Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review
Abstract
:1. Introduction
2. Aquaculture Production Systems
3. Aquaculture Waste
3.1. Gas Emission from Aquaculture
3.2. Aquaculture Effluents
3.3. Aquaculture Particulate Fraction
4. Aquaculture Waste Effect on the Environment
5. Aquaculture Waste Treatment
5.1. Biofiltration of Aquaculture Waste
5.2. Bioremediation of Aquaculture Waste
5.3. Biodigestion of Aquaculture Waste
5.4. Biomineralization of Aquaculture Waste
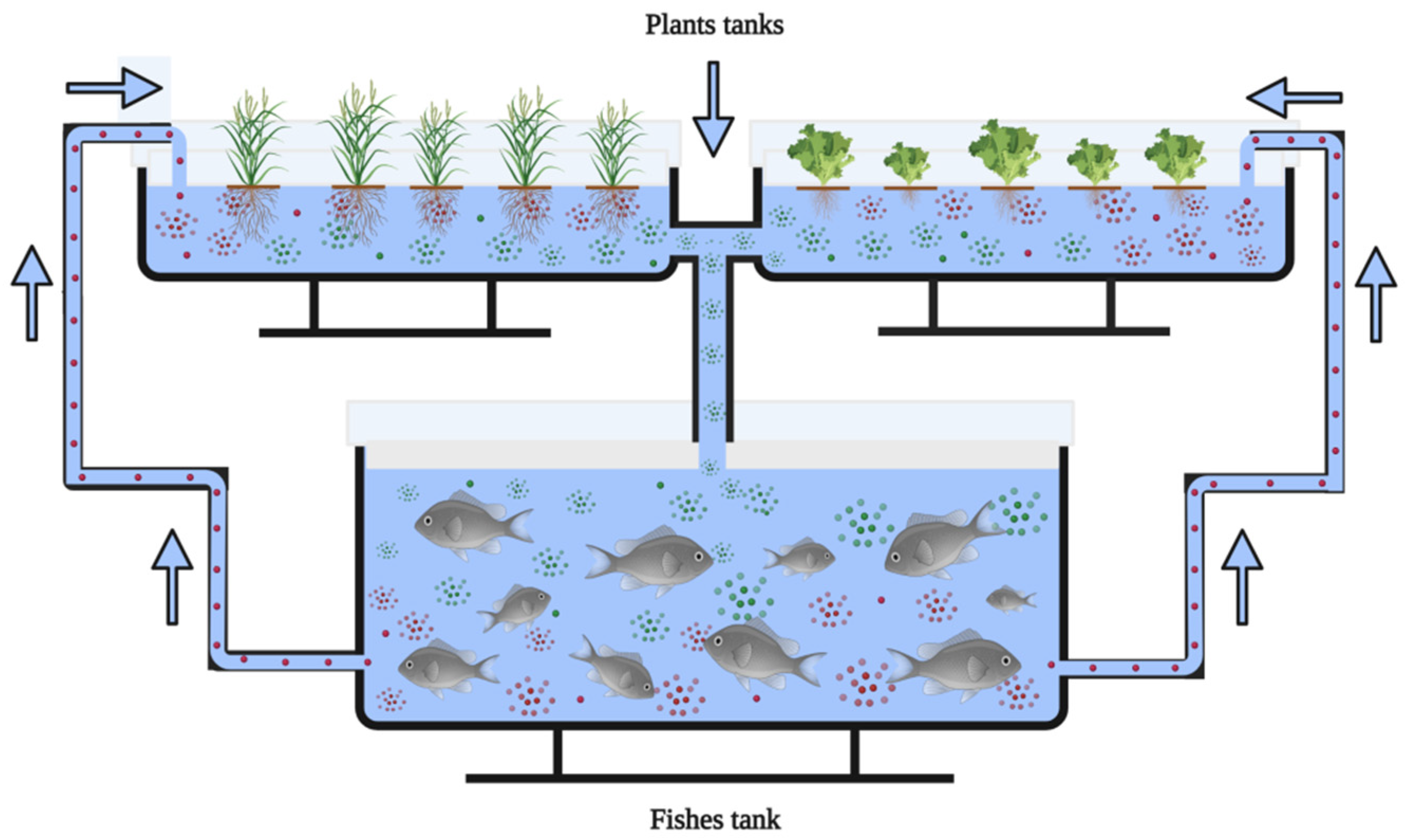
6. Aquaponics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef] [PubMed]
- El Estado Mundial de la Pesca y la Acuicultura (SOFIA). Cumplir los Objetivos de Desarrollo Sostenible. Available online: www.fao.org/3/i9540es/i9540es.pdf (accessed on 1 March 2022).
