Abstract
In recent years, the number of motor vehicles in circulation has increased in proportion to Brazil’s economic growth, resulting in an increase in emissions of toxic gases from combustion, such as nitrogen oxide, particulate matter, carbon dioxide and volatile organic compounds, among other polluting compounds. This type of pollution has its impacts potentiated in large cities, accumulating due to the configuration of streets and buildings in large urban centers, and can even penetrate indoor environments, having harmful effects on the health of residents. To minimize the emission of these gases, catalytic converters can be used in the vehicle exhausts. Catalytic converters are a promising technology used to reduce exhaust emissions from the engine. In this context, this paper presents an overview of the emission of toxic gases by heavy transport powered by diesel oil and the influence of the use of automotive catalysts in reducing the emission of toxic gases. Additionally, a proposal for monitoring the useful life of automotive catalysts is presented through an electronic sensing system, which makes it possible to determine the catalyst efficiency and the appropriate point for its reactivation or replacement.
1. Introduction
In recent years, the number of motor vehicles in circulation has increased, and consequently, there has been an increase in emissions of gases from combustion. Thus, it is necessary to monitor the emissions of these gases and study methods and technologies to reduce such emissions [1,2,3].
Road transport is a significant source of urban pollutant emissions and will likely continue for decades [4]. Diesel engines have the advantages of high engine efficiency and low fuel consumption, contributing to their wide use. However, the exhaust gas from the combustion of these engines contains a large amount of nitrogen oxide (NOx), particulate matter (PM), carbon dioxide (CO2) and volatile organic compounds (VOC), among other polluting compounds [5,6].
The transport sector ranks second in the production of global CO2 emissions, with a range of 22% [7,8]. According to Giechaskiel et al. [9], data from 2008 show that although vehicles with diesel engines represent less than 5% of the vehicle population in California (USA), China, and Brazil, these vehicles contributed from 44% to 57% of all pollutants emissions, such as particulate matter from road traffic and NOx gas emissions. In addition to NOx, emission from motor vehicles contains greenhouse gases.
In this way, pollution in large cities has become a matter of urgency. This concern is further compounded by the fact that in urban centers, due to their configuration, it is common to find areas of street canyons (streets surrounded by buildings on both sides), in which ventilation is reduced, favoring the accumulation of pollutants and particles which may be very harmful to human health [10,11]. These pollutants can enter buildings through windows and doors or even through cracks in them, thus reducing indoor air quality [10,12]. Lawrence et al. [13], for example, identified a positive correlation between the outdoor and indoor concentration of NO and NO2 in an urban site in India, and correlated this to the traffic pollution [13]. In this sense, Salonen et al. [14], through their review study, gathered reports that indicated that schools located close to highways, industrial areas, or in urban centers had considerably higher concentrations of NO2 than those located in less intense traffic locations, suggesting the level of pollution outdoors can affect indoor air quality [14]. This scenario stimulated the search for ways to mitigate atmospheric pollution, some of which are quite innovative, such as the incorporation of photocatalysts in building materials in order to promote the reduction in NOx present in the air [15,16]. Even so, in view of the large contribution of heavy-duty vehicles (HDVs) for NOx pollution, it is estimated that the application of Euro VI standards could lead to a substantial reduction in NOx emissions (up to 80–90% in comparison to 2040 baseline) in places where these standards have not yet been fully implemented [17]. The addition of pollutant-conversion technologies by the action of catalysts (such as Selective Catalytic Reduction (SCR), Ammonia Slip Catalyst (ASC) and Diesel Oxidation Catalysts (DOC)) to heavy duty trucks exhaust has promising potential to contribute to meeting these emission limits [18].
Catalytic converters are a technology used to reduce gas emissions from the engine. A catalytic converter (or simply catalytic converter) is a device that converts toxic gases from combustion into other non-harmful gases. Catalysts are highly active and efficient in reducing emissions, and for this reason, they were developed for use in different types of vehicles. Today, many car manufacturers can meet emission standards through the use of catalytic converters. The lifespan of catalytic converters is up to 36 months, so they must be replaced at least every three years [19,20].
SCR is the most promising technology in reducing NOx emissions from combustion in boilers and diesel engines [21,22]. The SCR system uses ammonia as a reducing agent. An aqueous solution of urea is injected onto the catalyst’s surface and, when it comes into contact with the hot exhaust gas, it evaporates and decomposes into ammonia NO3, which promotes the reduction of NOx into nitrogen N2 and water H2O. The conversion of NOx in an SCR system occurs at a high rate and is highly dependent on the catalyst temperature [23,24,25].
