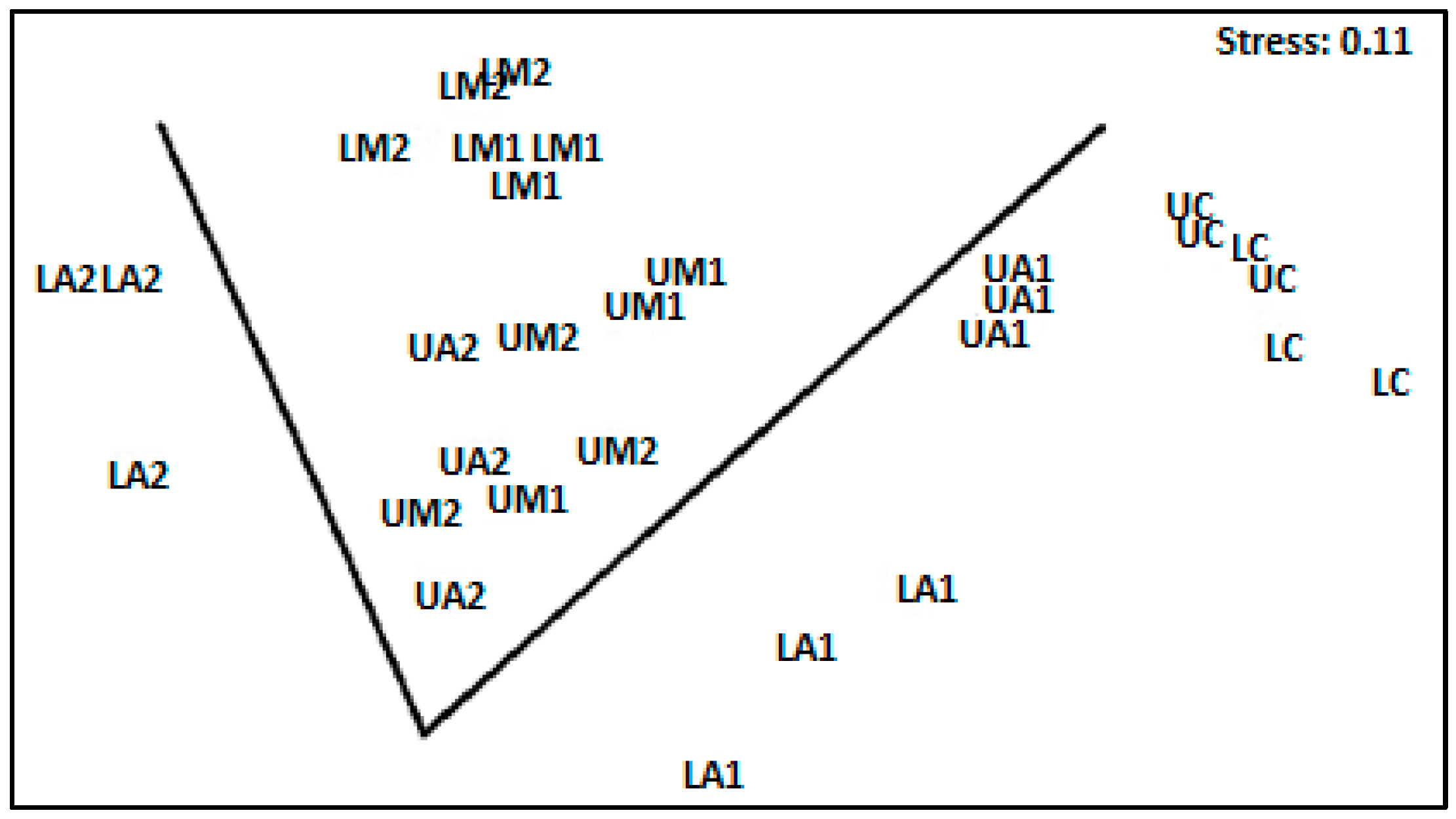
3.3. Multivariate Indices
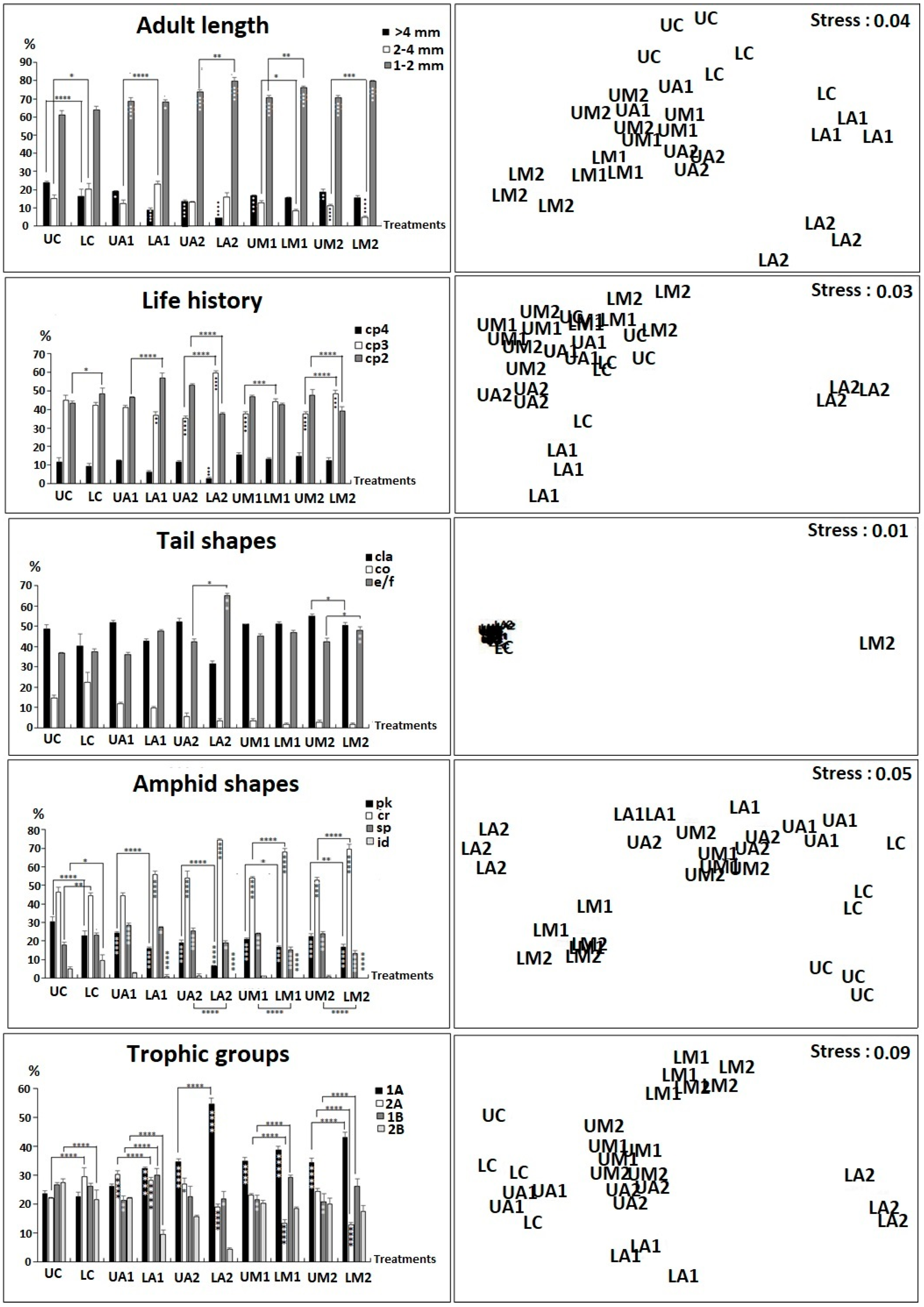
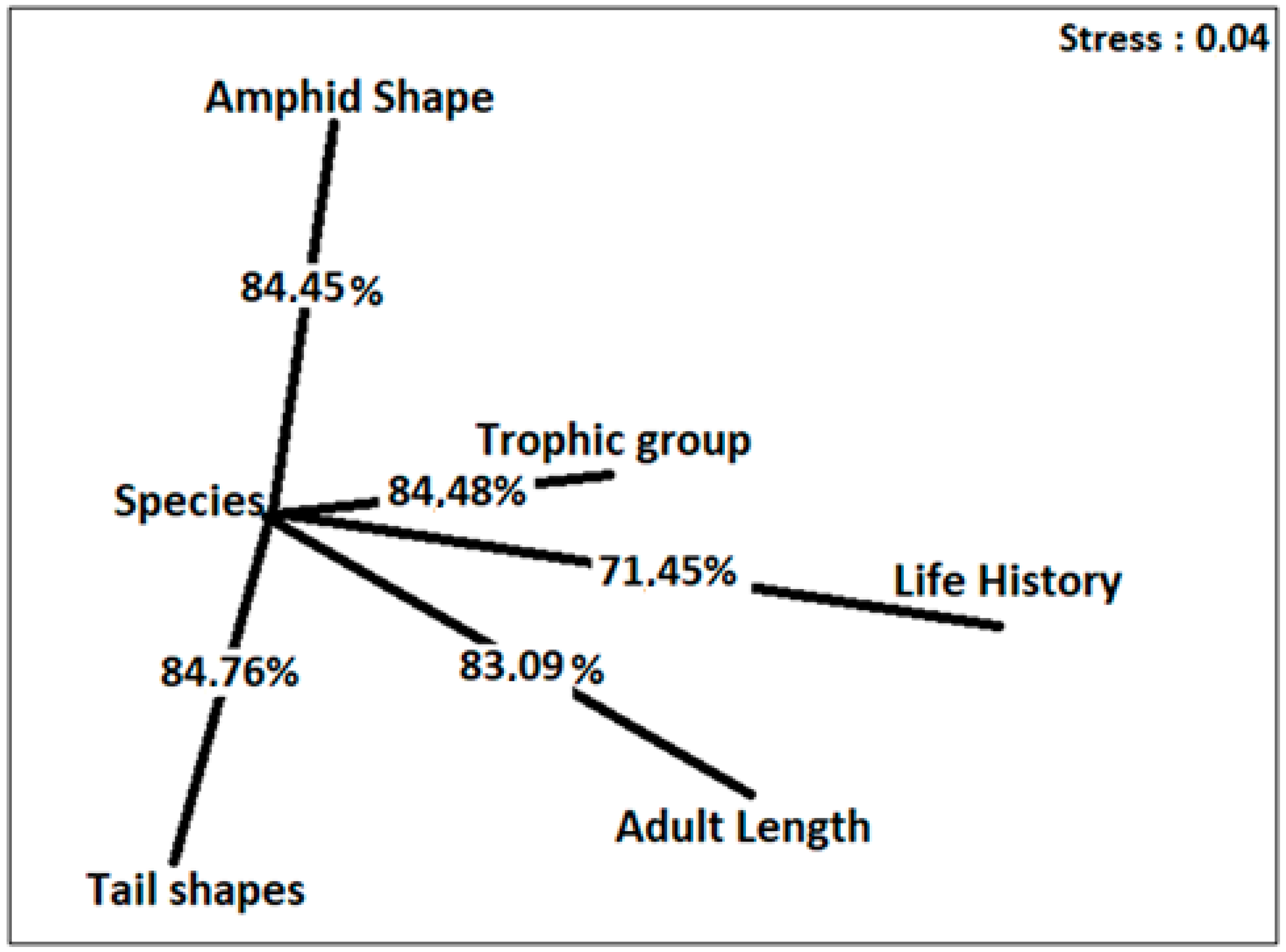
3.4. Diversity of Functional Traits
- The UC community feeding groups were dominated by non-selective deposit feeders (1B) and omnivorous ones (2B), comprising 26.7 ± 0.7% and 27.4 ± 1.35% of the nematofauna, respectively. The experiment showed a significant increase in groups 1A in all compartments, except for UA1. The group 2A in LA2 and the group 1B in UA1, LA2, UM1 and UM2 decreased significantly compared to control. The nMDS results indicate that LA2 treatment was the furthest from LC, followed by LM2 and LM1, whereas UA1 and UA2 treatments were the closest to UC.
- The amphid shapes of the control community (UC) were dominated by circular (cr) and pocket-shaped (pk) amphides, representing 46.4 ± 2.5% and 30.5 ± 2.7% of the nematofauna. A significant decrease was observed in the cr amphid shape in all compartments, except for UA1. In addition, the id amphid shape significantly decreased in LA1, LA2, LM1 and LM2 compared to control. The pk amphid shape percentage decreased in all compartments compared to control. The nMDS results indicate that LA2 was the furthest from LC, whereas UA1 and UA2 treatments were the closest to UC.
- Tail shapes were dominated by elongated/filiform (e/f) and clavate (cla) tails, representing 36.6 ± 0.5% and 48.8 ± 1.9% of the control nematofauna (UC), respectively. The contamination induced a significant increase in the shape of the e/f tails in LA2 and LM2. The shape of the cla tail showed significant differences between UM2 vs. LM2 and the e/f shape between UA2 vs. LA2 and UM2 vs. LM2. The nMDS indicated that the LM2 community was the furthest from LC, whereas all types of treatments were close to UC.