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, A.; Schumann, M.; Brinker, A. Water pollution from fish farms. Encycl. Water Sci. Technol. Soc. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Bao, W.; Zhu, S.; Jin, G.; Ye, Z. Generation, characterization, perniciousness, removal and reutilization of solids in aquaculture water: A review from the whole process perspective. Rev. Aquac. 2018, 11, 1342–1366. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Brod, E.; Hanserud, O.S.; Gracey, E.O.; Vestrum, M.I.; Bøen, A.; Brattebø, H. Investigating cross-sectoral synergies through integrated aquaculture, fisheries, and agriculture phosphorus assessments: A case study of Norway. J. Ind. Ecol. 2016, 20, 867–881. [Google Scholar] [CrossRef]
- Gál, D.; Pekár, F.; Kerepeczki, É. A survey on the environmental impact of pond aquaculture in Hungary. Aquacult. Int. 2016, 24, 1543–1554. [Google Scholar] [CrossRef]
- Srithongouthai, S.; Tada, K. Impacts of organic waste from a yellowtail cage farm on surface sediment and bottom water in Shido Bay (the Seto Inland Sea, Japan). Aquaculture 2017, 471, 140–145. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Álvarez-Borrego, S.; Ruiz-Fernández, A.C.; García-Hernández, J.; Jara-Marini, M.E.; Bergés-Tiznado, M.E.; Ruelas-Inzunza, J.R. Environmental status of the Gulf of California: A pollution review. Earth Sci. Rev. 2017, 166, 181–205. [Google Scholar] [CrossRef]
- Ezziddine, M.; Liltved, H.; Homme, J.M. A method for reclaiming nutrients from aquacultural waste for use in soilless growth systems. Water Sci. Technol. 2020, 81, 81–90. [Google Scholar] [CrossRef]
- Stabili, L.; Di Salvo, M.; Alifano, P.; Talà, A. An integrative, multiparametric approach for the comprehensive assessment of microbial quality and pollution in aquaculture systems. Microb. Ecol. 2021, 83, 271–283. [Google Scholar] [CrossRef]
- Fornshell, G.; Hinshaw, J.; Tidwell, J.H. Flow-through raceways. In Aquaculture Production Systems, 1st ed.; Tidwell, J.H., Ed.; Wiley-Blackwell: Oxford, UK, 2012; Volume 1, pp. 173–189. [Google Scholar]
- Liu, X.G.; Wang, J.; Wu, Z.F.; Cheng, G.F.; Gu, Z.J. Anaerobic ammonium oxidation bacteria in a freshwater recirculating pond aquaculture system. Int. J. Environ. Res. Public Health 2021, 18, 4941. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E. Hydrology and pond construction of freshwater catfish. In Channel Catfish Culture, 1st ed.; Tucker, C.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1, pp. 107–134. [Google Scholar]
- Cremer, M.; Chappell, J.; Zhang, J.; Zhou, E.H. New intensive pond aquaculture technology demonstrated in China. In Global Aquaculture Advocate; Global Aquaculture Alliance: Portsmouth, NH, USA, 2014; pp. 60–62. [Google Scholar]
- Drønen, K.; Roalkvam, I.; Dahle, H.; Olsen, A.B.; Nilsen, H.; Wergeland, H. Microbiome dataset from a marine recirculating aquaculture system (RAS) for salmon post-smolt production in Norway. Data Brief 2021, 40, 107767. [Google Scholar] [CrossRef] [PubMed]
- Espinal, C.A.; Matulić, D. Recirculating aquaculture technologies. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future, 1st ed.; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 35–76. [Google Scholar] [CrossRef] [Green Version]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Masser, M.P. Cage culture in freshwater and protected marine areas. In Aquaculture Production Systems, 1st ed.; Tidwell, J.H., Ed.; Wiley-Blackwell: Oxford, UK, 2012; Volume 1, pp. 119–130. [Google Scholar]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Cultivo de bivalvos en criadero: Un manual práctico. In FAO Documento Técnico de Pesca, 1st ed.; Helm, M.M., Bourne, N., Lovatelli, A., Eds.; FAO: Rome, Italy, 2006; Volume 471, pp. 31–82. (In Spanish) [Google Scholar]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Schumann, M.; Brinker, A. Understanding and managing suspended solids in intensive salmonid aquaculture: A review. Rev. Aquac. 2020, 12, 2109–2139. [Google Scholar] [CrossRef]