According to Mardani et al. [3], understanding the relationship between CO2 emissions and economic growth can help to develop energy policies and energy resources sustainably. In the study developed by Vieira et al. [15], the authors evaluated the influence of extreme events on electricity consumption and Gross Domestic Product (GDP). Figure 1 shows data from 1970 to 2014 for GDP per capita, per capita CO2 emissions, and electricity consumption in Brazil.
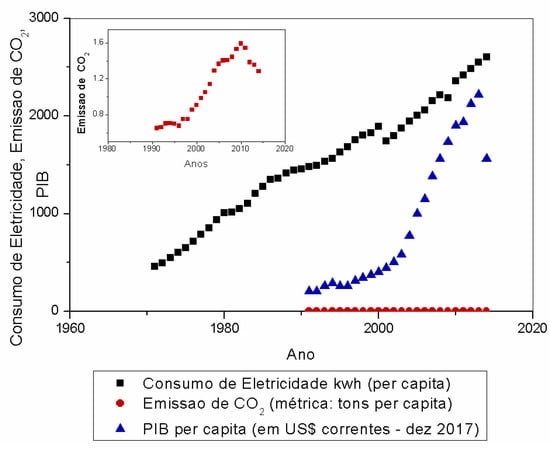
Figure 1.
Gross Domestic Product (GDP), CO2 emission, and electricity consumption in Brazil in per capita values in a time series [26].
The authors identified that, over the years, there was an increase in all the variables studied, which indicates that there is a correlation between them. The authors also observed that, between 2011 and 2014, there was a drop in the values of CO2 emissions and GDP. This study emphasizes the importance of monitoring and studying the gases present in pollutant emissions, as they can affect the health of living beings, the ecosystem, and the economy.
In this context, this study presents the influence of automotive catalysts in reducing the emission of toxic gases from the internal combustion of diesel engines. The study also provides a proposal for monitoring the useful life of automotive catalysts through sensing, which makes it possible to determine the efficiency of the catalyst, as well as the appropriate point for its reactivation or replacement.
2. Pollutant Emission by Diesel Vehicles in Brazil
Since 2012, Brazil has followed the EURO V standard, which provides information for implementing SCR systems for heavy vehicles that use diesel as fuel. The adoption of this technology has contributed to reducing pollutant gas emissions over the years. However, there are still many vehicles with obsolete technology in circulation. In addition, the increase in the vehicle fleet in Brazil, mainly in metropolitan regions, caused a rise in the number and extent of traffic jams and, consequently, in the release of pollutants into the atmosphere [27,28].
The creation of PROCONVE (Program for Control of Air Pollution by Motor Vehicles) by CONAMA (National Council for the Environment) in 1986 allowed Brazil to make progress in controlling emissions from motor vehicles. After the first phase of the program, in 1992, a reduction of approximately 70% was achieved for pollutants emitted by cars. The main objective of PROCONVE is to reduce pollutant emissions from new vehicles through the establishment of emission limits. Thus, with the progressive implementation of phases, technological improvements are induced in the automotive industry to reduce emissions from new vehicles placed on the market [29,30].
According to Benvenutti et al. [31], within the energy sector’s greenhouse gas emissions, road transport is responsible for 91% of total CO2 emissions. In Figure 2, the variation in CO2 emission and the variation in the consumption of petroleum-derived diesel oil are presented, considering only vehicular activity.
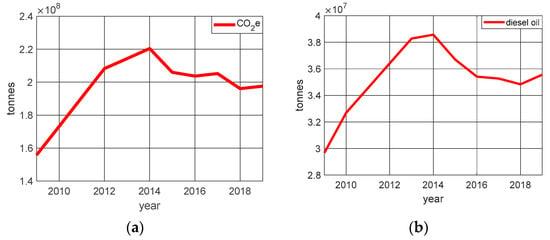
Figure 2.
Relation of the inclusion of catalysts in vehicle exhausts: (a) CO2 emission (b) diesel oil consumption.
We can see in Figure 2 that the introduction of catalysts in vehicle exhausts from 2012 onwards, combined with the reduction in diesel consumption, contributed to a reduction in the growth rate of CO2 emissions.
Figure 3 shows the number of truck-type vehicles in circulation in Brazil, as well as their rate of degradation according to the time of use.
