Optimal K Management Improved Potato Yield and Soil Microbial Community Structure
Abstract
:1. Introduction
2. Materials and Methods
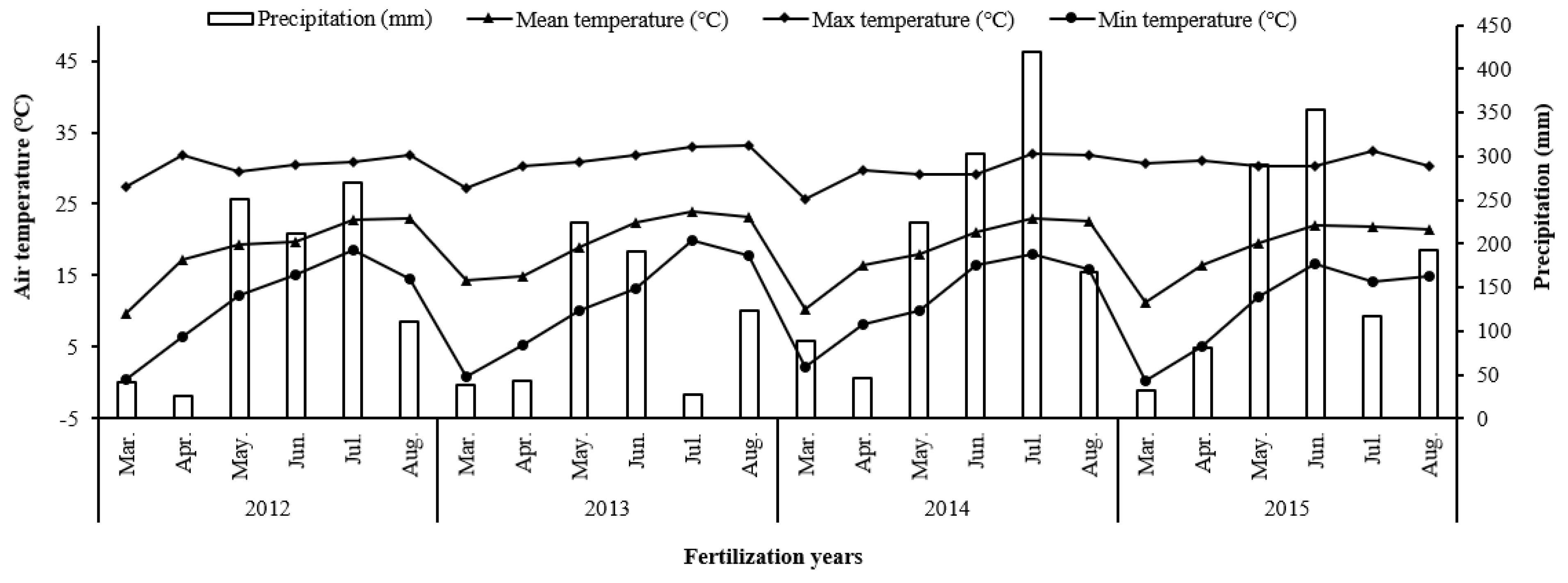
2.1. Site Description
2.2. Experimental Design
2.3. Crop and Soil Sampling and Analysis
2.4. Analysis of PLFAs of Microbial Communities
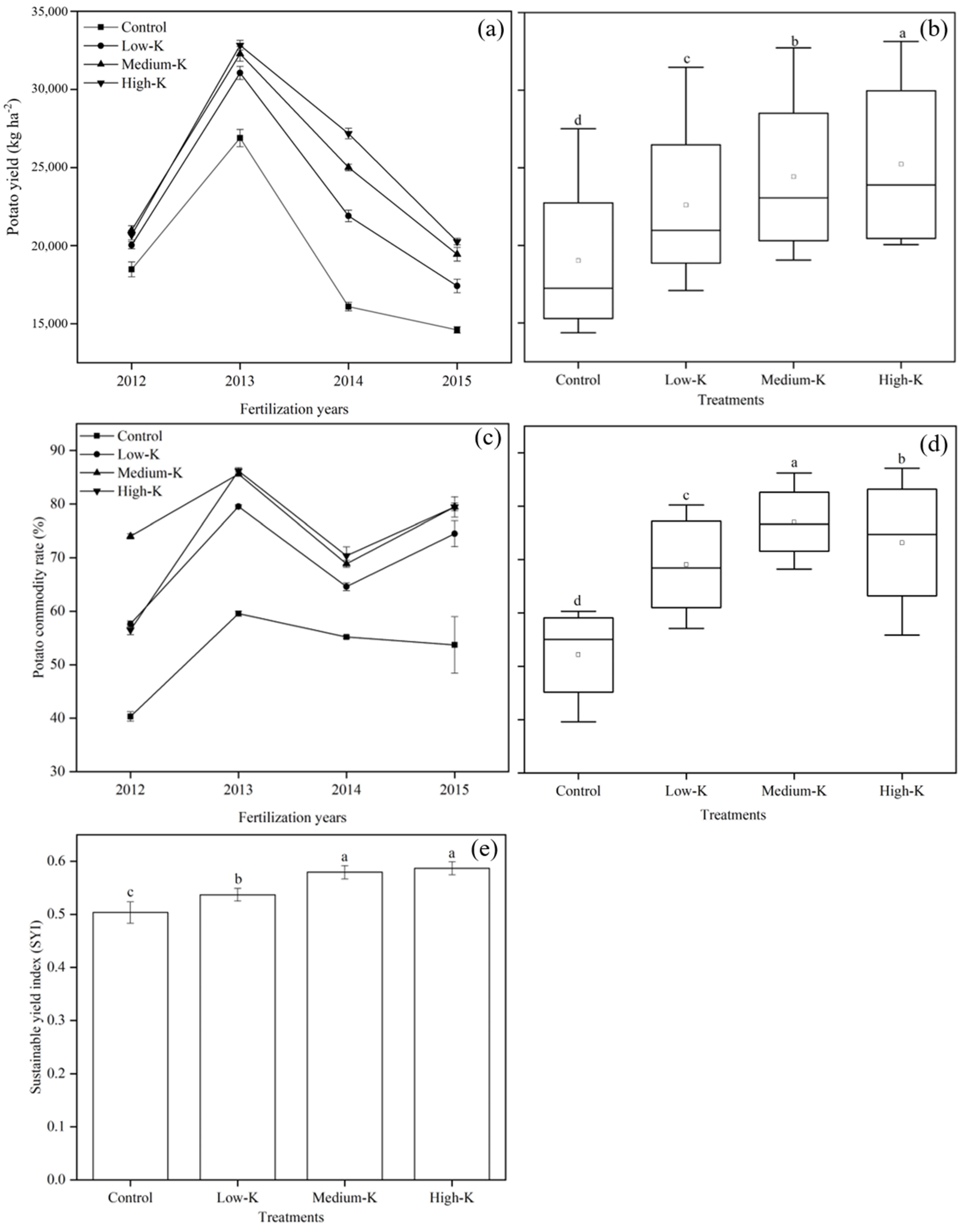
2.5. Commodity Rates (CRs) of Potato
2.6. Determination of the Sustainable Yield Index (SYI)
2.7. Statistical Analyses
3. Results
3.1. Potato Yield, CRs and the SYI
3.2. Soil Microbial PLFA Content and Composition
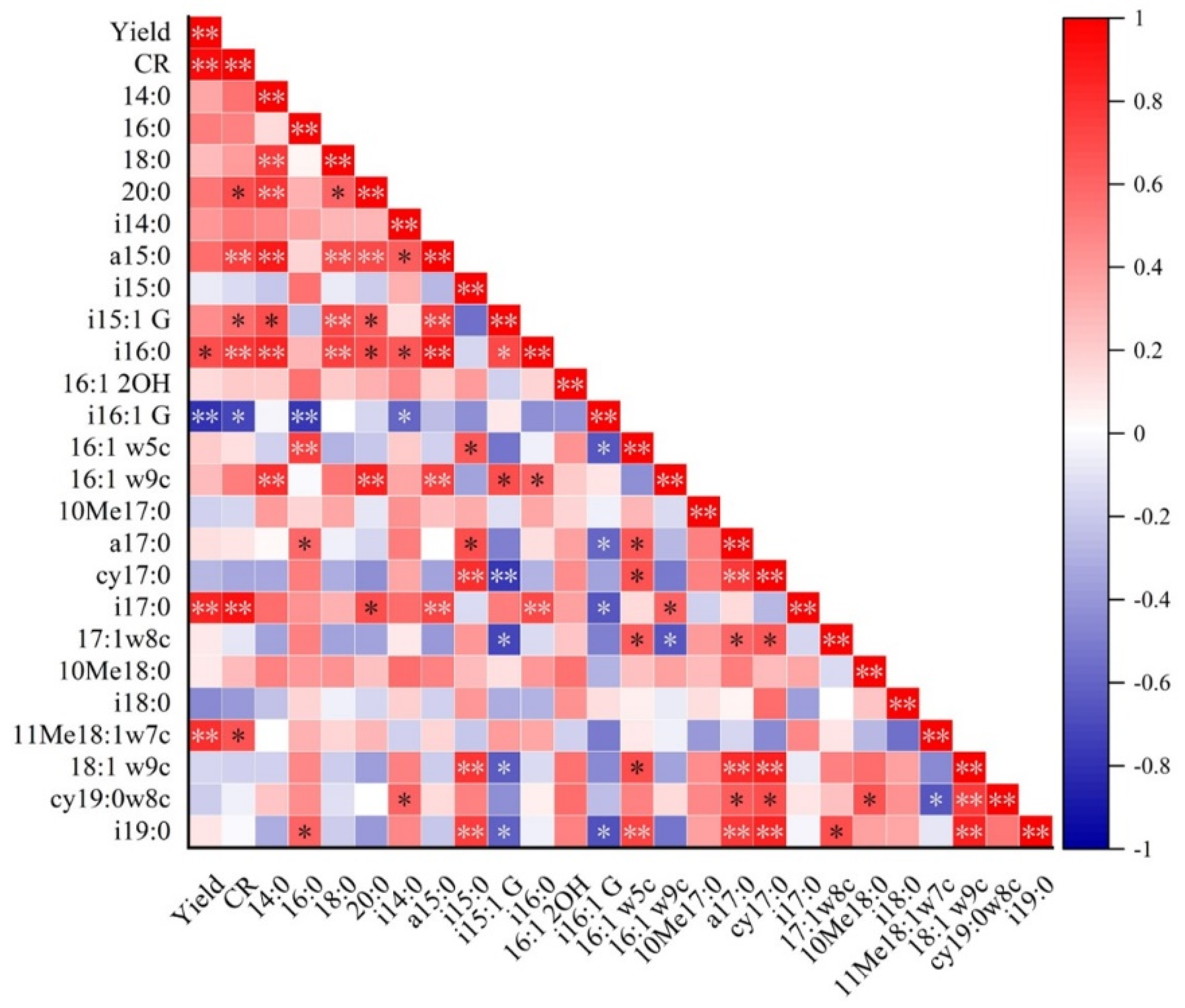
3.3. Principal Component Analysis (PCA)
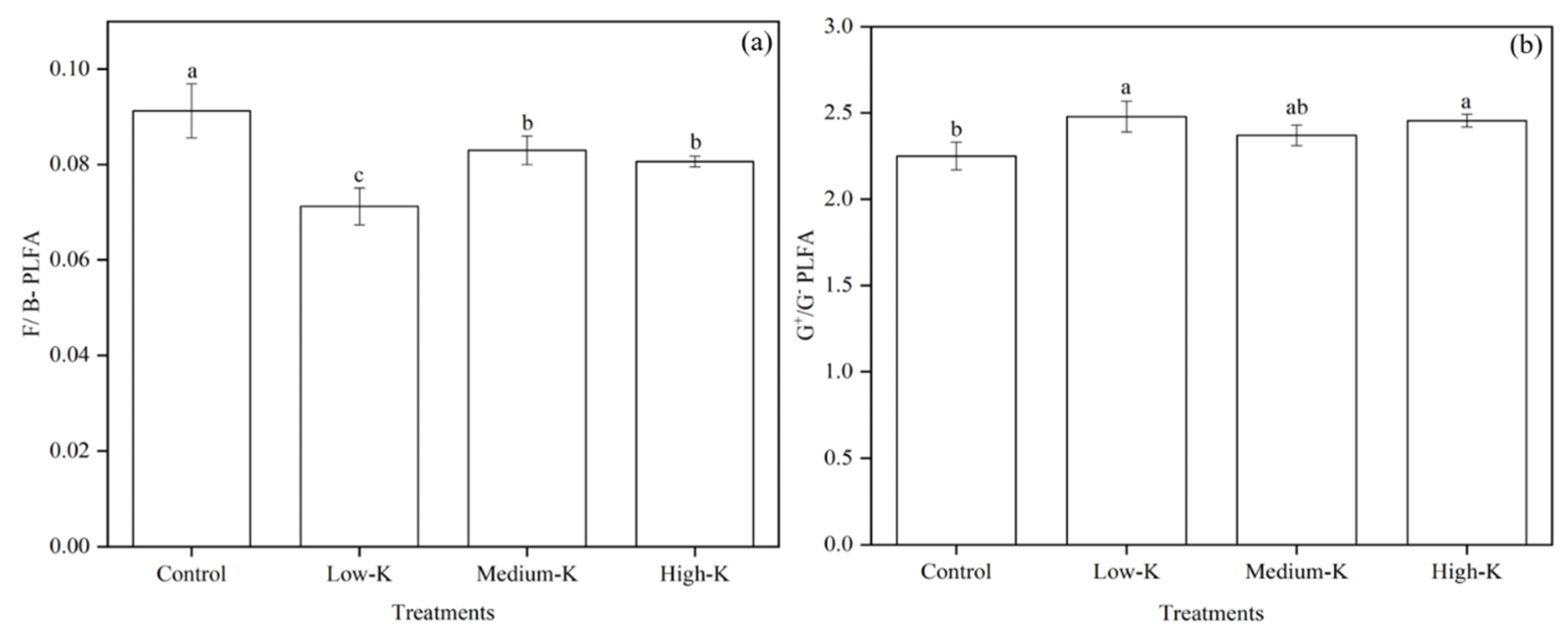
3.4. Soil Microbial PLFA Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| K | Potassium |
| PFLA | Phospholipid fatty acid |
| B | Bacteria |
| F | Fungal |
| G+ | Gram-positive bacteria |
| G− | Gram-negative bacteria |
| CRs | Potato commodity rates |
| SYI | System sustainability |
References
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture—Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Farhangi-Abriz, S.; Qin, R.; Noulas, C.; Sathuvalli, V.; Charlton, B.; Loka, D.A. Potassium: A Vital Macronutrient in Potato Production—A Review. Agronomy 2021, 11, 543. [Google Scholar] [CrossRef]
- Huber, S.C. Biochemical Basis for Effects of K-Deficiency on Assimilate Export Rate and Accumulation of Soluble Sugars in Soybean Leaves. Plant Physiol. 1984, 76, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H.; Cakmak, I. High Light Intensity Enhances Chlorosis and Necrosis in Leaves of Zinc, Potassium, and Magnesium Deficient Bean (Phaseolus vulgaris) Plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, J.; Jiang, X.; Ji, F.; Zhang, J.; Yu, Z.; Lin, X. Long-Term Fertilization Regimes Influence FAME Profiles of Microbial Communities in an Arable Sandy Loam Soil in Northern China. Pedobiologia 2013, 56, 179–183. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on Potassium in Agriculture: Needs and Prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Smil, V. Crop Residues: Agriculture’s Largest Harvest. BioScience 1999, 49, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Goulding, K.W.T.; Loveland, P.J. The Classification and Mapping of Potassium Reserves in Soils of England and Wales. J. Soil Sci. 1986, 37, 555–565. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.M.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of Potassium Solubilizing Bacteria (Bacillus cereus) on Growth and Yield of Potato. J. Plant Nutr. 2020, 44, 411–420. [Google Scholar] [CrossRef]
- Awad, M.; Ali, A.M.; Hegab, S.A.; El Gawad, A.M.A. Organic Fertilization Affects Growth and Yield of Potato (Cara. Cv) Plants Grown on Sandy Clay Loam. Commun. Soil Sci. Plant Anal. 2022, 53, 688–698. [Google Scholar] [CrossRef]
- Kang, W.Q.; Fan, M.S.; Ma, Z.; Shi, X.H.; Zheng, H.L. Luxury Absorption of Potassium by Potato Plants. Am. J. Potato Res. 2014, 91, 573–578. [Google Scholar] [CrossRef]
- Allison, M.F.; Fowler, J.H.; Allen, E.J. Responses of Potato (Solanum tuberosum) to Potassium Fertilizers. J. Agric. Sci. 2001, 136, 407–426. [Google Scholar] [CrossRef] [Green Version]
- Karam, F.; Massaad, R.; Skaf, S.; Breidy, J.; Rouphael, Y. Potato Response to Potassium Application Rates and Timing under Semi-Arid Conditions. Adv. Hortic. Sci. 2011, 25, 265–268. [Google Scholar]
- Khan, M.Z.; Akhtar, M.E.; Mahmood-ul-Hassan, M.; Mahmood, M.M.; Safdar, M.N. Potato Tuber Yield and Quality as Affected by Rates and Sources of Potassium Fertilizer. J. Plant Nutr. 2012, 35, 664–677. [Google Scholar] [CrossRef]
- Li, S.T.; Duan, Y.; Guo, T.W.; Zhang, P.L.; He, P.; Johnston, A.; Shcherbakov, A. Potassium Management in Potato Production in Northwest Region of China. Field Crops 2015, 174, 48–54. [Google Scholar] [CrossRef]
- Zhao, H.; Gou, J.L.; Zhao, L.X.; Wu, Q.Y.; He, J.F.; Zhao, P.Y.; Wang, Z.Y.; Li, Z.L.; Xiao, H.J. Analysis on Status of Soil Potassium and the Effects of Potassium Fertilizer in Dryland Soil in Guizhou. J. Plant Nutr. Fertil. 2016, 22, 277–285. (In Chinese) [Google Scholar]