- Boyd, C.E. Hydrogen Sulfide Toxic, But Manageable. In Global Aquaculture Advocate; Global Aquaculture Alliance: Portsmouth, NH, USA, 2014; pp. 34–36. [Google Scholar]
- Boyd, C.E. Aquaculture effluent management at the farm level. Aquaculture 2003, 226, 101–112. [Google Scholar] [CrossRef]
- Herath, S.S.; Satoh, S. Environmental impact of phosphorus and nitrogen from aquaculture. In Feed and Feeding Practices in Aquaculture; Allen, D., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; Volume 1, pp. 369–386. [Google Scholar] [CrossRef]
- Ngo, H.H.; Guo, W.; Vo, T.T.; Nghiem, L.D.; Hai, F.I. Aerobic treatment of effluents from the aquaculture industry. In Current Developments in Biotechnology and Bioengineering: Biological Treatment of Industrial Effluents; Larroche, C., Sanroman, M., Guocheng, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 35–77. [Google Scholar] [CrossRef]
- Madariaga, S.T.; Marín, S.L. Sanitary and environmental conditions of aquaculture sludge. Aquac. Res. 2017, 48, 1744–1750. [Google Scholar] [CrossRef]
- Kokou, F.; Fountoulaki, E. Aquaculture waste production associated with antinutrient presence in common fish feed plant ingredients. Aquaculture 2018, 495, 295–310. [Google Scholar] [CrossRef]
- Chen, S.; Coffin, D.E.; Malone, R.F. Sludge production and management for recirculating aquacultural systems. J. World Aquacult. Soc. 1997, 28, 303–315. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Shi, H.; Lee, C.T.; Hashim, H.; Zhang, Z.; Wu, W.M.; Li, C. Recovery of nutrients from fish sludge in an aquaponic system using biological aerated filters with ceramsite plus lignocellulosic material media. J. Clean. Prod. 2020, 258, 120886. [Google Scholar] [CrossRef]
- Fernandes, P.; Pedersen, L.F.; Pedersen, P.B. Microscreen effects on water quality in replicated recirculating aquaculture systems. Aquac. Eng. 2015, 65, 17–26. [Google Scholar] [CrossRef]
- Bannister, R.J.; Johnsen, I.A.; Hansen, P.K.; Kutti, T.; Asplin, L. Near-and far-field dispersal modelling of organic waste from Atlantic salmon aquaculture in fjord systems. ICES J. Mar. Sci. 2016, 73, 2408–2419. [Google Scholar] [CrossRef] [Green Version]
- King, O.C.; Smith, R.A.; Warne, M.S.J.; van de Merwe, J.P.; Connolly, R.M.; Brown, C.J. Combined impacts of photosystem II-inhibiting herbicides and light availability on seagrass and marine microalgae. Mar. Ecol. Prog. Ser. 2021, 668, 215–230. [Google Scholar] [CrossRef]
- Sugiura, S.H. Phosphorus, aquaculture, and the environment. Rev. Fish. Sci. Aquac. 2018, 26, 515–521. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Scheuren-Acevedo, S.M.; Martínez-Córdova, L.R.; Gollas-Galvan, T.; Barraza-Guardado, R.H.; Enríquez-Ocaña, F.; Cortés-Jacinto, E.; Porchas-Cornejo, M.A. Physiological and sanitary condition of the white clam Dosinia ponderosa collected from a coastal area impacted by shrimp farm effluent. Aquacult. Int. 2016, 24, 243–256. [Google Scholar] [CrossRef]
- Sikder, M.N.A.; Min, W.W.; Ziyad, A.O.; Kumar, P.P.; Kumar, R.D. Sustainable treatment of aquaculture effluents in future—A review. Int. Res. J. Adv. Eng. Sci. 2016, 1, 190–193. [Google Scholar]