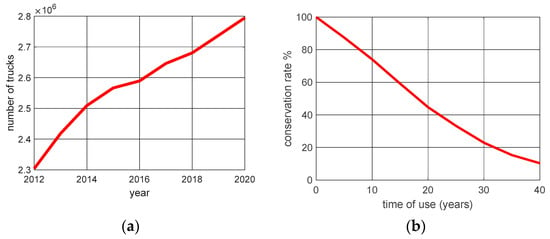
Figure 3.
The number of trucks emitting toxic gases and their state of conservation. (a) The number of trucks. (b) State of conservation.
By analyzing the data in Figure 3a, we can observe a growth in the truck fleet in Brazil between 2012 and 2020. Considering the growth as linear, we can estimate a truck fleet of approximately 2,837,452 in 2022. According to CONAMA resolution 490/2018 [32], from 2023, new models of trucks and other vehicles will have new maximum limits for exhaust gas emissions, in addition to the mandatory installation of on-board diagnostic systems and measurement of emissions in real traffic. However, after the resolution, 2,837,452 trucks will still be running with unmonitored catalytic converter exhausts. Control of catalytic converter replacement at the appropriate time will depend solely on the owner. The automotive catalytic converter should be replaced approximately every three years, which according to Figure 2b, represents a vehicle with approximately a 90% conservation status.
By analyzing the results presented in Figure 3a,b, we can see that there was a reduction of approximately 20% in CO2 emissions per truck compared to the number of trucks in 2012 (before the catalyst was mandatory) and 2019 (use of the catalyst in large part of the fleet).
Considering the 2019 fleet, it can be estimated that a vehicle emitted approximately 90,374 tons of CO2 per year. Making a projection of these data, considering a scenario where the maintenance or replacement of the catalyst is not performed, we can estimate a possible emission of more than 250 million tons of CO2 in 2022 by the fleet that will be in circulation. According to an estimate by the World Health Organization (WHO), 4.2 million deaths occur every year as a result of exposure to polluting compounds present in the air, a fact that characterizes air pollution as the fourth largest risk factor for death in the world [33].
3. Sensing the Catalytic Activities in the Vehicle Exhaust
Monitoring of the catalyst’s efficiency in reducing toxic gas emissions from the vehicle’s exhaust can be carried out in two ways, namely by controlling the catalyst temperature, or by determining the amount of ammonia at the output of the catalyst.
3.1. Monitoring by the Temperature Sensor
The conversion of NOx into N2 and H2O is highly dependent on the catalyst temperature; that is, it is susceptible to engine operating conditions [25]. For the temperature values to be corrected throughout the process, it is necessary to integrate sensing elements and electronic control devices. The most commonly used temperature sensors are the thermocouple and the RTD (Resistance Temperature Detector) sensors. Figure 4 shows the temperature variations in an exhaust gas treatment system of an internal combustion engine.

Figure 4.
Temperature values necessary for the performance of the gas treatment system.
3.2. Monitoring by NOx Sensor
In SCR systems, ammonia (NH3) is used as a reducing agent when converting NOx gases. In practice, NH3 cannot be transported directly in vehicles, so an aqueous urea solution is injected into the system, which is decomposed into NH3. However, if the urea concentration in the system is low, a reduction in NOx gases will be insufficient. On the other hand, if the urea concentration in the system is high, it causes NH3 to escape, making this compound a secondary pollutant. To avoid this problem and control NOx emissions, the use of NOx sensors has become common in vehicles. The purpose of these sensors is, by means of the NOx dosage, to control the amount of NH3 in the catalyst, and thus, proceed with the correct adjustment of the urea dosage in the stoichiometric proportion [34,35]. Figure 5 shows the graphic representation of the sensor.
Figure 5.
Graphic representation of the NOx sensor.
The sensor consists of two measuring Nernst cells. In addition to controlling the reactions within the chambers, the oxygen concentration in each cavity is also controlled by changing the pumping currents and comparing the reference cell potential with a setpoint value. To obtain the desired concentration, oxygen is pumped into or out of the cavities [36]. Figure 6 provides a schematic representation of the NOx electrical sensor.
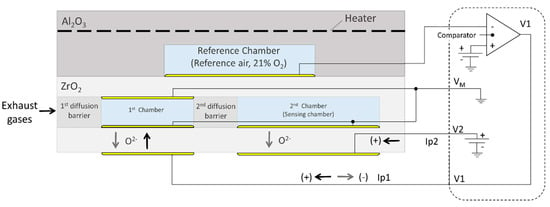
Figure 6.
NOx sensor wiring diagram.
4. Performance Sensing Proposal of the SCR Catalyst Used in Vehicle Exhausts
For technical and legal reasons, modern exhaust after-treatment systems must be monitored [37]. The proposed mode of sensing comprises an electronic device composed of a microcontroller, NOx gas sensors, EGTS (Exhaust Gas Temperature Sensor) and an LCD display.