- Mokrani, K.; Hamdi, K.; Tarchoun, N. Potato (Solanum tuberosum L.) Response to Nitrogen, Phosphorus and Potassium Fertilization Rates. Commun. Soil Sci. Plant Anal. 2018, 49, 1314–1330. [Google Scholar] [CrossRef]
- Zhao, H.; Gou, J.L.; He, J.F.; Zhao, L.X.; Zhao, P.Y.; Xiao, H.J.; Wang, Z.Y.; Li, Z.L. Effects of Potassium Fertilizer on Dry Matter Accumulation, Potassium Absorption and Utilization Efficiency of Potato. Southwest China J. Agric. Sci. 2015, 28, 644–649. (In Chinese) [Google Scholar]
- Myvan, F.F.; Jami Al-Ahmadi, M.J.; Eslami, S.V.; Noferest, K.S. Role of Potassium in Modifying the Potato Physiological Responses to Irrigation Regimes Under Different Planting Patterns. Potato Res. 2022, 1–22. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.Y.; Classen, A.T.; Zhao, K.; Chen, L.T.; Shi, Y.; Jiang, Y.X.; He, J.S. The Links between Ecosystem Multifunctionality and Above- and Belowground Biodiversity Are Mediated by Climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [Green Version]
- Montalba, R.; Arriagada, C.; Alvear, M.; Zúñiga, G.E. Effects of Conventional and Organic Nitrogen Fertilizers on Soil Microbial Activity, Mycorrhizal Colonization, Leaf Antioxidant Content, and Fusarium Wilt in Highbush Blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2010, 125, 775–778. [Google Scholar] [CrossRef]
- Pan, Y.; Cassman, N.; de Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.E.M.; van Veen, J.A.; Kuramae, E.E. Impact of Long-Term N, P, K, and NPK Fertilization on the Composition and Potential Functions of the Bacterial Community in Grassland Soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zarraonaindia, I.; Martínez-Goñi, X.S.; Liñero, O.; Muñoz-Colmenero, M.; Aguirre, M.; Abad, D.; Baroja-Careaga, I.; de Diego, A.; Gilbert, J.A.; Estonba, A. Response of Horticultural Soil Microbiota to Different Fertilization Practices. Plants 2020, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.G.D. Pyrosequencing Reveals the Influence of Organic and Conventional Farming Systems on Bacterial Communities. PLoS ONE 2012, 7, e51897. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-Biome Metagenomic Analyses of Soil Microbial Communities and Their Functional Attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [Green Version]