- Minnikova, T.; Kolesnikov, S.; Minkina, T.; Mandzhieva, S. Assessment of ecological condition of haplic chernozem calcic contaminated with petroleum hydrocarbons during application of bioremediation agents of various natures. Land 2021, 10, 169. [Google Scholar] [CrossRef]
- Gómez, S.; Hurtado, C.F.; Orellana, J.; Valenzuela-Olea, G.; Turner, A. Abarenicola pusilla (Quatrefages, 1866): A novel species for fish waste bioremediation from marine recirculating aquaculture systems. Aquac. Res. 2018, 49, 1363–1367. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.Y.; Liu, R.Z.; Wang, G.Y. Culturable microorganisms associated with sea cucumbers and microbial natural products. Mar. Drugs 2021, 19, 461. [Google Scholar] [CrossRef]
- Robinson, G.; Caldwell, G.S.; Jones, C.L.W.; Stead, S.M. The effect of resource quality on the growth of Holothuria scabra during aquaculture waste bioremediation. Aquaculture 2019, 499, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.; Caldwell, G.S.; Wade, M.J.; Free, A.; Jones, C.L.W.; Stead, S.M. Profiling bacterial communities associated with sediment-based aquaculture bioremediation systems under contrasting redox regimes. Sci. Rep. 2016, 6, 38850. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, K. Process performance of anaerobic co-digestion of waste activated sludge and aquaculture sludge. Aquac. Eng. 2020, 90, 1–6. [Google Scholar] [CrossRef]
- Delaide, B.; Monsees, H.; Gross, A.; Goddek, S. Aerobic and anaerobic treatments for aquaponic sludge reduction and mineralisation. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 247–266. [Google Scholar] [CrossRef] [Green Version]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Woo-Lee, J.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Monsees, H.; Keitel, J.; Paul, M.; Kloas, W.; Wuertz, S. Potential of aquacultural sludge treatment for aquaponics: Evaluation of nutrient mobilization under aerobic and anaerobic conditions. Aquacult. Environ. Interact. 2017, 9, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Wu, X.; Li, L.; Lv, Z.; Zhang, Z.; Hao, A.; Iseri, Y.; Kuba, T.; Zhang, X.; Wu, W.M.; et al. Pollution control and in situ bioremediation for lake aquaculture using an ecological dam. J. Clean. Prod. 2018, 172, 2256–2265. [Google Scholar] [CrossRef]
- Godoy-Olmos, S.; Jauralde, I.; Monge-Ortiz, R.; Milián-Sorribes, M.C.; Jover-Cerdá, M.; Tomás-Vidal, A.; Martínez-Llorens, S. Influence of diet and feeding strategy on the performance of nitrifying trickling filter, oxygen consumption and ammonia excretion of gilthead sea bream (Sparus aurata) raised in recirculating aquaculture systems. Aquac. Int. 2022, 30, 581–606. [Google Scholar] [CrossRef]
- Resende, L.; Flores, J.; Moreira, C.; Pacheco, D.; Baeta, A.; Garcia, A.C.; Rocha, A.C.S. Effective and low-maintenance IMTA system as effluent treatment unit for promoting sustainability in coastal aquaculture. Appl. Sci. 2022, 12, 398. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.; Gomes, C.R.; Almeida, C.M.R.; Silva, A.M.T. Constructed wetland microcosms for the removal of organic micropollutants from freshwater aquaculture effluents. Sci. Total Environ. 2018, 644, 1171–1180. [Google Scholar] [CrossRef]
- Ouyang, X.; Guo, F. Paradigms of mangroves in treatment of anthropogenic wastewater pollution. Sci. Total Environ. 2016, 544, 971–979. [Google Scholar] [CrossRef]
- Daneshvar, E.; Antikainen, L.; Koutra, E.; Kornaros, M.; Bhatnagar, A. Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: Treatment of wastewater and lipid extraction. Bioresour. Technol. 2018, 255, 104–110. [Google Scholar] [CrossRef]