Through sensing, it will be possible to estimate the performance of the SCR catalyst and its useful life, in addition to diagnosing if there is a failure in the activation of the SCR catalyst. The data make it possible to anticipate maintenance solutions, thus preventing emissions from reaching a critical level of toxicity.
Another variable that was evaluated is the internal temperature of the SCR catalyst, which has a minimum difference of 10% between the inlet and outlet of the gases. The minimum temperature required for the process is in the range of 170 °C to 200 °C. Suppose the exhaust does not reach the appropriate temperature for the activation process of the SCR catalyst. In that case, the reduction of NOx gases will not occur due to the inefficiency of the chemical reaction between the noble metals of the catalyst, the gases from the exhaust, and the liquid urea injected at the inlet of the catalytic device. Failure in the activation process can be due to:
- ✓
- SCR, DOC and cDPF (Catalyzed Diesel Particulate Filter) filter devices showing internal or external cracks;
- ✓
- SCR devices, DOC and cDPF filter clogged by soot;
- ✓
- Failure to control the air/fuel mixture;
- ✓
- Low fuel-burning power in the combustion chamber;
- ✓
- Pipe leaks;
- ✓
- Low chemical reaction (which is exothermic) of DOC and SCR.
Figure 7 shows the sensing proposal used in the treatment system for exhaust gases from diesel engines.
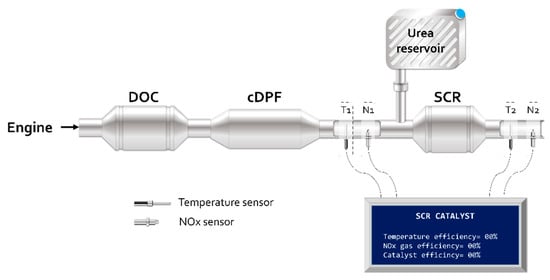
Figure 7.
Implementation of the onboard monitoring system.
In Figure 7, the integration of NOx sensors, temperature, and the electronic device for conditioning and data acquisition in the post exhaust gas treatment system is presented. Figure 8 provides the electrical diagram of the temperature monitoring sensor and the NOx sensor, used to analyze the SCR catalyst performance.
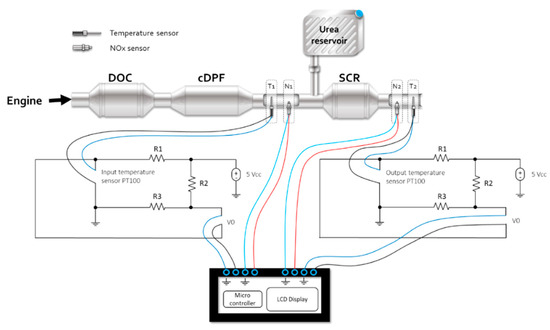
Figure 8.
Integration of electronic components in the SCR catalyst monitoring system.
The T1 and T2 temperature sensors provide the electrical signals corresponding to the temperature values measured at the ends of the SCR catalyst. Figure 9 shows the catalyst failure analysis flowchart of temperature monitoring.
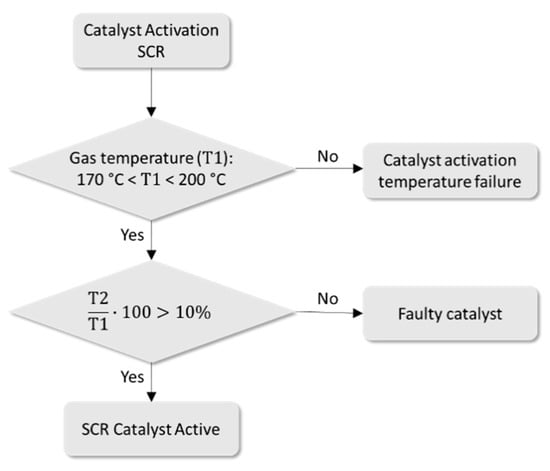
Figure 9.
Flowchart for temperature monitoring.
Figure 10 presents the flowchart for evaluating catalyst failure by monitoring the reduction of NOx gases.