- The control nematofauna (UC) life history was dominated by cp3 and cp2 types, representing 45.1 ± 2.7%, and 43.3 ± 1.3. A significant decrease was observed in cp3 in the LA2 and LM2 compartments. Conversely, the results show a significant decrease in cp3 in the uncontaminated compartments UA2, UM1 and UM2. The cp2 showed significant differences between UC and LC; UA1 and LA1 and UA2 and LA2, the cp3 between UA2 and LA2 as well as UM1 and LM1, and UM2 and LM2, and the cp4 between LC and LA2. The nMDS ordination showed that LA2 and LM2 comprised a clear different cluster compared with UC, whereas UA1 treatment was the closest.
- The body-length intervals were dominated by 1–2 mm and >4 mm size-classes, comprising 61.1 ± 2.4% and 23.8 ± 0.8% of the control nematofauna (UC), respectively. The contamination induced a significant increase in the body-size class 1–2 mm in all compartments compared to control and a significant decrease in the 2–4 mm size-class between UC vs. UM2 and LC vs. LM2, as well as of the >4 mm size-class in all compartments compared to control, except for LM1 and LM2. The body-size interval 1–2 mm significantly differed between the control (UC and LC) and all other analyzed compartments, as well as between UA2 vs. LA2 and UM1 vs. LM1. The 2–4 mm size-class showed significant differences between UC and UM2 and between LC and LM2 and the >4 mm class between UC and LC. The results of the nMDS indicated that LA2 and LM2 were the furthest from LC, whereas the UA1 community the closest to UC.