- Beyer, C.P.; Gómez, S.; Lara, G.; Monsalve, J.P.; Orellana, J.; Hurtado, C.F. Sarcocornia neei: A novel halophyte species for bioremediation of marine aquaculture wastewater and production diversification in integrated systems. Aquaculture 2021, 543, 736971. [Google Scholar] [CrossRef]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic biofilms in freshwater and interactions with macrophytes: Current understanding and future directions. Aquat. Bot. 2022, 176, 103467. [Google Scholar] [CrossRef]
- Hua, Z.L.; Li, X.Q.; Zhang, J.Y.; Gu, L. Removal potential of multiple perfluoroalkyl acids (PFAAs) by submerged macrophytes in aquatic environments: Tolerance of Vallisneria natans and PFAA removal in submerged macrophyte-microbiota systems. J. Hazard. Mater. 2022, 424, 127695. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Doeser, A.; Cao, Y.; Lv, X.; Li, W.; Liu, F. Regional macrophyte diversity is shaped by accumulative effects across waterbody types in southern China. Aquat. Bot. 2022, 176, 103468. [Google Scholar] [CrossRef]
- Barnharst, T.; Rajendran, A.; Hu, B. Bioremediation of synthetic intensive aquaculture wastewater by a novel feed-grade composite biofilm. Int. Biodeterior. Biodegrad. 2018, 126, 131–142. [Google Scholar] [CrossRef]
- Musyoka, S.N. Concept of microbial bioremediation in aquaculture wastes—Review. Int. J. Adv. Sci. Tech. Res. 2016, 5, 1–10. [Google Scholar]
- Dong, D.; Sun, H.; Qi, Z.; Liu, X. Improving microbial bioremediation efficiency of intensive aquacultural wastewater based on bacterial pollutant metabolism kinetics analysis. Chemosphere 2021, 265, 129151. [Google Scholar] [CrossRef]
- Irhayyim, T.; Beliczky, G.; Bercsényi, M. Nutrient bioremediation efficiency of bacterial biofilms and plant based biofilters in a recirculating common carp (Cyprinus carpio L.) culture system. Iran. J. Fish. Sci. 2021, 20, 828–845. [Google Scholar]
- Zadinelo, I.V.; dos Santos, L.D.; Cagol, L.; de Muniz, G.I.B.; Ellendersen, L.D.S.N.; Alves, H.J.; Bombardelli, R.A. Adsorption of aquaculture pollutants using a sustainable biopolymer. Environ. Sci. Pollut. Res. 2018, 25, 4361–4370. [Google Scholar] [CrossRef]
- Bernardi, F.; Zadinelo, I.V.; Alves, H.J.; Meurer, F.; dos Santos, L.D. Chitins and chitosans for the removal of total ammonia of aquaculture effluents. Aquaculture 2018, 483, 203–212. [Google Scholar] [CrossRef]
- Calderini, M.L.; Stevčić, Č.; Taipale, S.; Pulkkinen, K. Filtration of Nordic recirculating aquaculture system wastewater: Effects on microalgal growth, nutrient removal, and nutritional value. Algal Res. 2021, 60, 102486. [Google Scholar] [CrossRef]
- Kafil, M.; Berninger, F.; Koutra, E.; Kornaros, M. Utilization of the microalga Scenedesmus quadricauda for hexavalent chromium bioremediation and biodiesel production. Bioresour. Technol. 2022, 346, 126665. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Gouveia, L.; Gonçalves, M. Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy. J. Environ. Manag. 2021, 286, 112–187. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae bioremediation: A perspective towards wastewater treatment along with industrial carotenoids production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