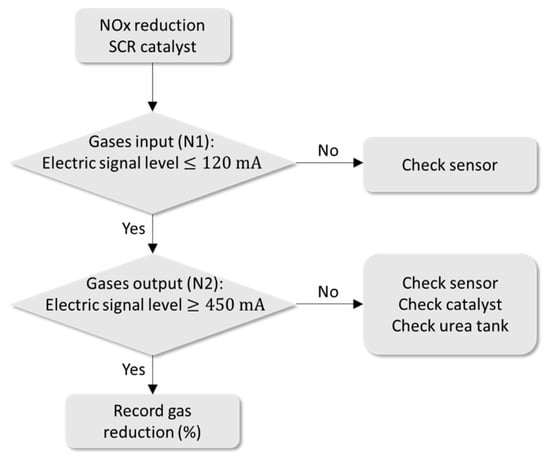
Figure 10.
Flowchart for monitoring the NOx gas sensor.
For NOx sensors, if N1 = N2 then the catalyst is faulty.
Computer Simulation of the Monitoring System
To obtain the temperature a PT 100—platinum (Pt)-based sensor with 100 Ω resistance at 0 °C was used, as represented in Equation (1).
where R2 = Internal resistance of the sensor as a function of the variable T; R0 = Value of the minimum range of the PT100 sensor; T = Temperature of the internal catalyst gases; Ø = Pt (platinum) sensor temperature coefficient 100 (0.00385 IEC 60751).
For the SCR catalyst to be considered active, a temperature differential of the gases between the inlet and outlet of the device is required. This differential is the result of the exothermic reaction inside the catalyst. For the catalytic converter to be active, this differential must be at least 10% greater during the exit of the gases.
The temperature differential can be obtained with Equation (2).
where Dt represents the catalyst temperature differential, Te represents the temperature of the gases at the inlet of the catalyst, and Ts the gas temperature in the catalyst exit.
Another method to determine the temperature of the catalyst gases is to identify the potential difference (ddp), where the output voltage (Vout) is collected at the resistive bridge. By identifying the variable Rs (PT 100 sensor resistance), it is possible to identify the ddp at the output (Vout) of the bridge.
R1, R2, R0 and Vin values are considered constant in the resistive bridge, while Vout can be obtained using Equation (3).
The internal resistance of the sensor can be obtained from Equation (4), as a function of ddp Vout of the resistive bridge.
Figure 11 shows a graph of the temperature variation (T) of a PT 100 sensor, considering Equations (1), (3) and (4), for Vin = [0:5] Volts.
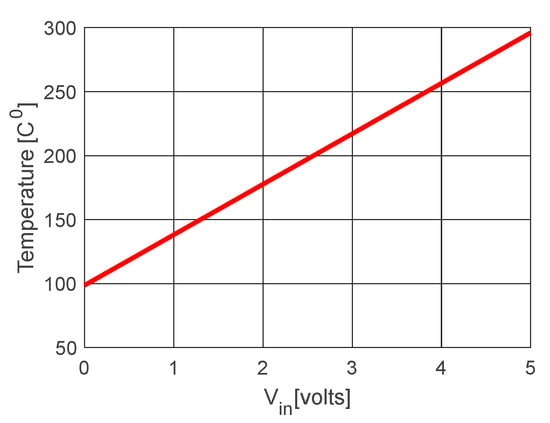
Figure 11.
Temperature variation (T) of a PT 100 sensor versus input voltage.
To evaluate the efficiency of the SCR catalyst in the reduction of NOx gases, the electrical response of the sensors was considered, which were installed at the inlet and outlet of the gases in the catalyst, to perform an efficiency calculation in the reduction of gases. For an optimal state of reduced NOx gas emissions, the electrical output signal of the NOx sensor must have a stoichiometric value of 450 mV. For a low-efficiency reduction, the value indicated is 120 Mv, which is the value that the sensor can identify at the inlet of the catalyst immediately after combustion.
Equation (5) presents the calculation implemented to identify the value, as a percentage, of the reduction of NOx gases in relation to the inlet and outlet of gases in a diesel SCR catalyst.
where ddpout represents the electrical voltage value in mV presented by the output sensor of gases in the catalyst, ddpin represents the constant value of 120 Mv and span represents the theoretical range of the sensor for measuring NOx gases. For Equation (5), the value is a constant of 330.
Another possibility for analyzing the efficiency of the reduction of NOx gases in an SCR catalyst is to evaluate the ddp signal provided by the sensor installed at the output of the SCR catalyst, using the desired value of 450 mV as a reference, using Equation (6).
where ddpout represents the electrical voltage value in mV presented by the gas output sensor in the catalyst and ddpw represents the constant value of 450 mV.
In Figure 12, we can see the electronic circuit diagram of the efficiency of the SCR catalyst in relation to NOx gases.