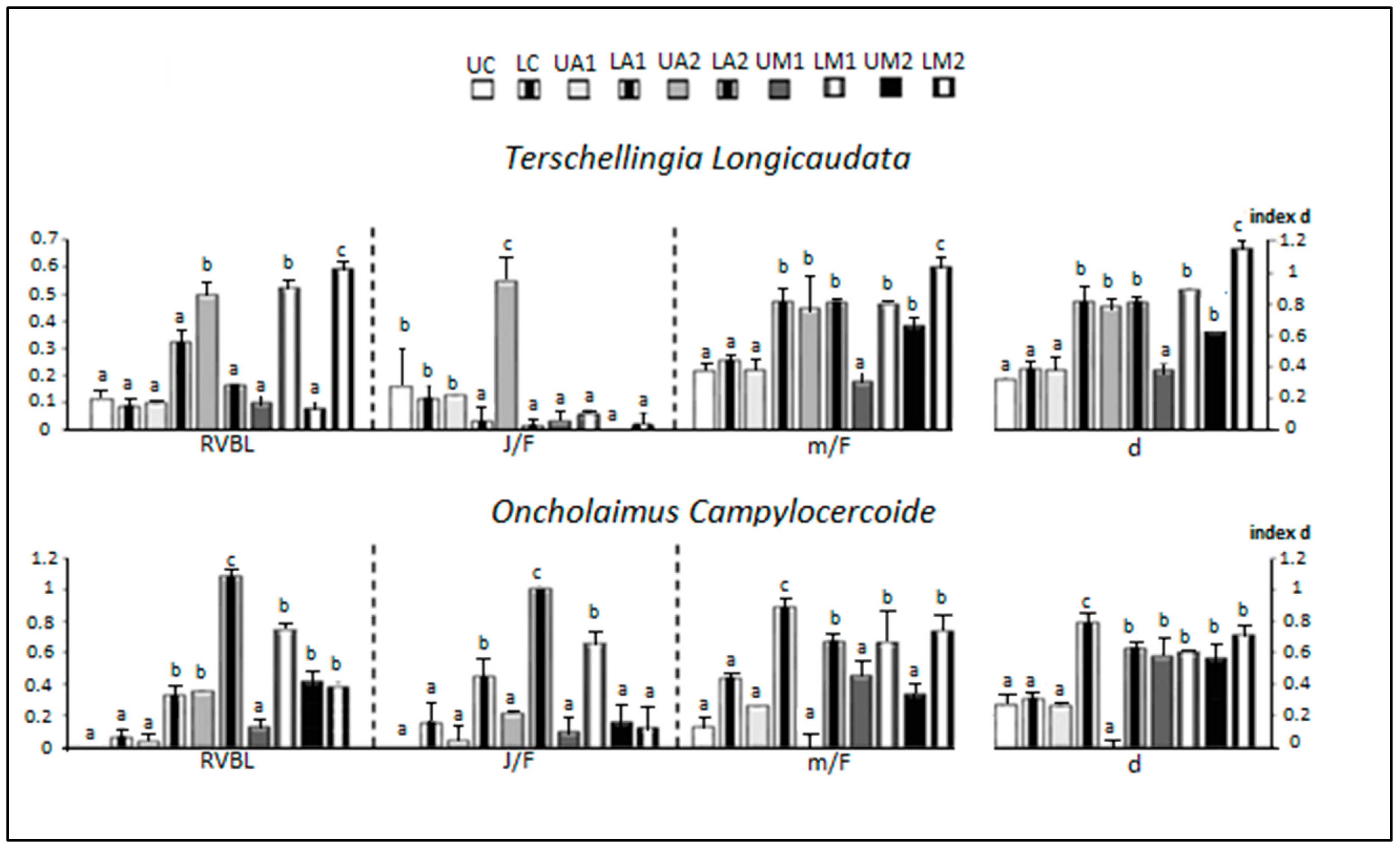
3.5. Sex Ratio and Maturity Status
3.6. Taxon-Functional Traits
4. Discussion
4.1. What Is the Effect of Contamination with Amitriptyline on Nematodes?
4.2. How Does the Mixture of Amitriptyline and Paroxetine Affect Nematodes?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schultz, M.M.; Furlong, E.T. Trace analysis of antidepressant pharmaceuticals and their select degradates in aquatic matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Lützhøft, H.C.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment. A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Mills, D.S. Medical paradigms for the study of problem behavior: A critical review. Appl. Anim. Behav. Sci. 2003, 81, 265–277. [Google Scholar] [CrossRef]
- Hignite, C.; Azarnoff, D.L. Drugs and drug metabolites as environmental contaminants: Chlorophenoxyisobutyrate and salicylic acid in sewage water effluent. Life Sci. 1977, 20, 337–341. [Google Scholar] [CrossRef]
- Brooks, N.; Adger, W.N.; Kelly, P.M. The determinants of vulnerability and adaptive capacity at the national level and the implications for adaptation. Glob. Environ. Chang. 2005, 15, 151–163. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef]
- Henry, T.B.; Kwon, J.W.; Armbrust, K.L.; Black, M.C. Acute and chronic toxicity of five selective serotonin reuptake inhibitors in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2004, 23, 2229–2233. [Google Scholar] [CrossRef]
- Fong, P.P.; Molnar, N. Norfluoxetine induces spawning and parturition in estuarine and freshwater bivalves. Bull. Environ. Contam. Toxicol. 2008, 81, 535–538. [Google Scholar] [CrossRef]
- Schultz, M.M.; Bartell, S.E.; Schoenfuss, H.L. Effects of Triclosan and Triclocarban, Two Ubiquitous Environmental Contaminants, on Anatomy, Physiology, and Behavior of the Fathead Minnow (Pimephales promelas). Arch. Environ. Contam. Toxicol. 2012, 63, 114–124. [Google Scholar] [CrossRef]
- Nyman, A.M.; Hintermeister, A.; Schirmer, K.; Ashaueral, R. The Insecticide Imidacloprid Causes Mortality of the Freshwater Amphipod Gammarus pulex by Interfering with Feeding Behavior. PLoS ONE 2013, 8, e62472. [Google Scholar] [CrossRef]
- Joss, A.; Keller, E.; Alder, A.C. Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res. 2005, 39, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Sweeger, S.; Glauner, T. Metabolites from the biodegradation of pharmaceutical residues of ibuprofen in biofilm reactor and batch experiment. Anal. Bioanal. Chem. 2002, 372, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, S.A.; Smyth, B.K.; Barclay, B.S.; Sauvé, C.C.; Gagnon, A. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Furlong, E.T.; Kolpin, D.W.; Werner, S.L.; Schoenfuss, H.L.; Barber, L.B.; Blazer, V.S.; Norris, D.O.; Vajda, A.M. Antidepressant Pharmaceuticals in Two U.S. Effluent-Impacted Streams: Occurrence and Fate in Water and Sediment, and Selective Uptake in Fish Neural Tissue. Environ. Sci. Technol. 2010, 44, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Marques, R.; Noronha, J.P.; Mexia, J.T.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludge plant. Environ. Pollut. 2011, 159, 2359–2367. [Google Scholar] [CrossRef]
- Boufahja, F.; Semprucci, F. Stress-induced selection of a single species from an entire meiobenthic nematode assemblage: Is it possible using iron enrichment and does pre-exposure affect the ease of the process? Environ. Sci. Pollut. Res. 2015, 22, 1979–1998. [Google Scholar] [CrossRef]
- Moreno, M.; Semprucci, F.; Vezzulli, L.; Balsamo, M.; Fabiano, M.; Albertelli, G. The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol. Indic. 2011, 11, 328–336. [Google Scholar] [CrossRef]
- Semprucci, F.; Balsamo, M. Key role of free-living nematodes in the marine ecosystem. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Jordan, A.C., Eds.; NOVA Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 109–134. [Google Scholar]
- Ishak, S.; Allouche, M.; Harrath, A.H.; Alwasel, S.; Beyrem, H.; Pacioglu, O.; Badraoui, R.; Boufahja, F. Effects of the antidepressant paroxetine on migratory behaviour of meiobenthic nematodes: Computational and open experimental microcosm approach. Mar. Pollut. Bull. 2022, 177, 113558. [Google Scholar] [CrossRef]
- Austen, M.C.; McEvoy, A.J.; Warwick, R.M. The specificity of meiobenthiccommunity responses to different pollutants: Results from microcosm experiments. Mar. Pollut. Bull. 1994, 28, 557–563. [Google Scholar] [CrossRef]
- Hedfi, A.; Boufahja, F.; Ben Ali, M.; Aïssa, P.; Mahmoudi, E.; Beyrem, H. Do trace metals (chromium, copper and nickel) influence toxicity of diesel fuel for free-living marine nematodes? Environ. Sci. Pollut. Res. 2013, 20, 3760–3770. [Google Scholar] [CrossRef]