- Andreotti, V.; Chindris, A.; Brundu, G.; Vallainc, D.; Francavilla, M.; García, J. Bioremediation of aquaculture wastewater from Mugil cephalus (Linnaeus, 1758) with different microalgae species. Chem. Ecol. 2017, 33, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Tossavainen, M.; Lahti, K.; Edelmann, M.; Eskola, R.; Lampi, A.M.; Piironen, V.; Korvonen, P.; Ojala, A.; Romantschuk, M. Integrated utilization of microalgae cultured in aquaculture wastewater: Wastewater treatment and production of valuable fatty acids and tocopherols. J. Appl. Phycol. 2019, 31, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, K.; Ahmad, M.F.; Yaacob, N.S.; Ikram, W.M.; Maniyam, M.N.; Abdullah, H.; Katayama, T.; Komatsu, K.; Kuwahara, V.S. Enhancement of targeted microalgae species growth using aquaculture sludge extracts. Heliyon 2020, 6, 45–56. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Sahu, D.; Rath, B. Microalgal bioremediation: Current practices and perspectives. J. Biochem. Tech. 2012, 3, 299–304. [Google Scholar]
- Amosu, A.O.; Robertson-Andersson, D.V.; Kean, E.; Maneveldt, G.W.; Cyster, L. Biofiltering and uptake of dissolved nutrients by Ulva armoricana (Chlorophyta) in a land-based aquaculture system. Int. J. Agric. Biol. 2016, 18, 298–304. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, C.; Gao, T.; Peng, X.; Ma, S.; Sun, Q.; Xia, B.; Xie, X.; Bai, Z.; Xu, S.; et al. Improvement of fish production and water quality in a recirculating aquaculture pond enhanced with bacteria-microalgae association. Aquaculture 2022, 547, 737420. [Google Scholar] [CrossRef]
- Jasmin, M.Y.; Syukri, F.; Kamarudin, M.S.; Karim, M. Potential of bioremediation in treating aquaculture sludge: Review article. Aquaculture 2019, 519, 734–905. [Google Scholar] [CrossRef]
- Suhr, K.I.; Letelier-Gordo, C.O.; Lund, I. Anaerobic digestion of solid waste in RAS: Effect of reactor type on the biochemical acidogenic potential (BAP) and assessment of the biochemical methane potential (BMP) by a batch assay. Aquac. Eng. 2015, 65, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.Z.; Ma, N.; Li, P.; Tan, H.X.; Liu, W. Enhancement of anaerobic digestion to treat saline sludge from recirculating aquaculture systems. Sci. World J. 2015, 2015, 479101. [Google Scholar] [CrossRef] [Green Version]
- González-Hermoso, J.P.; Segovia, M. Effect of four different pretreatments in anaerobic digestion and nutrient removal of effluents from a recirculating aquaculture system. Lat. Am. J. Aquat. Res. 2017, 45, 276–292. [Google Scholar] [CrossRef]
- Brod, E.; Oppen, J.; Kristoffersen, A.Ø.; Haraldsen, T.K.; Krogstad, T. Drying or anaerobic digestion of fish sludge: Nitrogen fertilisation effects and logistics. Ambio 2017, 46, 852–864. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tao, Y.; Hu, J.; Liu, G.; Spanjers, H.; van Lier, J.B. Biomethanation and microbial community changes in a digester treating sludge from a brackish aquaculture recirculation system. Bioresour. Technol. 2016, 214, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Khiari, Z.; Kaluthota, S.; Savidov, N. Aerobic bioconversion of aquaculture solid waste into liquid fertilizer: Effects of bioprocess parameters on kinetics of nitrogen mineralization. Aquaculture 2019, 500, 492–499. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Keesman, K.; Jijakli, M.H. A methodology to quantify aerobic and anaerobic sludge digestion performances for nutrient recycling in aquaponics. Biotechnol. Agron. Soc. Environ. 2018, 22, 106–112. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.P.L.; Joyce, A.; Wuertz, S.; Jijakli, M.H.; Gross, A.; Eding, E.H.; Bläser, I.; Reuter, M.; Keizer, L.C.P.; et al. Nutrient mineralization and organic matter reduction performance of RAS-based sludge in sequential UASB-EGSB reactors. Aquac. Eng. 2018, 83, 10–19. [Google Scholar] [CrossRef]