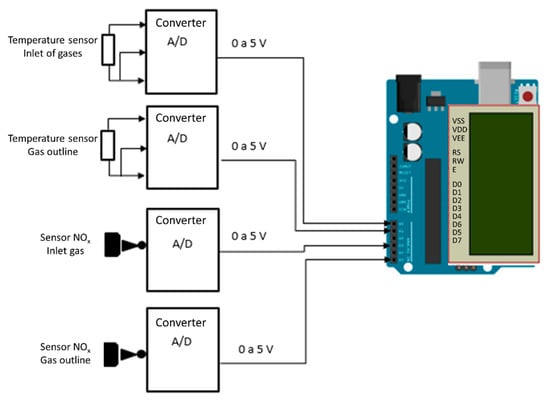
Figure 12.
Electronic circuit for virtual simulation.
Figure 13 shows the results for Equations (5) and (6), obtained in simulations in the Proteus virtual environment.
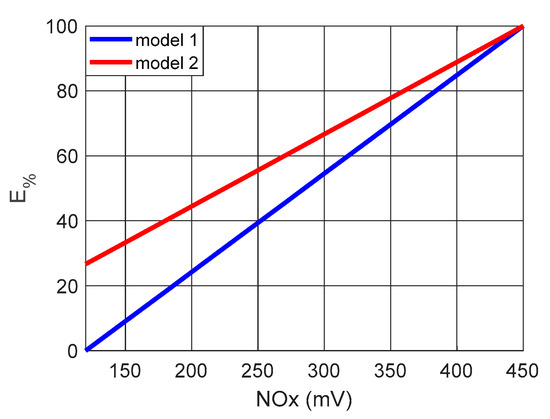
Figure 13.
Analysis of catalyst performance for NOx variations.
As shown in Figure 13, the efficiencies calculated using Equations (5) and (6) present curves of equal evolution and can be used in parallel to guarantee robustness in the evaluation of the catalyst’s performance.
5. Conclusions
The results presented demonstrate the relationship of the emission of toxic gases with exhaust systems without the catalytic system or with failure in the catalyst. The levels observed are worrying for the respiratory health of the population. As can be seen, with the proper use of the catalyst, it is possible to reduce the release levels of NOx, PM, CO2, and VOC, among other polluting compounds. Additionally, with the proposal of the catalyst efficiency sensor, this paper proposes a model for monitoring the catalyst, enabling better planning and maintenance or replacement of the catalytic system, thus reducing the emission of toxic gases and thus improving the quality of life, reducing the costs of treating respiratory problems caused by pollution.
Considering that the NOx sensor can be used at low or high temperatures, and is widely used in the automotive industry due to its small size, fast response, low price and long service life, the monitoring system proposed in this paper can be easily produced because it uses sensors that are already commercially available and used in the automotive industry [1,38].
Author Contributions
J.F., D.I.A., M.E.K.F. and L.N.B.d.A. carried out the methodology and investigation; L.M.S.C., M.E.K.F., G.G.L. and A.M.T. conceived of the project and shared in the writing, reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Capes, Fundação Araucária, and CNPq agency. The seventh author thanks CNPq for the financial support (Process: 310562/2021-0).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Srivastava, S. Study of gas sensor detection for NOx gas: A review. Mater. Today Proc. 2021, 37, 3709–3712. [Google Scholar] [CrossRef]
- Paleczek, A.; Szafraniak, B.; Fuśnik, Ł.; Brudnik, A.; Grochala, D.; Kluska, S.; Jurzecka-Szymacha, M.; Maciak, E.; Kałużyński, P.; Rydosz, A. The Heterostructures of CuO and SnOx for NO2 Detection. Sensors 2021, 21, 4387. [Google Scholar] [CrossRef]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon Dioxide (CO2) Emissions and Economic Growth: A Systematic Review of Two Decades of Research from 1995 to 2017. Sci. Total Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Xu, P.; Zhang, Y. Moving towards Sustainability: Road Grades and On-Road Emissions of Heavy-Duty Vehicles—A Case Study. Sustainability 2015, 7, 12644–12671. [Google Scholar] [CrossRef] [Green Version]
- Phugot, S.; Wongchang, T.; Sawatmongkhon, B.; Theinnoi, K. Effect of Diesel—Biodiesel—Ethanol Fuel Blends on Low Temperature NOx Reduction Activity over a Lean NOx Catalyst. In Proceedings of the 2018 Third International Conference on Engineering Science and Innovative Technology (ESIT), Mariott Khaolak, Thailand, 19–22 April 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Ao, C.; Ruan, S.; He, W.; He, C.; Xu, K.; Zhang, L. Theoretical Investigation of Chemical Reaction Kinetics of CO2 and Vinyl Radical under Catalytic Combustion. Fuel 2021, 305, 121566. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.; Wang, Y.; Zhao, L.; He, C. Prediction of Transient NOx Emission from Diesel Vehicles Based on Deep-Learning Differentiation Model with Double Noise Reduction. Atmosphere 2021, 12, 1702. [Google Scholar] [CrossRef]
