Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mortar Prisms
2.1.1. Bacterial Isolates
2.1.2. Growth Medium (GM)
2.1.3. Aerated Concrete Granules (ACGs)
2.1.4. Casting of Mortar Prisms
2.2. Re-Cracking of Previously Healed 20-Month-Old Mortar Prisms
2.3. New Crack Produced on 22-Month-Old Mortar Prisms
3. Results
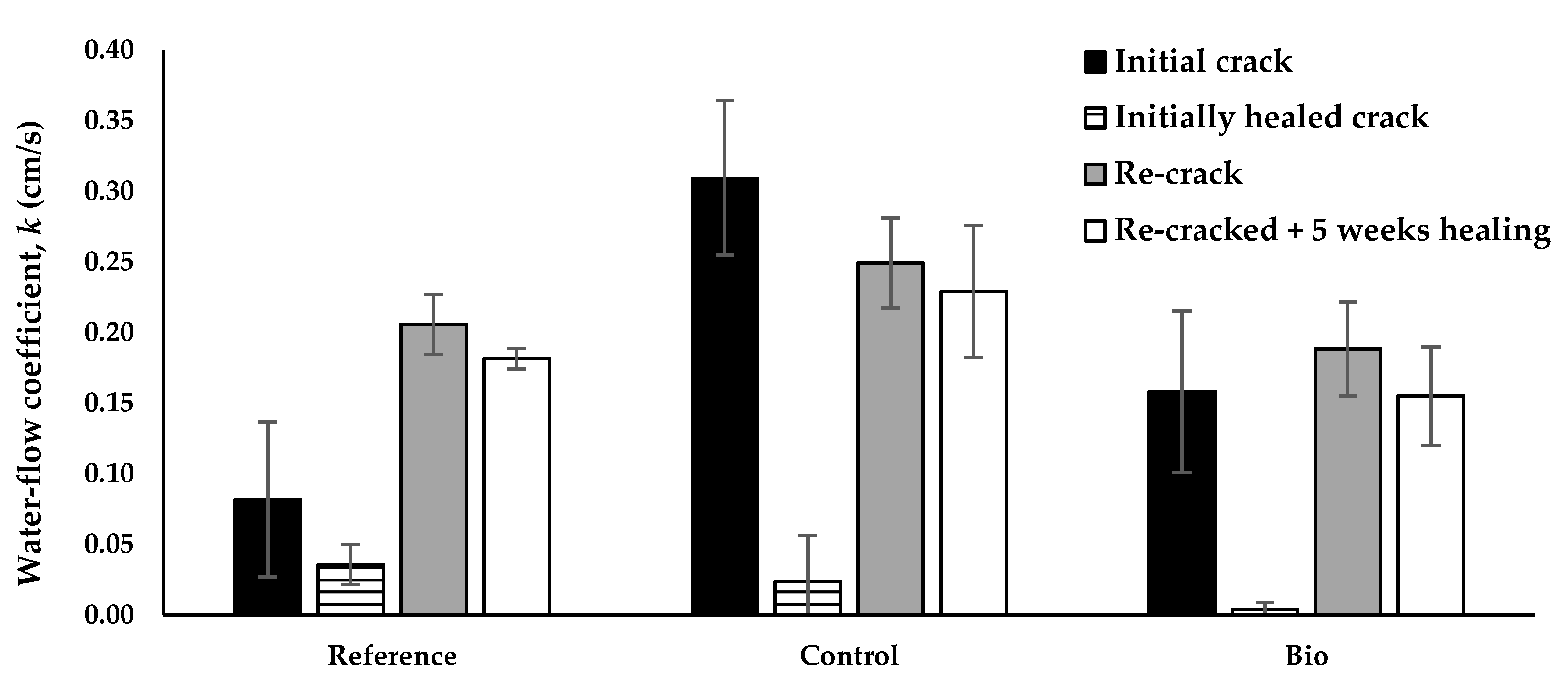
3.1. Re-Cracking of Previously Healed 20-Month-Old Mortar Prisms
3.1.1. Crack Area Quantification
3.1.2. Water Tightness
3.2. New Cracks Produced on 22-Month-Old Mortar Prisms
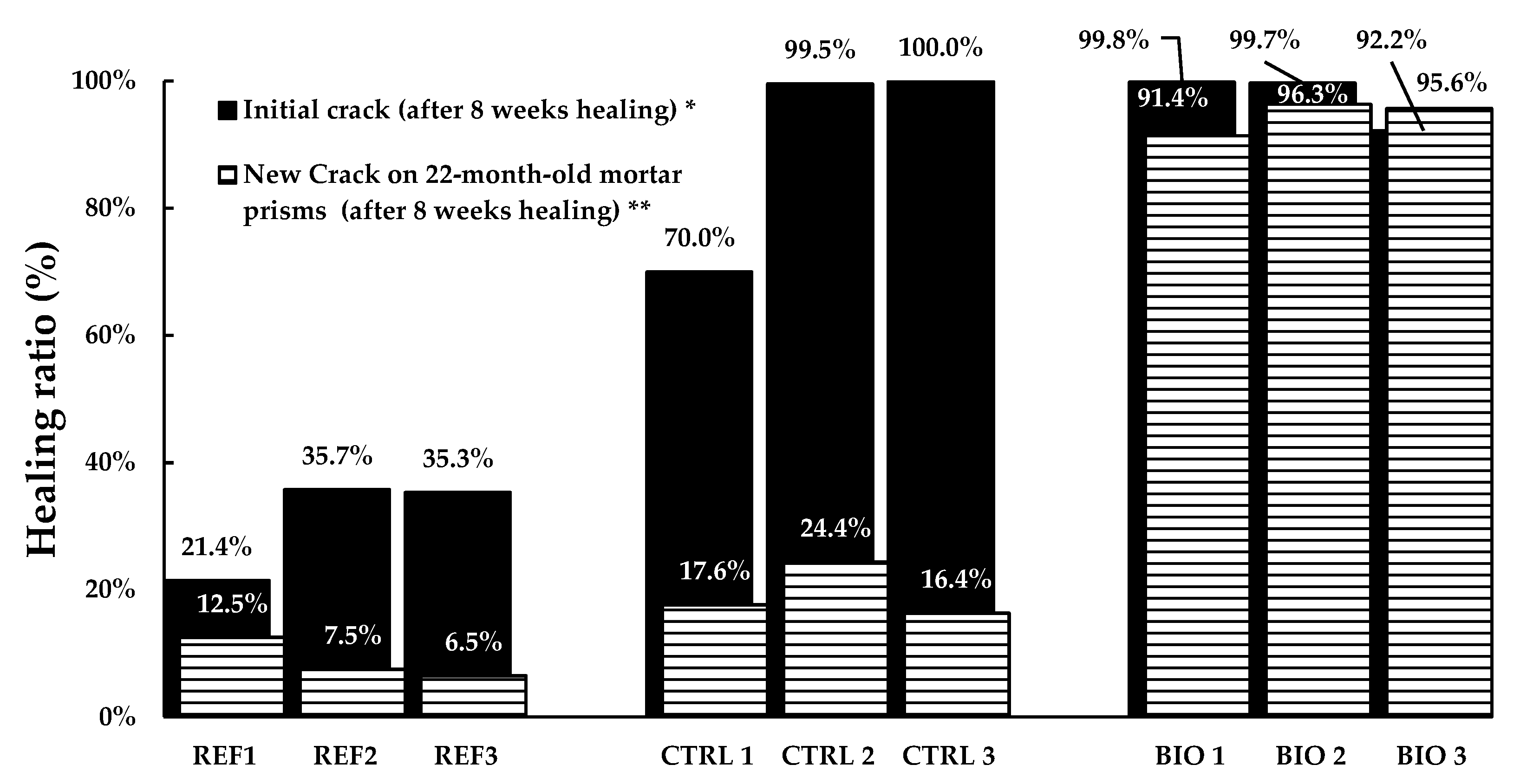
3.2.1. Crack Area Quantification
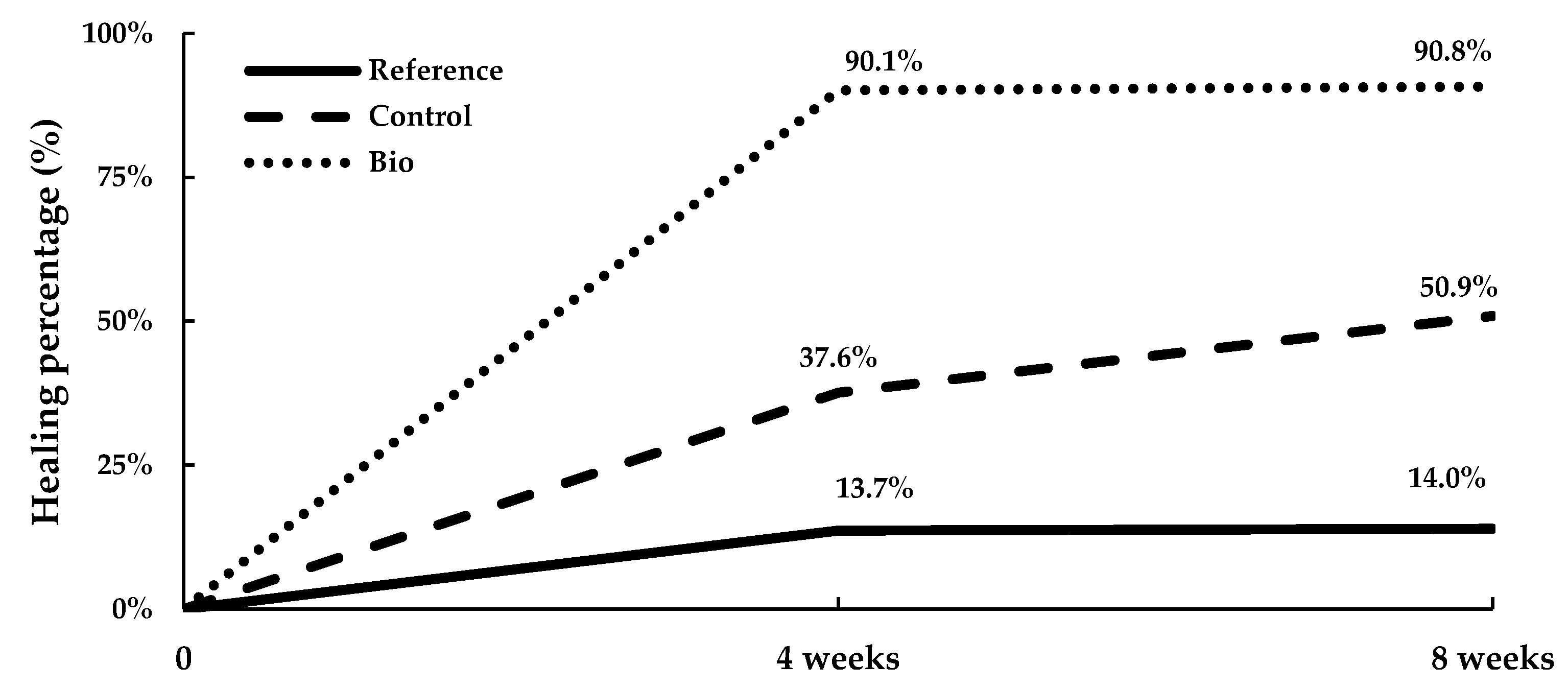
3.2.2. Water Tightness
4. Discussion
4.1. Re-Cracking of Previously Healed 20-Month-Old Mortar Prisms
4.2. New Cracks Produced on 22-Month-Old Mortar Prisms
5. Conclusions
- It has been demonstrated that bacterial spores encapsulated into ACGs remain viable within the cement matrix and can heal later-formed cracks (at 22 months) if proper conditions are supplied (i.e., nutrients, additional calcium, and humidity).
- The BBSH mortar formulation investigated, where spores from a bacterium closely related to Bacillus licheniformis were encapsulated into aerated concrete granules (ACGs), is not effective for re-healing previously healed cracks when these are reformed at a later age (i.e., 20 months).