- Hedfi, A.; Mahmoudi, E.; Boufahja, F.; Beyrem, H.; Aїssa, P. Effects of Increasing Levels of Nickel Contamination on Structureof Offshore Nematode Communities in Experimental Microcosms. Bull. Environ. Contam. Toxicol. 2007, 79, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Hedfi, A.; Mahmoudi, E.; Beyrem, H.; Boufahja, F.; Essid, N.; Aïssa, P. Réponse d’une communauté de nématodes libres marins à une contamination par le cuivre: Étude microcosmique. Bull. Soc. Zool. Fr. 2008, 133, 97–106. [Google Scholar]
- Boufahja, F.; Hedfi, A.; Amorri, J.; Aïssa, P.; Beyrem, H.; Mahmoudi, E. An Assessment of the Impact of Chromium-Amended Sediment on a Marine Nematode Assemblage Using Microcosm Bioassays. Biol. Trace Elem. Res. 2011, 142, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Boufahja, F.; Sellami, B.; Dellali, M.; Aïssa, P.; Mahmoudi, E.; Beyrem, H. A microcosm experiment on the effects of permethrin on a free-living nematode assemblage. Nematology 2011, 13, 901–909. [Google Scholar] [CrossRef]
- Boufahja, F.; Hedfi, A.; Amorri, J.; Aïssa, P.; Mahmoudi, E.; Beyrem, H. Experimental validation of the “relative volume of the pharyngeal lumen (RVPL)” of free-living nematodes as a biomonitoring index using sediment-associated metals and/or Diesel Fuel in microcosms. Exp. Mar. Biol. Ecol. 2011, 399, 142–150. [Google Scholar] [CrossRef]
- Essid, N.; Boufahja, F.; Beyrem, H.; Aïssa, P.; Mahmoudi, E. Effects of 17-α-estradiol on a free-living marine nematode community: A microcosm experiment. Afr. J. Aquat. Sci. 2013, 38, 305–311. [Google Scholar] [CrossRef]
- Kang, T.; Kim, D.; Oh, J.H.; Yoo, S. Effect of oxytetracycline on the community structure of nematodes: Results from microcosm experiments. Plankton Benthos Res. 2019, 14, 105–113. [Google Scholar] [CrossRef]
- Nasri, A.; Hannachi, A.; Allouche, M.; Barhoumi, B.; Saidi, I.; Dallali, M.; Harrath, A.H.; Mansour, L.; Mahmoudi, E.; Beyrem, H. Chronic ecotoxicity of ciprofloxacin exposure on taxonomic diversity of a meiobenthic nematode community in microcosm experiments. J. King Saud Univ.-Sci. 2020, 32, 1470–1475. [Google Scholar] [CrossRef]
- Wakkaf, T.; Allouche, M.; Harrath, A.H.; Mansour, L.; Alwasel, S.; Mohamed, T.A.; Kapul, G.; Beyrem, H.; Sellami, B.; Boufahja, F. The individual and combined effects of cadmium, polyvinyl chloride (PVC) microplastics and their polyalkylamines modified forms on meiobenthic features in a microcosm. Environ. Pollut. 2020, 266, 115–263. [Google Scholar] [CrossRef]
- Ristau, K.; Steinfartz, S.; Traunspurger, W. First evidence of cryptic species diversity and significant population structure in a widespread freshwater nematode morphospecies (Tobrilus gracilis). Mol. Ecol. 2013, 22, 4562–4575. [Google Scholar] [CrossRef]
- Armenteros, M.; Pérez-García, A.J.; Ruiz-Abierno, A.; Díaz-Asencio, L.; Helguera, Y.; Vincx, M.; Decraemerde, W. Effects of organic enrichment on nematode assemblages in a microcosm experiment. Mar. Environ. Res. 2010, 70, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Gingold, R.; Moens, T.; Rocha-Olivares, A. Assessing the Response of Nematode Communities to Climate Change-Driven Warming: A Microcosm Experiment. PLoS ONE 2013, 8, e66653. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Oh, J.H.; Hong, J.S.; Kim, D. Response of Intertidal Meiofaunal Communities to Heavy Metal Contamination in Laboratory Microcosm Experiments. J. Coast. Res. 2018, 85, 361–365. [Google Scholar] [CrossRef]
- Ullberg, J.; Ólafsson, E. Free-living marine nematodes actively choose habitat when descending from the water column. Mar. Ecol. Prog. Ser. 2003, 260, 141–149. [Google Scholar] [CrossRef]
- Zhou, H. Effects of leaf litter addition on meiofaunal colonization of azoic sediments in a subtropical mangrove in Hong Kong. Exp. Mar. Biol. Ecol. 2001, 256, 99–121. [Google Scholar] [CrossRef]
- Ptatscheck, C.; Traunspurger, W. The ability to get everywhere: Dispersal modes of free-living, aquatic nematodes. Hydrobiologia 2020, 847, 3519–3547. [Google Scholar] [CrossRef]
- Pacioglu, O.; Duţu, F.; Pavel, A.B.; Duţu, L.T. The influence of hydrology and sediment grain-size on the spatial distribution of macroinvertebrate communities in two submerged dunes from the Danube Delta (Romania). Limnetica 2022, 41, 85–100. [Google Scholar] [CrossRef]
- Pacioglu, O.; Shaw, P.; Robertson, A. Patch scale response of hyporheic invertebrates to fine sediment removal in two chalk rivers. Fundam. Appl. Limnol.-Archiv. für Hydrobiol. 2012, 181, 283. [Google Scholar] [CrossRef]