- Reşitoğlu, İ.A.; Altinişik, K.; Keskin, A. The Pollutant Emissions from Diesel-Engine Vehicles and Exhaust Aftertreatment Systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Giechaskiel, B.; Lähde, T.; Schwelberger, M.; Kleinbach, T.; Roske, H.; Teti, E.; van den Bos, T.; Neils, P.; Delacroix, C.; Jakobsson, T.; et al. Particle Number Measurements Directly from the Tailpipe for Type Approval of Heavy-Duty Engines. Appl. Sci. 2019, 9, 4418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chen, G.; Zhang, Y.; Liu, S.; Wang, X.; Wang, B.; Hang, J. Integrated Impacts of Turbulent Mixing and NOx-O3 Photochemistry on Reactive Pollutant Dispersion and Intake Fraction in Shallow and Deep Street Canyons. Sci. Total Environ. 2020, 712, 135553. [Google Scholar] [CrossRef]
- Scungio, M.; Stabile, L.; Rizza, V.; Pacitto, A.; Russi, A.; Buonanno, G. Lung Cancer Risk Assessment Due to Traffic-Generated Particles Exposure in Urban Street Canyons: A Numerical Modelling Approach. Sci. Total Environ. 2018, 631, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, B.; Zhou, W.; Jiang, X.; Tan, Z. A Methodology for Predicting Particle Penetration Factor through Cracks of Windows and Doors for Actual Engineering Application. Build. Environ. 2012, 47, 339–348. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Masih, A.; Taneja, A. Indoor/Outdoor Relationships of Carbon Monoxide and Oxides of Nitrogen in Domestic Homes with Roadside, Urban and Rural Locations in a Central Indian Region. Indoor Air 2005, 15, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Salonen, H.; Salthammer, T.; Morawska, L. Human Exposure to NO2 in School and Office Indoor Environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.V.S.; Trichês, G. Evaluation of the Influence of Environmental Conditions on the Efficiency of Photocatalytic Coatings in the Degradation of Nitrogen Oxides (NOx). Build. Environ. 2012, 49, 117–123. [Google Scholar] [CrossRef]
- Hakki, A.; Yang, L.; Wang, F.; Elhoweris, A.; Alhorr, Y.; Macphee, D.E. Photocatalytic Functionalized Aggregate: Enhanced Concrete Performance in Environmental Remediation. Buildings 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and Mitigation of Excess Diesel-Related NO x Emissions in 11 Major Vehicle Markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef]
- Vressner, A.; Gabrielsson, P.; Gekas, I.; Senar-Serra, E. Meeting the EURO VI NOx Emission Legislation Using a EURO IV Base Engine and a SCR/ASC/DOC/DPF Configuration in the World Harmonized Transient Cycle. SAE Tech. Pap. 2010. [Google Scholar] [CrossRef]
- Majidi-Jirandehi, A.A.; Soleymani, M.M.; Dehghani, H. Determine the Useful Life of Catalytic Converter and Standard Revision of Technical Inspection Centers. Automot. Sci. Eng. 2021, 11, 3614–3619. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Automobile Pollution Control Using Catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Wang, B.; Wang, M.; Han, L.; Hou, Y.; Bao, W.; Zhang, C.; Feng, G.; Chang, L.; Huang, Z.; Wang, J. Improved Activity and SO Resistance by Sm-Modulated Redox of MnCeSmTiOxMesoporous Amorphous Oxides for Low-Temperature NH3-SCR of NO. ACS Catal. 2020, 10, 9034–9045. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Feng, G.; Chang, L.; Bao, W. In Situ Synthesis of CuSAPO-34/Cordierite and Its Selective Catalytic Reduction of Nitrogen Oxides in Vehicle Exhaust: The Effect of HF. Fuel 2013, 109, 101–109. [Google Scholar] [CrossRef]