- The lack of healing observed when the cracks were reformed is likely due to the absence of enough viable bacterial spores available within the reopened cracks. In this regard, further research is needed to elucidate whether the bacteria, capable of healing the cracks in the first instance, can form new spores and whether these new spores can remain protected within the calcium carbonate precipitates formed during the first healing cycle.
- Therefore, for one-time healing events, independent encapsulation of spores and nutrients might allow efficient healing. However, for successful cyclic healing, additional research should be conducted on developing systems capable of effective repetitive delivery of spores and nutrients (e.g., vascular networks).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, P.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials, 3rd ed.; Mc Graw Hill: New York, NY, USA, 2006; p. 659. [Google Scholar]
- Zongjin, L. Advanced Concrete Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T. A review of self-healing concrete for damage management of structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Heath, A.; Gebhard, S.; Paine, K. Aerobic non-ureolytic bacteria-based self-healing cementitious composites: A comprehensive review. J. Build. Eng. 2021, 42, 102834. [Google Scholar] [CrossRef]
- Farhadi, S.; Ziadloo, S. Self-Healing Microbial Concrete—A Review. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2020; Volume 990, pp. 8–12. [Google Scholar]
- Kim, H.; Son, H.; Seo, J.; Lee, H.-K. Recent advances in microbial viability and self-healing performance in bacterial-based cementitious materials: A review. Constr. Build. Mater. 2021, 274, 122094. [Google Scholar] [CrossRef]
- Tziviloglou, E.; Wiktor, V.; Jonkers, H.M.; Schlangen, E. Selection of nutrient used in biogenic healing agent for cementitious materials. Front. Mater. 2017, 4, 15. [Google Scholar] [CrossRef]
- Reeksting, B.J.; Hoffmann, T.D.; Tan, L.; Paine, K.; Gebhard, S. In-depth profiling of calcite precipitation by environmental bacteria reveals fundamental mechanistic differences with relevance to application. Appl. Environ. Microbiol. 2020, 86, e02739-19. [Google Scholar] [CrossRef]
- Yoonhee, J.; Wonjae, K.; Wook, K.; Woojun, P. Complete Genome and Calcium Carbonate Precipitation of Alkaliphilic bacillus sp. AK13 for Self-Healing Concrete. J. Microbiol. Biotechnol. 2020, 30, 404–416. Available online: http://kiss.kstudy.com/search/detail_page.asp?key=3761121 (accessed on 22 April 2022).