- Pacioglu, O.; Moldovan, O.T. Response of invertebrates from the hyporheic zone of chalk rivers to eutrophication and land use. Environ. Sci. Pollut. Res. 2016, 23, 4729–4740. [Google Scholar] [CrossRef]
- Pacioglu, O.; Pârvulescu, L. The chalk hyporheic zone: A true ecotone? Hydrobiologia 2017, 790, 1–12. [Google Scholar] [CrossRef]
- Béjaoui-Omri, A.; Béjaoui, B.; Harzallah, A.; Aloui-Béjaoui, N.; El Bour, M.; Aleya, L. Dynamic energy budget model: A monitoring tool for growth and reproduction performance of Mytilus galloprovincialis in Bizerte Lagoon (Southwestern Mediterranean Sea). Environ. Sci. Pollut. Res. 2014, 21, 13081–13094. [Google Scholar] [CrossRef] [PubMed]
- Calleja, M.C.; Persoone, G.; Geladi, P. Human acute toxicity prediction of the first 50 MEIC chemicals by a banery of ecotoxicological tests and physicochernical properties. Food Chem. Toxicol. 1994, 32, 173–187. [Google Scholar] [CrossRef]
- Benchaouala, A. Ecotoxicity, Cytotoxicity and Androgenic Potential of Residues Pharmaceuticals on the Two Biological Models: Hydra Attenuata and MDA-kb2 Cells. Ecosystems; University of Lorraine: Metz, France, 2017; pp. 57–58. [Google Scholar]
- Yalkowsky, S.H.; Dannenfelser, R.M. The Aquasol Database of Aqueous Solubility; Ver 5; College of Pharmacy, University of Arizona: Tucson, AZ, USA, 1992. [Google Scholar]
- Troemel, E.R.; Chou, J.H.; Dwyer, N.D.; Colbert, H.A.; Bargmann, C.I. Divergent seven transmembrane receptors are candidate chemosensory receptors in Coenorhabditis elegans. Cell 1995, 83, 207–218. [Google Scholar] [CrossRef]
- Vitiello, P.; Dinet, A. Definition and sampling of meiobenthos. Rapp. Comm. Int. Mer. Medit. 1979, 25, 279–283. [Google Scholar]
- Elarbaoui, S.; Richard, M.; Boufahja, F.; Mahmoudi, E.; Thomas-Guyon, H. Effect of crude oil exposure and dispersant application on meiofauna: Anintertidal mesocosm experiment. Environ. Sci. Process. Impact. 2015, 17, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to an hydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part II. British Chromadorids; Synopsis of the British fauna; Field Studies Council: Shrewsbury, UK, 1988; Volume 38. [Google Scholar]
- Bezerra, T.N.; Eisendle, U.; Hodda, M.; Holovachov, O.; Leduc, D.; Mokievsky, V.; Peña Santiago, R.; Sharma, J.; Smol, N.; Tchesunov, A.; et al. Nemys: World Database of Nematodes. 2021. Available online: http://nemys.ugent.be (accessed on 20 March 2022).
- Semprucci, F.; Balsamo, M.; Appolloni, L.; Sandulli, R. Assessment of ecological quality status along the Apulian coasts (eastern Mediterranean Sea) based on meiobenthic and nematode assemblages. Mar. Biodivers. 2018, 48, 105–115. [Google Scholar] [CrossRef]
- Thistle, D.; Lambshead, P.J.D.; Sherman, K.M. Nematode tail-shape groups respond to environmental differences in the deep-sea. Life Environ. 1995, 45, 107–115. [Google Scholar]
- Alves, A.S.; Veríssimo, H.; Costa, M.J.; Marques, J.C. Taxonomic resolution and Biological Traits Analysis (BTA) approaches in estuarine free-living nematodes. Estuar. Coast. Shelf Sci. 2014, 138, 69–78. [Google Scholar] [CrossRef][Green Version]
- Schratzberger, M.; Warr, K.; Rogers, S.I. Functional diversity of nematodecommunities in the southwestern North Sea. Mar. Environ. Res. 2007, 63, 368–389. [Google Scholar] [CrossRef]
- Bongers, T. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Bradbury, I.R.; Laurel, B.; Snelgrove, P.V.R.; Bentzen, P.; Campana, S.E. Global patterns in marine dispersal estimates: The influence of geography, taxonomic category and life history. Proc. R. Soc. B Biol. Sci. 2008, 275, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Clarke, K.R.; Gorley, R.; Carman, K.R.; Fleeger, J.W.; Pomarico, S.M. Does historical exposure to hydrocarbon contamination alter the response of benthic communities to diesel contamination? Mar. Environ. Res. 2000, 49, 255–278. [Google Scholar]
- Pelissolo, A.; Boyer, P.; Lepine, J.P.; Bisserbe, J.C. Epidemiology of the consumption of anxiolytics and hypnotics in France and in the world. L’encephale 1996, 22, 187–196. [Google Scholar]
- Moore, M.; Yuen, H.M.; Dunn, N.; Mullee, M.A.; Maskell, J.; Kendrick, T. Explaining the rise in antidepressant prescribing: A descriptive study using the general practice research database. BMJ 2009, 339, b3999. [Google Scholar] [CrossRef]
- Noordam, R.; Aarts, N.; Verhamme, K.M.; Sturkenboom, M.C.M.; Stricker, B.H.; Visser, L.E. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: A dynamic population-based study. Eur. J. Clin. Pharmacol. 2015, 71, 369–375. [Google Scholar] [CrossRef]
- National Centre for Health Statistics. 2014, Special Feature Onprescription Drugs. Available online: http://www.cdc.gov/nchs/hus.htm (accessed on 20 May 2020).