- Kurzydym, D.; Żmudka, Z.; Perrone, D.; Klimanek, A. Experimental and Numerical Investigation of Nitrogen Oxides Reduction in Diesel Engine Selective Catalytic Reduction System. Fuel 2022, 313, 122971. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Tan, D.; Feng, Z.; Luo, J.; Tan, Y.; Huang, Y. The Effects of Fe2O3 Based DOC and SCR Catalyst on the Combustion and Emission Characteristics of a Diesel Engine Fueled with Biodiesel. Fuel 2021, 290, 120039. [Google Scholar] [CrossRef]
- McCaffery, C.; Zhu, H.; Tang, T.; Li, C.; Karavalakis, G.; Cao, S.; Oshinuga, A.; Burnette, A.; Johnson, K.C.; Durbin, T.D. Real-World NOx Emissions from Heavy-Duty Diesel, Natural Gas, and Diesel Hybrid Electric Vehicles of Different Vocations on California Roadways. Sci. Total Environ. 2021, 784, 147224. [Google Scholar] [CrossRef]
- Vieira, F.; Ribeiro, M.; Francisco, A.; Gonçalves Lenzi, G. Influence of Extreme Events in Electric Energy Consumption and Gross Domestic Product. Sustainability 2019, 11, 672. [Google Scholar] [CrossRef] [Green Version]
- Policarpo, N.A.; Silva, C.; Lopes, T.F.A.; dos Santos Araújo, R.; Cavalcante, F.S.Á.; Pitombo, C.S.; de Oliveira, M.L.M. Road Vehicle Emission Inventory of a Brazilian Metropolitan Area and Insights for Other Emerging Economies. Transp. Res. Part D Transp. Environ. 2018, 58, 172–185. [Google Scholar] [CrossRef]
- De Melo, C.A.; Jannuzzi, G.D.M.; Santana, P.H.D.M. Why Should Brazil to Implement Mandatory Fuel Economy Standards for the Light Vehicle Fleet? Renew. Sustain. Energy Rev. 2018, 81, 1166–1174. [Google Scholar] [CrossRef]
- Carvalho, V.S.B.; Freitas, E.D.; Martins, L.D.; Martins, J.A.; Mazzoli, C.R.; de Fatima Andrade, M. Air Quality Status and Trends over the Metropolitan Area of São Paulo, Brazil as a Result of Emission Control Policies. Environ. Sci. Policy 2015, 47, 68–79. [Google Scholar] [CrossRef]
- Szwarcfiter, L.; Mendes, F.E.; La Rovere, E.L. Enhancing the Effects of the Brazilian Program to Reduce Atmospheric Pollutant Emissions from Vehicles. Transp. Res. Part. D Transp. Environ. 2005, 10, 153–160. [Google Scholar] [CrossRef]
- Benvenutti, L.M.; Uriona-Maldonado, M.; Campos, L.M.S. The Impact of CO2 Mitigation Policies on Light Vehicle Fleet in Brazil. Energy Policy 2019, 126, 370–379. [Google Scholar] [CrossRef]
- CONAMA. Resolução CONAMA No 490 de 16 de Novembro de 2018. 2018. Available online: http://conama.mma.gov.br/?option=com_sisconama&task=arquivo.download&id=767 (accessed on 4 May 2022).
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of Air Pollution on Health: A Mapping Review of Systematic Reviews and Meta-Analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Cai, Y.; Wang, J. Experimental Studies on the Diesel Engine Urea-SCR System Using a Double NOx Sensor System. Environ. Eng. Res. 2015, 20, 397–402. [Google Scholar] [CrossRef]
- Bonfils, A.; Creff, Y.; Lepreux, O.; Petit, N. Closed-Loop Control of a SCR System Using a NOx Sensor Cross-Sensitive to NH3. J. Process. Control 2014, 24, 368–378. [Google Scholar] [CrossRef]
- Aliramezani, M.; Koch, C.R.; Hayes, R.E.; Patrick, R. Amperometric Solid Electrolyte NOx Sensors—The Effect of Temperature and Diffusion Mechanisms. Solid State Ion. 2017, 313, 7–13. [Google Scholar] [CrossRef]
- Motroniuk, I.; Stober, R.; Fischerauer, G. Transmitter Power Influence in a Wireless-Signal-Based System Used to Monitor Catalyst States. In Proceedings of the 2016 13th International Multi-Conference on Systems, Signals & Devices (SSD), Leipzig, Germany, 21–24 March 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 507–511. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, Z.; Zhu, R.; Zhou, Y.; Li, X. Modeling and analysis of pumping cell of NOx sensor—Part I: Main oxygen pumping cell. Sens. Actuators B Chem. 2020, 359, 131622. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).