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef]
- Shashank, B.; Dhannur, B.; Ravishankar, H.; Nagaraj, P. Study on Development of Strength Properties of Bio-concrete. In Sustainable Construction and Building Materials; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 423–437. [Google Scholar]
- Roig-Flores, M.; Formagini, S.; Serna, P. Self-healing concrete—What Is it Good For? Mater. Constr. 2021, 71, e237. [Google Scholar] [CrossRef]
- Wang, J.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Melchers, R.; de Belie, N.; Shi, X.; Van Tittelboom, K.; Perez, A.S. Eco-Efficient Repair and Rehabilitation of Concrete Infrastructures; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Tan, L.; Reeksting, B.; Ferrandiz-Mas, V.; Heath, A.; Gebhard, S.; Paine, K. Effect of carbonation on bacteria-based self-healing of cementitious composites. Constr. Build. Mater. 2020, 257, 119501. [Google Scholar] [CrossRef]
- Rauf, M.; Khaliq, W.; Khushnood, R.A.; Ahmed, I. Comparative performance of different bacteria immobilized in natural fibers for self-healing in concrete. Constr. Build. Mater. 2020, 258, 119578. [Google Scholar] [CrossRef]
- Han, S.; Jang, I.; Choi, E.K.; Park, W.; Yi, C.; Chung, N. Bacterial Self-Healing Performance of Coated Expanded Clay in Concrete. J. Environ. Eng. 2020, 146, 04020072. [Google Scholar] [CrossRef]
- Zhu, X.; Mignon, A.; Nielsen, S.D.; Zieger, S.E.; Koren, K.; Boon, N.; De Belie, N. Viability determination of Bacillus sphaericus after encapsulation in hydrogel for self-healing concrete via microcalorimetry and in situ oxygen concentration measurements. Cem. Concr. Compos. 2021, 119, 104006. [Google Scholar] [CrossRef]
- Gao, M.; Guo, J.; Cao, H.; Wang, H.; Xiong, X.; Krastev, R.; Nie, K.; Xu, H.; Liu, L. Immobilized bacteria with pH-response hydrogel for self-healing of concrete. J. Environ. Manag. 2020, 261, 110225. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Reeksting, B.J.; Hamley-Bennett, C.; Heath, A.; Gebhard, S.; Paine, K. Air-entraining admixtures as a protection method for bacterial spores in self-healing cementitious composites: Healing evaluation of early and later-age cracks. Constr. Build. Mater. 2022, 327, 126877. [Google Scholar] [CrossRef]
- Erşan, Y.Ç.; Da Silva, F.B.; Boon, N.; Verstraete, W.; De Belie, N. Screening of bacteria and concrete compatible protection materials. Constr. Build. Mater. 2015, 88, 196–203. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.; Crump, J.; Zhou, H.; Davies, D.G.; Zhou, G.; Zhang, N.; Jin, C. Interactions of fungi with concrete: Significant importance for bio-based self-healing concrete. Constr. Build. Mater. 2018, 164, 275–285. [Google Scholar] [CrossRef]
- Mors, R.; Jonkers, H. Effect on concrete surface water absorption upon addition of lactate derived agent. Coatings 2017, 7, 51. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, W. Current challenges and future directions for bacterial self-healing concrete. Appl. Microbiol. Biotechnol. 2018, 102, 3059–3070. [Google Scholar] [CrossRef]
- Kua, H.W.; Gupta, S.; Aday, A.N.; Srubar, W.V., III. Biochar-immobilized bacteria and superabsorbent polymers enable self-healing of fiber-reinforced concrete after multiple damage cycles. Cem. Concr. Compos. 2019, 100, 35–52. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Stefanidou, M.; Tsampali, E.; Karagiannis, G.; Amanatiadis, S.; Ioakim, A.; Kassavetis, S. Techniques for recording self-healing efficiency and characterizing the healing products in cementitious materials. Mater. Des. Process. Commun. 2021, 3, e166. [Google Scholar] [CrossRef][Green Version]