- Ma, L.D.; Li, J.; Li, J.J.; Liu, M.; Yan, D.Z.; Shi, W.Y.; Xu, G. Occurrence and source analysis of selected antidepressants and their metabolites in municipal wastewater and receiving surface water. Environ. Sci. Process. Impacts 2018, 7, 991–1082. [Google Scholar] [CrossRef]
- Muir, D.; Simmons, D.; Wang, X.; Peart, T.; Villella, M.; Miller, J.; Sherry, J. Bioaccumulation of pharmaceuticals and personal care product chemicals in fish exposed to wastewater effluent in an urban wetland. Sci. Rep. 2017, 7, 16999. [Google Scholar] [CrossRef]
- Konstantin, A.D.; Tatiana, O.K.; Sergey, L.K.; Darya, A.M.; Evgeniya, V.E.; Yuri, Y.M.; Allan, V.K. Acute effects of amitriptyline on adult zebrafish: Potential relevance to antidepressant drug screening and modeling human toxidromes. Neurotoxicol. Teratol. 2017, 62, 27–33. [Google Scholar]
- Sophie, L.G.; Matthew, J.W.; William, H.J.N.; Charles, R.T. The potential for adverse effects in fish exposed to antidepressants in the aquatic environment. Environ. Sci. Technol. 2021, 55, 16299–16312. [Google Scholar]
- Leire, M.; Martin, K.; Laura, D.M.; Haizea, Z.; Maitane, O.; Olatz, Z.; Urtzi, I.; Tobias, S.; Werner, B.; Ailette, P.; et al. Application of the Sea Urchin Embryo Test in Toxicity Evaluation and Effect-Directed Analysis of Wastewater Treatment Plant Effluents. Environ. Sci. Technol. 2020, 54, 8890–8899. [Google Scholar]
- Lilius, H.; Hästbacka, T.; Isomaa, B. Short Communication: A comparison of the toxicity of 30 reference chemicals to Daphnia Magna and Daphnia Pulex. Environ. Toxicol. Chem. 1995, 14, 2085–2088. [Google Scholar]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems—Impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [PubMed]
- Allouche, M.; Ishak, S.; BenAli, M.; Hadfi, A.; Almalki, M.; Karachle, K.P.; Harrath, A.H.; Abu-zied, H.R.; Badraoui, R.; Boufahja, F. Molecular interactions of polyvinyl chloride microplastics and beta-blockers (Diltiazem and Bisoprolol) and their effects on marine meiofauna: Combined in vivo and modeling study. Hazard. Mater. 2022, 431, 128609. [Google Scholar] [CrossRef]
- Burr, A.H.; Burr, C. The amphid of the nematode Oncholaimus vesicarius: Ultrastructural evidence for a dual function as chemoreceptor and photoreceptor. J. Ultrastruct. Res. 1975, 51, 1–15. [Google Scholar] [CrossRef]
- Schratzberger, M.; Harrison, J.P.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef]
- Gray, J.; Lissmann, H.W. The locomotion of nematodes. Exp. Biol. 1964, 41, 135–154. [Google Scholar] [CrossRef]
- Vanaverbeke, J.; Soetaert, K.; Vincx, M. Changes in morphometric characteristics of nematode communities during a spring phytoplankton bloom deposition. Mar. Ecol. Prog. Ser. 2004, 273, 139–146. [Google Scholar] [CrossRef][Green Version]
- Gingold, R.; Ibarra-Obando, S.E.; Rocha-Olivares, A. Spatial aggregation patterns of free-living marine nematodes in contrasting sandy beach microhabitats. J. Mar. Biol. Assoc. UK 2011, 91, 615–622. [Google Scholar] [CrossRef]
- Saburova, M.A.; Polikarpov, I.G. Diatom activity within soft sediments: Behavioral and physiological processes. Mar. Ecol. Prog. Ser. 2003, 251, 115–126. [Google Scholar] [CrossRef]
- Mitbavkar, S.; Anil, A.C. Vertical migratory rhythms of benthic diatoms in a tropical intertidal sand flat: Influence of irradiance and tides. Mar. Biol. 2004, 145, 9–20. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Wastewater treatment plants as a pathway foraquatic contamination by pharmaceuticals in the Ebro river basin (Northeast Spain). Environ. Toxicol. Chem. 2007, 26, 1553–1562. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]


| Functional Traits | Treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | c-p | TI | Am | FG | AL | UC | LC | UA1 | LA1 | UA2 | LA2 | UM1 | LM1 | UM2 | LM2 |
| Terschellingia sp. | 2 | e/f | pk | 1A | 2–4 mm | 2.37 ± 1.17 | 1.68 ± 0.56 | 1.67 ± 0.58 | 4.33 ± 0.58 | 1.67 ± 0.58 | 1 ± 0.58 | 1 ± 0.08 | 1.33 ± 0.58 | ||
| Terschellingia longicaudata | 3 | e/f | cr | 1A | 1–2 mm | 19.67 ± 1.72 | 18.18 ± 0.75 | 24 ± 1 | 22.67 ± 0.58 | 31.33 ± 1.15 | 50.33 ± 1.53 | 27 ± 2 | 41.70 ± 5.56 | 23.67 ± 0.58 | 42.33 ± 2.52 |
| Metalinhomoeus numidicus | 2 | e/f | cr | 1B | 2–4 mm | 6.44 ± 0.61 | 6.74 ± 0.65 | 5.33 ± 0.58 | 12.33 ± 0.58 | 5 ± 1.73 | 4.67 ± 0.58 | 5 ± 1.02 | 5 ± 0.70 | 4.67 ± 0.58 | 2.33 ± 0.58 |
| Paracomesoma dubium | 2 | cla | sp | 2A | 1–2 mm | 7.44 ± 1.43 | 8.43 ± 0.74 | 20.33 ± 1.53 | 17.33 ± 0.58 | 25.67 ± 1.15 | 13 ± 1 | 18 ± 1 | 14.56 ± 1.15 | 17.33 ± 0.58 | 12.66 ± 1.53 |
| Marylynnia puncticaudata | 3 | e/f | sp | 2A | 2–4 mm | 1.36 ± 0.61 | 1.69 ± 0.60 | 0.67 ± 0.58 | 0.33 ± 0.58 | 5.33 ± 1.15 | |||||
| Daptonema trabeculosum | 2 | cla | cr | 1B | 1–2 mm | 2.37 ± 0.55 | 2.35 ± 0.56 | 3.67 ± 0.58 | 2 ± 0.2 | 4.33 ± 2.08 | 3.33 ± 1.15 | 3 ± 1 | 4.66 ± 0.85 | 3 ± 1 | 3.33 ± 0.58 |
| Paramonohystera wieseri | 2 | cla | cr | 1B | 1–2 mm | 6.10 ± 1.00 | 6.05 ± 0.92 | 4.33 ± 0.58 | 5 ± 1.73 | 5 ± 1 | 7 ± 1 | 3.67 ± 0.58 | 8.62 ± 2.30 | 3.67 ± 0.58 | 7.33 ± 0.58 |