- Allard, S.M.; Walsh, C.S.; Wallis, A.E.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Solanum lycopersicum (Tomato) Hosts Robust Phyllosphere and Rhizosphere Bacterial Communities When Grown in Soil Amended with Various Organic and Synthetic Fertilizers. Sci. Total Environ. 2016, 573, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.P.; Yang, J.; Chen, Y.Y.; Han, H.H.; Liu, X.; Yi, X.P.; Wang, J.C.; Lyu, C.W. Screening High Potassium Efficiency Potato Genotypes and Physiological Responses at Different Potassium Levels. Not. Bot. Horti. Agrobot. 2021, 49, 12190. [Google Scholar] [CrossRef]
- He, P.; Yang, L.P.; Xu, X.P.; Zhao, S.C.; Chen, F.; Li, S.T.; Tu, S.H.; Jin, J.Y.; Johnston, A.M. Temporal and Spatial Variation of Soil Available Potassium in China (1990–2012). Field Crops Res. 2015, 173, 49–56. [Google Scholar] [CrossRef]
- Söderberg, K.H.; Probanza, A.; Jumpponen, A.; Bååth, E. The Microbial Community in the Rhizosphere Determined by Community-Level Physiological Profiles (CLPP) and Direct Soil– and Cfu–PLFA Techniques. Appl. Soil Ecol. 2004, 25, 135–145. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Ge, T.D.; Gunina, A.; Li, Y.H.; Zhu, Z.K.; Peng, P.Q.; Wu, J.S.; Kuzyakov, Y. Carbon and Nitrogen Availability in Paddy Soil Affects Rice Photosynthate Allocation, Microbial Community Composition, and Priming: Combining Continuous C-13 Labeling with PLFA Analysis. Plant Soil 2019, 445, 137–152. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Janus, L.R.; Angeloni, N.L.; McCormack, J.; Rier, S.T.; Tuchman, N.C.; Kelly, J.J. Elevated Atmospheric CO2 Alters Soil Microbial Communities Associated with Trembling Aspen (Populus tremuloides) Roots. Microb. Ecol. 2005, 50, 102–109. [Google Scholar] [CrossRef] [Green Version]