- Luo, M.; Qian, C.-X.; Li, R.-Y. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- RILEM. RILEM Test Method 11.4: Measurement of Water Absorption under Low Pressure; RILEM: Paris, France, 1987. [Google Scholar]
- Roig-Flores, M.; Pirritano, F.; Serna, P.; Ferrara, L. Effect of crystalline admixtures on the self-healing capability of early-age concrete studied by means of permeability and crack closing tests. Constr. Build. Mater. 2016, 114, 447–457. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, B.; Cai, T.; Luo, J.; Wu, W.; Liu, B.; Han, N.; Xing, F.; Deng, X. Optimization of a binary concrete crack self-healing system containing bacteria and oxygen. Materials 2017, 10, 116. [Google Scholar] [CrossRef]
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
- De Nardi, C.; Gardner, D.; Jefferson, T. Advanced 3D Printed Mini-Vascular Network for Self-Healing Concrete; Maddalena, R., Wright-Syed, M., Eds.; Cardiff University: Cardiff, UK, 2021; p. 292. [Google Scholar]
- De Nardi, C.; Gardner, D.; Jefferson, A.D. Development of 3D printed networks in self-healing concrete. Materials 2020, 13, 1328. [Google Scholar] [CrossRef]
- Indhumathi, S.; Dinesh, A.; Pichumani, M. Diverse perspectives on self healing ability of Engineered Cement Composite—All-inclusive insight. Constr. Build. Mater. 2022, 323, 126473. [Google Scholar]
- Zheng, T.; Qian, C. Self-healing of later-age cracks in cement-based materials by encapsulation-based bacteria. J. Mater. Civ. Eng. 2020, 32, 04020341. [Google Scholar] [CrossRef]
- Skevi, L.; Reeksting, B.; Gebhard, S.; Paine, K. Bacteria Based Self-healing of Later-Age Cracks in Concrete. In International RILEM Conference on Early-Age and Long-Term Cracking in RC Structures; Springer International Publishing: Cham, Switzerland, 2021; pp. 367–376. [Google Scholar]





| Mix | Cement (g) | Water (mL) | Sand (g) | Calcium Nitrate (g) | Yeast Extract (g) | Bacterial Spores (CFU) | PVA-Coated ACG-S (g) | PVA-Coated ACG-NS (g) |
|---|---|---|---|---|---|---|---|---|
| Reference | 92 | 46 | 276 | 0 | 0 | 0 | 0 | 0 |
| Control | 92 | 46 | 253 | 4.6 | 1.0 | 0 | 0 | 4.6 |
| Bio | 92 | 46 | 253 | 4.6 | 1.0 | 2.1 × 1010 | 4.6 | 0 |
| Mortar Mix | Sample | Healing Ratio (%) |
|---|---|---|
| Reference | Ref 1 | 21.4 |
| Ref 2 | 35.7 | |
| Ref 3 | 35.3 | |
| Control | Ctrl 1 | 70.0 |
| Ctrl 2 | 99.5 | |
| Ctrl 3 | 99.9 | |
| Bio | Bio 1 | 99.8 |
| Bio 2 | 99.7 | |
| Bio 3 | 92.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justo-Reinoso, I.; Reeksting, B.J.; Heath, A.; Gebhard, S.; Paine, K. Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites. Sustainability 2022, 14, 6845. https://doi.org/10.3390/su14116845
Justo-Reinoso I, Reeksting BJ, Heath A, Gebhard S, Paine K. Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites. Sustainability. 2022; 14(11):6845. https://doi.org/10.3390/su14116845
Chicago/Turabian StyleJusto-Reinoso, Ismael, Bianca J. Reeksting, Andrew Heath, Susanne Gebhard, and Kevin Paine. 2022. "Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites" Sustainability 14, no. 11: 6845. https://doi.org/10.3390/su14116845
APA StyleJusto-Reinoso, I., Reeksting, B. J., Heath, A., Gebhard, S., & Paine, K. (2022). Evaluation of Cyclic Healing Potential of Bacteria-Based Self-Healing Cementitious Composites. Sustainability, 14(11), 6845. https://doi.org/10.3390/su14116845