- Greenfeld, A.; Becker, N.; Bornman, J.F.; Spatari, S.; Angel, D.L. Monetizing environmental impact of integrated aquaponic farming compared to separate systems. Sci. Total Environ. 2021, 792, 59–84. [Google Scholar] [CrossRef] [PubMed]
- Goddek, S.; Espinal, C.A.; Delaide, B.; Jijakli, M.H.; Schmautz, Z.; Wuertz, S.; Keesman, K.J. Navigating towards decoupled aquaponic systems: A system dynamics design approach. Water 2016, 8, 303. [Google Scholar] [CrossRef]
- Lennard, W.; Goddek, S. Aquaponics: The basics. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 1, pp. 113–144. [Google Scholar] [CrossRef] [Green Version]
- Abusin, S.A.A.; Mandikiana, B.W. Towards sustainable food production systems in Qatar: Assessment of the viability of aquaponics. Glob. Food Sec. 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Goddek, S.; Körner, O. A fully integrated simulation model of multi-loop aquaponics: A case study for system sizing in different environments. Agric. Syst. 2019, 171, 143–154. [Google Scholar] [CrossRef]
- Goddek, S.; Keesman, K.J. The necessity of desalination technology for designing and sizing multi-loop aquaponics systems. Desalination 2018, 428, 76–85. [Google Scholar] [CrossRef]
- Van-Gorcum, B.; Goddek, S.; Keesman, K.J. Gaining market insights for aquaponically produced vegetables in Kenya. Aquac. Inter. 2019, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Love, D.C.; Uhl, M.S.; Genello, L. Energy and water use of a small-scale raft aquaponics system in Baltimore, Maryland, United States. Aquacult. Eng. 2015, 68, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Yogev, U.; Barnes, A.; Gross, A. Nutrients and energy balance analysis for a conceptual model of three loops off grid, aquaponics. Water 2016, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Schiebe, G.; Schmidt, U. Advanced Aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled systems on trial: Eliminating bottlenecks to improve aquaponic processes. PLoS ONE 2017, 12, e0183056. [Google Scholar] [CrossRef] [Green Version]
- Goddek, S.; Vermeulen, T. Comparison of Lactuca sativa growth performance in conventional and RAS-based hydroponic systems. Aquac. Int. 2018, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lennard, W.; Ward, J. A comparison of plant growth rates between an NFT hydroponic system and an NFT aquaponic System. Horticulturae 2019, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Kim, H.J. Comparisons of nitrogen and phosphorus mass balance for tomato-, basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122–135. [Google Scholar] [CrossRef]
- Lenz, G.L.; Loss, A.; Lourenzi, C.R.; Lopes, D.L.D.A.; Siebeneichler, L.D.M.; Brunetto, G. Common chicory production in aquaponics and in soil fertilized with aquaponic sludge. Sci. Hortic. 2021, 281, 109–146. [Google Scholar] [CrossRef]
- Barrett, L.T.; Theuerkauf, S.J.; Rose, J.M.; Alleway, H.K.; Bricker, S.B.; Parker, M.; Petrolia, D.R.; Jones, R.C. Sustainable growth of non-fed aquaculture can generate valuable ecosystem benefits. Ecosyst. Serv. 2022, 53, 101396. [Google Scholar] [CrossRef]
| Aquaculture System | Characteristics | Species Production | Waste Production | Reference |
|---|---|---|---|---|
| Flow | This system has rectangular canals with an outlet drop at the end of the structure allowing elevating O concentration and releasing CO2. The flow or canal system use run-off waters coming from rivers or springs. |
| The water is not retained the sufficient time for significant OM biological decomposition processes to develop, thus continuous waste produced is discharged to the environment. | [12] |