- McKinley, V.L.; Peacock, A.D.; White, D.C. Microbial Community PLFA and PHB Responses to Ecosystem Restoration in Tallgrass Prairie Soils. Soil Biol. Biochem. 2005, 37, 1946–1958. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, K.; Liu, W.T.; Gao, T.P.; Li, G.; Han, H.F.; Li, Z.J.; Ning, T.Y. Responses of Soil Carbon, Nitrogen, and Wheat and Maize Productivity to 10 Years of Decreased Nitrogen Fertilizer under Contrasting Tillage Systems. Soil Tillage Res. 2020, 196, 104444. [Google Scholar] [CrossRef]
- Tang, Z.H.; Zhang, A.J.; Wei, M.; Chen, X.G.; Liu, Z.H.; Li, H.M.; Ding, Y.F. Physiological Response to Potassium Deficiency in Three Sweet Potato (Ipomoea batatas [L.] Lam.) Genotypes Differing in Potassium Utilization Efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Kassim, A.M.; Nawar, S.; Mouazen, A.M. Potential of On-the-Go Gamma-Ray Spectrometry for Estimation and Management of Soil Potassium Site Specifically. Sustainability 2021, 13, 661. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.W.; Wang, Q.L.; Zhang, H.Q.; Li, M.F.; Song, B.T.; Zhao, Z.Q. Effects of Potassium Fertilization on Potato Starch Physicochemical Properties. Int. J. Biol. Macromol. 2018, 117, 467–472. [Google Scholar] [CrossRef]
- Heenan, D.P.; Campbell, L.C. Influence of Potassium and Manganese on Growth and Uptake of Magnesium by Soybeans (Glycine max (L.) Merr. Cv. Bragg). Plant Soil 1981, 61, 447–456. [Google Scholar] [CrossRef]
- Seggewiss, B.; Jungk, A. Einfluss der Kaliumdynamik im wurzelnahen Boden auf die Magnesiumaufnahme von Pflanzen. Z. Pflanzenernaehr. Bodenk 1988, 151, 91–96. [Google Scholar] [CrossRef]
- Westermann, D.T.; James, D.W.; Tindall, T.A.; Hurst, R.L. Nitrogen and Potassium Fertilization of Potatoes: Sugars and Starch. Am. Potato J. 1994, 71, 433–453. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil Water Content and Organic Carbon Availability Are Major Determinants of Soil Microbial Community Composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in Soil Microbial Community Composition in Response to Fertilization of Paddy Soils in Subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Sheng, X.F.; He, L.Y.; Huang, W.Y. The Conditions of Releasing Potassium by a Silicate Dissolving Bacterial Strain NBT. Agric. Sci. China 2002, 1, 662–666. (In Chinese) [Google Scholar]