| Paramonohystera pilosa | 2 | cla | cr | 1B | 1–2 mm | 3.05 ± 1.04 | 3.03 ± 1.00 | 2.33 ± 0.58 | 1.67 ± 0.58 | ||||||
| Steineria sp. | 2 | cla | cr | 1B | 1–2 mm | 7.46 ± 0.62 | 5.06 ± 1.09 | 4.33 ± 0.58 | 3.67 ± 0.58 | 4 ± 1 | 2.67 ± 0.58 | 4.67 ± 0.58 | 11.51 ± 1.05 | 3.33 ± 0.58 | 11 ± 1 |
| Metoncholaimus pristiurus | 3 | cla | pk | 2B | >4 mm | 3.74 ± 1.23 | 3.36 ± 0.55 | 2 ± 0.2 | 2 ± 0.3 | 0.33 ± 0.58 | 0.33 ± 0.58 | 1.86 ± 0.39 | 1.67 ± 0.58 | 1.67 ± 0.58 | |
| Oncholaimelluscalvadocicus | 3 | cla | pk | 2B | >4 mm | 8.15 ± 1.14 | 5.41 ± 1.65 | 5 ± 1 | 0.33 ± 0.58 | 1 ± 1 | 1.33 ± 1.15 | 2 ± 0.38 | 1.97 ± 0.88 | 1.33 ± 0.58 | 1.67 ± 0.58 |
| Viscosia cobbi | 3 | e/f | pk | 2B | 1–2 mm | 4.06 ± 0.95 | 3.37 ± 0.65 | 3.33 ± 0.58 | 0.67 ± 0.58 | 2.67 ± 1.53 | 2 ± 0.2 | 1.86 ± 0.39 | 1 | 1.67 ± 0.58 | |
| Oncholaimus campylocercoides | 4 | cla | pk | 2B | >4 mm | 10.48 ± 2.39 | 6.41 ± 2.12 | 11.67 ± 0.58 | 5.33 ± 0.58 | 11.67 ± 0.58 | 2.67 ± 0.58 | 11.33 ± 0.58 | 12.38 ± 1.53 | 10.67 ± 0.58 | 11.67 ± 1.53 |
| Calomicrolaimus honestus | 3 | co | sp | 2A | 1–2 mm | 2.04 ± 1.04 | 3.03 ± 1.00 | 2 ± 0.19 | 4.67 ± 0.58 | ||||||
| Calomicrolaimus sp. | 3 | co | sp | 2A | 1–2 mm | 4.06 ± 1.01 | 4.37 ± 0.55 | 3.67 ± 0.58 | 1.67 ± 0.58 | ||||||
| Neochromadora sp. | 2 | co | Id | 2A | 1–2 mm | 2.38 ± 0.62 | 4.37 ± 1.52 | 1.67 ± 0.58 | 0.33 ± 0.58 | ||||||
| Chromadorina sp. | 2 | co | Id | 2A | 1–2 mm | 1.02 ± 0.02 | 2.34 ± 1.51 | 1 ± 0.02 | |||||||
| Chromadorina minor | 2 | co | Id | 2A | 1–2 mm | 1.70 ± 0.61 | 2.69 ± 0.54 | 0.33 ± 0.58 | 1.33 ± 1.15 | 1 ± 0.02 | 0.67 ± 0.58 | ||||
| Anticoma acuminata | 2 | e/f | pk | 1A | 2–4 mm | 1.69 ± 0.58 | 2.69 ± 1.14 | 1.33 ± 0.58 | 1.33 ± 0.58 | 1.67 ± 0.58 | 1 ± 0.5 | 1 ± 0.04 | 0.67 ± 0.58 | ||
| Ascolaimus sp. | 2 | co | cr | 1B | 2–4 mm | 1.36 ± 0.61 | 3.03 ± 1.00 | 2 ± 0.2 | 1.67 ± 0.58 | 4.33 ± 0.58 | 3.33 ± 1.15 | 1.67 ± 0.58 | 2 ± 0.91 | 1 ± 1 | 1.67 ± 0.58 |
| Cyatholaimus prinzi | 3 | co | sp | 2A | 1–2 mm | 2.03 ± 1.02 | 2.69 ± 0.54 | 2 ± 0.01 | 0.33 ± 0.58 | 0.33 ± 0.58 | |||||
| Synonchiella edax | 4 | e/f | sp | 2B | 2–4 mm | 1.02 ± 0.02 | 3.03 ± 1.00 | 1 ± 0.02 | 1.67 ± 0.58 | 1.99 ± 0.91 | 0.33 ± 0.58 | 0.67 ± 0.58 | |||
| UC vs. UA1 (20.35%) | UC vs. UA2 (37.83%) | UC vs. UM1 (32.25%) | UC vs. UM2 (32.84%) | ||
| Species | Paracomesoma dubium (31.66%) + | Paracomesoma dubium (24.45%) + | Paracomesoma dubium (18.23%) + | Paracomesoma dubium (17.61%) + | |
| Terschellingia longicaudata (11.34%) + | Terschellingia longicaudata (15.99%) + | Terschellingia longicaudata (13.08%) + | Oncholaimellus calvadocicus (11.75%) − | ||
| Oncholaimellus calvadocicus (7.34%) − | Oncholaimellus calvadocicus (9.34%) − | Oncholaimellus calvadocicus (10.25%) − | Terschellingia longicaudata (7.62%) + | ||
| Steineria sp. (7.33%) − | Calomicrolaimus sp. (5.33%) − | Calomicrolaimus sp. (6.83%) − | Steineria sp. (7.05%) − | ||
| Oncholaimus campylocercoides (5.43%) + | Metoncholaimus pristiurus (4.45%) − | Metoncholaimus pristiurus (6.27%) − | Calomicrolaimus sp. (7.04%) − | ||
| Metoncholaimus pristiurus (4.07%) − | Steineria sp. (4.44%) − | Paramonohystera pilosa (5.13%) − | Paramonohystera pilosa (5.29%) − | ||
| Chromadorina minor (4.07%) − | Paramonohystera pilosa (4.00%) − | Steineria sp. (4.55%) − | Viscosia cobbi (5.27%) − | ||
| Paramonohystera wieseri (3.99%) − | Paramonohystera wieseri (4.11%) − | ||||
| Neochromadora sp. (4.11%) − | |||||
| Feeding groups | 10.66% | 15.90% | 12.27% | 13.17% | |
| 2A + | 2B − | 1A + | 1A + | ||
| 1B − | 2B − | ||||
| Tail shape | 3.32% | 9.03% | 11.02% | 11.94% | |
| cla + | co − | co − | co − | ||
| Amphid shape | 11.03% | 8.16% | 18.56% | 12.36% | |
| sp + | sp + | cr + | cr + | ||
| pk − | pk − | pk − | |||
| Adult length | 7.81% | 12.63% | 9.80% | 9.40% | |
| 1–2 mm + | 1–2 mm + | 1–2 mm + | 1–2 mm + | ||
| c-p score | 4.43% | 10.58% | 7.49% | 7.69% | |
| c-p3 − | c-p3 − | c-p3 − | c-p3 − | ||
| LC vs. LA1 (32.26%) | LC vs. LA2 (46.22%) | LC vs. LM1 (39.08%) | LC vs. LM2 (43.46%) | ||
| Species | Paracomesoma dubium (14.93%) + | Terschellingia longicaudata (35.88%) + | Terschellingia longicaudata (24.32%) + | Terschellingia longicaudata (28.40%) + | |
| Metalinhomoeus numidicus (9.40%) + | Paracomesoma dubium (5.19%) + | Steineria sp. (6.62%) + | Steineria sp. (6.99%) + | ||
| Oncholaimellus calvadocicus (8.31%) − | Calomicrolaimus sp. (4.81%) − | Calomicrolaimus sp. (5.75%) − | Oncholaimus campylocercoides (6.19%) + | ||
| Terschellingia longicaudata (7.75%) + | Neochromadora sp. (4.81%) − | Neochromadora sp. (5.75%) − | Neochromadora sp. (5.06%) − | ||
| Neochromadora sp. (6.63%) − | Oncholaimellus calvadocicus (4.47%) − | Oncholaimus campylocercoides (5.74%) + | Calomicrolaimus sp. (5.06%) − | ||
| Synonchiella edax (4.98%) − | Oncholaimus campylocercoides (4.07%) − | Paracomesoma dubium (5.73%) + | Metalinhomoeus numidicus (5.05%) − | ||
| Calomicrolaimus sp. (4.44%) − | Marylynnia puncticaudata (4.05%) + | Oncholaimellus calvadocicus (4.88%) − | Paracomesoma dubium (5.02%) + | ||
| Cyatholaimus sp. (4.42%) − | Viscosia cobbi (3.70%) − | Calomicrolaimus honestus (3.99%) − | Oncholaimellus calvadocicus (4.29%) − | ||