| Pond | This system is made up of artificial structures covered with high-density plastic to retain water for long periods of time, water quality is controlled by natural, chemical, and biological processes that occur in ponds. A constant water source is necessary to guarantee sufficient capacity to achieve a daily recharge of at least 10% of total pond volume to allow eliminating NH4+ and OM excess. |
| Around 80 to 90% of dry matter and C, as well as 70 to 80% of N and P end up as waste. From 1 to 100 kg/ha of daily feed rate, approximately 350 mg/m2/day is excreted by fish as waste. | [13,14,15] |
| Recirculating aquaculture system (RAS) | This system consists of intensive fish production that uses water treatments to facilitate recycling. RAS generally include: (1) Settlers and micro-screens for collecting sediment and suspended particles, (2) Nitrifying biofilters and (3) Gas exchange devices to eliminate dissolved CO2 and add the O. |
| RAS consume a small quantity of water (only 5% per day to compensate for the loss caused by evaporation, solid elimination, and plant absorption) and generate pollutants of small volume but with a high nutrient concentrate. | [16,17,18] |
| Open-net pen or net cage | This system basically represents “fencing” a portion of water. Net cages are systems that retain farmed species in a confined area, excluding unwanted animals from the surrounding water body, this system depends on the water course where this type of system is located, in which the number of pollutants dumped in the environment cannot be controlled. |
| Sites with bad circulation imply low DO concentration conditions, and the accumulation of metabolic waste promotes algal growth and many other benthic organisms that adhere and colonize around the cage, reducing water movement through the cage severely and deteriorating water quality. | [19] |
| Floating and bottom | This system uses similar principles to those of open–net pen or cage-net systems, which is why they also depend on water movement as well as its natural quality to supply the necessary nutrients and conditions for the development of farming bivalves. | This system is those destined for bivalve mollusk production (oysters, mussels, clams, and scallops) | Likewise, they cannot also control the number of pollutants dumped in the environment | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiquito-Contreras, R.G.; Hernandez-Adame, L.; Alvarado-Castillo, G.; Martínez-Hernández, M.d.J.; Sánchez-Viveros, G.; Chiquito-Contreras, C.J.; Hernandez-Montiel, L.G. Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review. Sustainability 2022, 14, 7257. https://doi.org/10.3390/su14127257
Chiquito-Contreras RG, Hernandez-Adame L, Alvarado-Castillo G, Martínez-Hernández MdJ, Sánchez-Viveros G, Chiquito-Contreras CJ, Hernandez-Montiel LG. Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review. Sustainability. 2022; 14(12):7257. https://doi.org/10.3390/su14127257
Chicago/Turabian StyleChiquito-Contreras, Roberto G., Luis Hernandez-Adame, Gerardo Alvarado-Castillo, María de J. Martínez-Hernández, Gabriela Sánchez-Viveros, César J. Chiquito-Contreras, and Luis G. Hernandez-Montiel. 2022. "Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review" Sustainability 14, no. 12: 7257. https://doi.org/10.3390/su14127257
APA StyleChiquito-Contreras, R. G., Hernandez-Adame, L., Alvarado-Castillo, G., Martínez-Hernández, M. d. J., Sánchez-Viveros, G., Chiquito-Contreras, C. J., & Hernandez-Montiel, L. G. (2022). Aquaculture—Production System and Waste Management for Agriculture Fertilization—A Review. Sustainability, 14(12), 7257. https://doi.org/10.3390/su14127257