- Han, H.S.; Supanjani; Lee, K.D. Effect of Co-Inoculation with Phosphate and Potassium Solubilizing Bacteria on Mineral Uptake and Growth of Pepper and Cucumber. Plant Soil Environ. 2006, 7, 130–136. [Google Scholar]
- Basak, B.B.; Biswas, D.R. Influence of Potassium Solubilizing Microorganism (Bacillus mucilaginosus) and Waste Mica on Potassium Uptake Dynamics by Sudan Grass (Sorghum vulgare Pers.) Grown under Two Alfisols. Plant Soil 2009, 317, 235–255. [Google Scholar] [CrossRef]
- Abou-el-Seoud, I.I.; Abdel-Megeed, A. Impact of Rock Materials and Biofertilizations on P and K Availability for Maize (Zea Maize) under Calcareous Soil Conditions. Saudi J. Biol. Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Pushkareva, E.; Sommer, V.; Barrantes, I.; Karsten, U. Diversity of Microorganisms in Biocrusts Surrounding Highly Saline Potash Tailing Piles in Germany. Microorganisms 2021, 9, 714. [Google Scholar] [CrossRef]
- Frey, S.D.; Six, J.; Elliott, E.T. Reciprocal Transfer of Carbon and Nitrogen by Decomposer Fungi at the Soil–Litter Interface. Soil Biol. Biochem. 2003, 35, 1001–1004. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schroth, M.N.; Miller, T.D. Effects of Rhizosphere Colonization by Plant Growth Promoting Rhizobacteria on Potato Plant Development and Yield. Ecol. Epidemiol. 1980, 70, 1078–1082. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gilani, S.S.M.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; Galagedara, L.; et al. The Potential of Corn-Soybean Intercropping to Improve the Soil Health Status and Biomass Production in Cool Climate Boreal Ecosystems. Sci. Rep. 2019, 9, 13148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/Bacterial Ratios in Grasslands with Contrasting Nitrogen