| Viscosia cobbi (4.42%) − | Paramonohystera pilosa (3.99%) − | Calomicrolaimus honestus (3.51%) − | |||
| Terschellingia sp. (4.42%) + | |||||
| Feeding groups | 13.81% | 32.04% | 19.36% | 21.78% | |
| 2B − | 1A + | 1A + | 1A + | ||
| Tail shape | 13.38% | 27.58% | 20.73% | 11.94% | |
| co − | e/f − | co − | co − | ||
| Amphid shape | 15.55% | 29.83% | 23.55% | 16.93% | |
| cr + | cr + | cr + | cr + | ||
| id − | id Φ | sp − | |||
| Adult length | 7.56% | 15.85% | 13.65% | 17.10% | |
| >4 mm − | 1–2 mm + | 1–2 mm + | 1–2 mm + | ||
| c-p score | 8.50% | 8.50% | 5.88% | 9.30% | |
| c-p2 + | c-p3 + | c-p2 − | c-p2 − | ||
| UC vs. LC (15.19%) | UA1 vs. LA1 (22.95%) | UA2 vs. LA2 (30.71%) | UM1 vs. LM1 (18.41%) | UM2 vs. LM2 (26.24%) | |
| Species | Oncholaimus campylocercoides (13.29%) − | Metalinhomoeus numidicus (15.96%) + | Terschellingia longicaudata (31.54%) + | Terschellingia longicaudata (28.57%) + | Terschellingia longicaudata (41.13%) + |
| Oncholaimellus calvadocicus (8.89%) − | Oncholaimus campylocercoides (14.45%) − | Paracomesoma dubium (21.03%) − | Paracomesoma dubium (16.44%) − | Steineria sp. (16.90%) + | |
| Steineria sp. (7.77%) − | Oncholaimellus calvadocicus (10.63%) − | Oncholaimus campylocercoides (14.95%) − | Steineria sp. (16.32%) + | Paracomesoma dubium (10.38%) − | |
| Synonchiella edax (6.67%) + | Paracomesoma dubium (6.82%) − | ||||
| Neochromadora sp. (6.66%) + | Calomicrolaimus honestus (6.08%) + | ||||
| Ascolaimus sp. (5.55%) + | Terschellingia sp. (6.07%) + | ||||
| Terschellingia longicaudata (5.21%) − | Viscosia cobbi (6.06%) − | ||||
| Paracomesoma dubium (4.84%) + | |||||
| Chromadorina sp. (4.43%) + | |||||
| Calomicrolaimus honestus (4.07%) + | |||||
| Feeding groups | 7.97% | 14.87% | 20.99% | 11.60% | 14.29% |
| 2A + | 2B − | 1A + | 2A − | 2A − | |
| Tail shape | 8.90% | 11.42% | 22.63% | 2.11% | 21.93% |
| cla − | e/f + | e/f + | e/f + | e/f + | |
| Amphid shape | 9.85% | 11.35% | 20.28% | 14.01% | 16.85% |
| pk − | cr + | cr + | cr + | cr + | |
| sp + | |||||
| Adult length | 7.93% | 11.31% | 8.84% | 5.36% | 9.17% |
| >4 mm − | 2–4 mm + | >4 mm − | 1–2 mm + | 1–2 mm + | |
| c-p score | 5.53% | 10.50% | 24.40% | 6.48% | 10.86% |
| c-p2 + | c-p2 + | c-p3 + | c-p3 + | c-p3 + | |
| % | UC | LC | UA1 | LA1 | UA2 | LA2 | UM1 | LM1 | UM2 | LM2 |
|---|---|---|---|---|---|---|---|---|---|---|
| m | 16.76 ± 4 | 21.41 ± 3.63 | 20.71 ± 2.77 | 26.34 ± 2.77 | 23 ± 5 | 32.37 ± 3.13 ** | 18.09 ± 2.35 | 31.59 ± 3.44 **** | 15.45 ± 1.33 | 37.61 ± 3.63 **** |
| f | 65.88 ± 1.16 | 62.09 ± 5.43 | 75.13 ± 4.70 | 37.48 ± 4.32 **** | 51.33 ± 4.73 | 52.32 ± 7.11 | 40.56 ± 1.94 | 44.13 ± 5.43 | 31.88 ± 2.20 | 49.51 ± 7.42 |
| gf | 6.55 ± 0.48 | 4.95 ± 0.09 | 5.51 ± 0.61 | 5.26 ± 0.91 | 4 | 5.43 ± 0.49 | 5.28 ± 0.55 | 1.87 ± 0.09 | 2.24 ± 0.07 | 2.94 ± 0.18 |
| J | 7.52 ± 1.39 | 9.59 ± 1.66 | 5.5 ± 0.44 | 7.86 ± 0.58 | 21.66 ± 1.53 | 2.22 ± 0.42 **** | 5.56 ± 0.67 | 10.27 ± 0.44 | 6.22 ± 0.49 | 6.21 ± 0.80 |
| % | UC | LC | UA1 | LA1 | UA2 | LA2 | UM1 | LM1 | UM2 | LM2 |
|---|---|---|---|---|---|---|---|---|---|---|
| gf/F | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.07 ± 0.01 | 0.14 ± 0.02 | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.13 ± 0.01 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| J/gf | 1.14 ± 0.14 | 1.57 ± 0.36 **** | 1.01 ± 0.18 | 1.54 ± 0.39 ** | 5.42 ± 0.38 | 0.41 ± 0.08 **** | 1.1 ± 0.09 | 5.5 ± 0.5 **** | 2.79 ± 0.32 | 2.11 ± 0.2 **** |
| m/F | 0.23 ± 0.06 | 0.22 ± 0.05 | 0.26 ± 0.02 | 0.62 ± 0.05 | 0.42 ± 0.13 | 0.56 ± 0.02 | 0.39 ± 0.03 | 0.69 ± 0.03 | 0.46 ± 0.07 | 0.72 ± 0.05 |
| J/F | 0.1 ± 0.02 | 0.13 ± 0.02 | 0.07 ± 0.01 | 0.19 ± 0.03 | 0.39 ± 0.02 | 0.04 ± 0.01 | 0.12 ± 0.01 | 0.23 ± 0.04 | 0.18 ± 0.03 | 0.12 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishak, S.; Allouche, M.; Nasri, A.; Harrath, A.H.; Alwasel, S.; Plăvan, G.; Beyrem, H.; Boufahja, F. The Antidepressants Amitriptyline and Paroxetine Induce Changes in the Structure and Functional Traits of Marine Nematodes. Sustainability 2022, 14, 6100. https://doi.org/10.3390/su14106100
Ishak S, Allouche M, Nasri A, Harrath AH, Alwasel S, Plăvan G, Beyrem H, Boufahja F. The Antidepressants Amitriptyline and Paroxetine Induce Changes in the Structure and Functional Traits of Marine Nematodes. Sustainability. 2022; 14(10):6100. https://doi.org/10.3390/su14106100
Chicago/Turabian StyleIshak, Sahar, Mohamed Allouche, Ahmed Nasri, Abdel Halim Harrath, Saleh Alwasel, Gabriel Plăvan, Hamouda Beyrem, and Fehmi Boufahja. 2022. "The Antidepressants Amitriptyline and Paroxetine Induce Changes in the Structure and Functional Traits of Marine Nematodes" Sustainability 14, no. 10: 6100. https://doi.org/10.3390/su14106100
APA StyleIshak, S., Allouche, M., Nasri, A., Harrath, A. H., Alwasel, S., Plăvan, G., Beyrem, H., & Boufahja, F. (2022). The Antidepressants Amitriptyline and Paroxetine Induce Changes in the Structure and Functional Traits of Marine Nematodes. Sustainability, 14(10), 6100. https://doi.org/10.3390/su14106100