Management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Ibekwe, A.M.; Kennedy, A.C.; Frohne, P.S.; Papiernik, S.K.; Crowley, D.E. Microbial Diversity along a Transect of Agronomic Zones. FEMS Microbiol. Ecol. 2002, 39, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kieft, T.L.; Ringelberg, D.B.; White, D.C. Changes in Ester-Linked Phospholipid Fatty Acid Profiles of Subsurface Bacteria during Starvation and Desiccation in a Porous Medium. Appl. Environ. Microbiol. 1994, 60, 3292–3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, P.; Wang, S.P.; Jia, S.G.; Gao, Q. Effect of 25-Year Fertilization on Soil Microbial Biomass and Community Structure in a Continuous Corn Cropping System. Arch. Agron. Soil Sci. 2015, 61, 1303–1317. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.Q.; Wang, H.; Bai, E.; Lou, Y.H.; Zhang, A.J.; Zhuge, Y.P. 35 Years of Manure and Chemical Fertilizer Application Alters Soil Microbial Community Composition in a Fluvo-Aquic Soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Soares, P.R.; Pato, R.L.; Dias, S.; Santos, D. Effects of Grazing Indigenous Laying Hens on Soil Properties: Benefits and Challenges to Achieving Soil Fertility. Sustainability 2022, 14, 3407. [Google Scholar] [CrossRef]


| PLFA Biomarker | Control | Low-K | Medium-K | High-K |
|---|---|---|---|---|
| 14:0 | 0.12 ± 0.02 b | 0.17 ± 0.04 a | 0.18 ± 0.02 a | 0.13 ± 0.01 b |
| 16:0 | 2.17 ± 0.22 bc | 2.08 ± 0.23 c | 2.49 ± 0.06 a | 2.39 ± 0.03 ab |
| 18:0 | 0.65 ± 0.08 a | 0.72 ± 0.11 a | 0.71 ± 0.01 a | 0.67 ± 0.02 a |
| 20:0 | 0.07 ± 0.00 b | 0.09 ± 0.01 a | 0.09 ± 0.00 a | 0.08 ± 0.00 ab |
| i14:0 | 0.11 ± 0.01 b | 0.10 ± 0.02 b | 0.15 ± 0.00 a | 0.11 ± 0.00 b |
| a15:0 | 0.70 ± 0.03 c | 0.88 ± 0.12 ab | 0.94 ± 0.04 a | 0.79 ± 0.04 bc |
| i15:0 | 1.77 ± 0.09 a | 1.43 ± 0.17 b | 1.77 ± 0.18 a | 1.69 ± 0.05 a |
| i15:1 G | 0.09 ± 0.01 c | 0.14 ± 0.02 a | 0.12 ± 0.00 ab | 0.11 ± 0.01 bc |
| i16:0 | 1.02 ± 0.05 b | 1.19 ± 0.17 a | 1.28 ± 0.05 a | 1.18 ± 0.02 ab |
| 16:1 2OH | 1.24 ± 0.10 ab | 1.14 ± 0.10 b | 1.41 ± 0.22 a | 1.20 ± 0.04 ab |
| i16:1 G | 0.15± 0.00 a | 0.17 ± 0.02 a | nd | nd |
| 16:1 w5c | 0.31 ± 0.00 ab | 0.25 ± 0.06 b | 0.33 ± 0.03 a | 0.32 ± 0.03 a |
| 16:1 w9c | 0.10 ± 0.01 b | 0.17 ± 0.01 a | 0.16 ± 0.02 a | 0.10 ± 0.01 b |
| 10Me17:0 | 0.32 ± 0.01 a | 0.30 ± 0.04 a | 0.32 ± 0.02 a | 0.30 ± 0.00 a |
| a17:0 | 0.52 ± 0.00 a | 0.45 ± 0.03 b | 0.56 ± 0.04 a | 0.52 ± 0.05 a |
| cy17:0 | 0.68 ± 0.02 a | 0.54 ± 0.03 c | 0.65 ± 0.01 ab | 0.62 ± 0.02 b |
| i17:0 | 0.96 ± 0.02 c | 1.11 ± 0.01 b | 1.20 ± 0.04 a | 1.11 ± 0.01 b |
| 17:1w8c | 0.12 ± 0.01 a | 0.10 ± 0.00 b | 0.11 ± 0.01 ab | 0.12 ± 0.00 a |
| 10Me18:0 | 0.58 ± 0.14 b | 0.54 ± 0.03 b | 0.74 ± 0.06 a | 0.51 ± 0.01 b |
| i18:0 | 0.09 ± 0.01 a | 0.07 ± 0.00 a | 0.08 ± 0.01 a | 0.07 ± 0.01 a |
| 11Me18:1w7c | 0.04 ± 0.01 d | 0.07 ± 0.00 b | 0.06 ± 0.00 c | 0.09 ± 0.00 a |
| 18:1 w9c | 1.10 ± 0.05 a | 0.84 ± 0.04 c | 1.12 ± 0.04 a | 0.99 ± 0.02 b |
| cy19:0w8c | 1.16 ± 0.07 b | 0.98 ± 0.04 c | 1.27 ± 0.03 a | 0.98 ± 0.04 c |
| i19:0 | 0.07 ± 0.01 a | nd | 0.08 ± 0.00 a | 0.07 ± 0.00 a |
| Total PLFA | 14.13 ± 0.56 b | 13.54 ± 0.70 b | 15.82 ± 0.06 a | 14.17 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Liu, H.; Xiao, H.; Hu, G.; Gao, M.; Wang, Z. Optimal K Management Improved Potato Yield and Soil Microbial Community Structure. Sustainability 2022, 14, 6579. https://doi.org/10.3390/su14116579
Zhao H, Liu H, Xiao H, Hu G, Gao M, Wang Z. Optimal K Management Improved Potato Yield and Soil Microbial Community Structure. Sustainability. 2022; 14(11):6579. https://doi.org/10.3390/su14116579
Chicago/Turabian StyleZhao, Huan, Hai Liu, Houjun Xiao, Gang Hu, Ming Gao, and Zhengyin Wang. 2022. "Optimal K Management Improved Potato Yield and Soil Microbial Community Structure" Sustainability 14, no. 11: 6579. https://doi.org/10.3390/su14116579
APA StyleZhao, H., Liu, H., Xiao, H., Hu, G., Gao, M., & Wang, Z. (2022). Optimal K Management Improved Potato Yield and Soil Microbial Community Structure. Sustainability, 14(11), 6579. https://doi.org/10.3390/su14116579






