Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Samples and Reagent Drugs
2.2. Study Area and Sample Collection
2.3. Antibiotic Determination
2.4. Determination of Antibiotic Resistance Genes
2.4.1. DNA Extraction
2.4.2. PCR Amplification Experiment
2.4.3. qPCR Quantitative Experiment
2.5. Determination of Water Quality Parameter
2.6. Ecological Risk Assessment of Antibiotics
2.7. Data Analysis Method
3. Results and Discussion
3.1. Distribution Characteristics of Antibiotics in the Water Environment
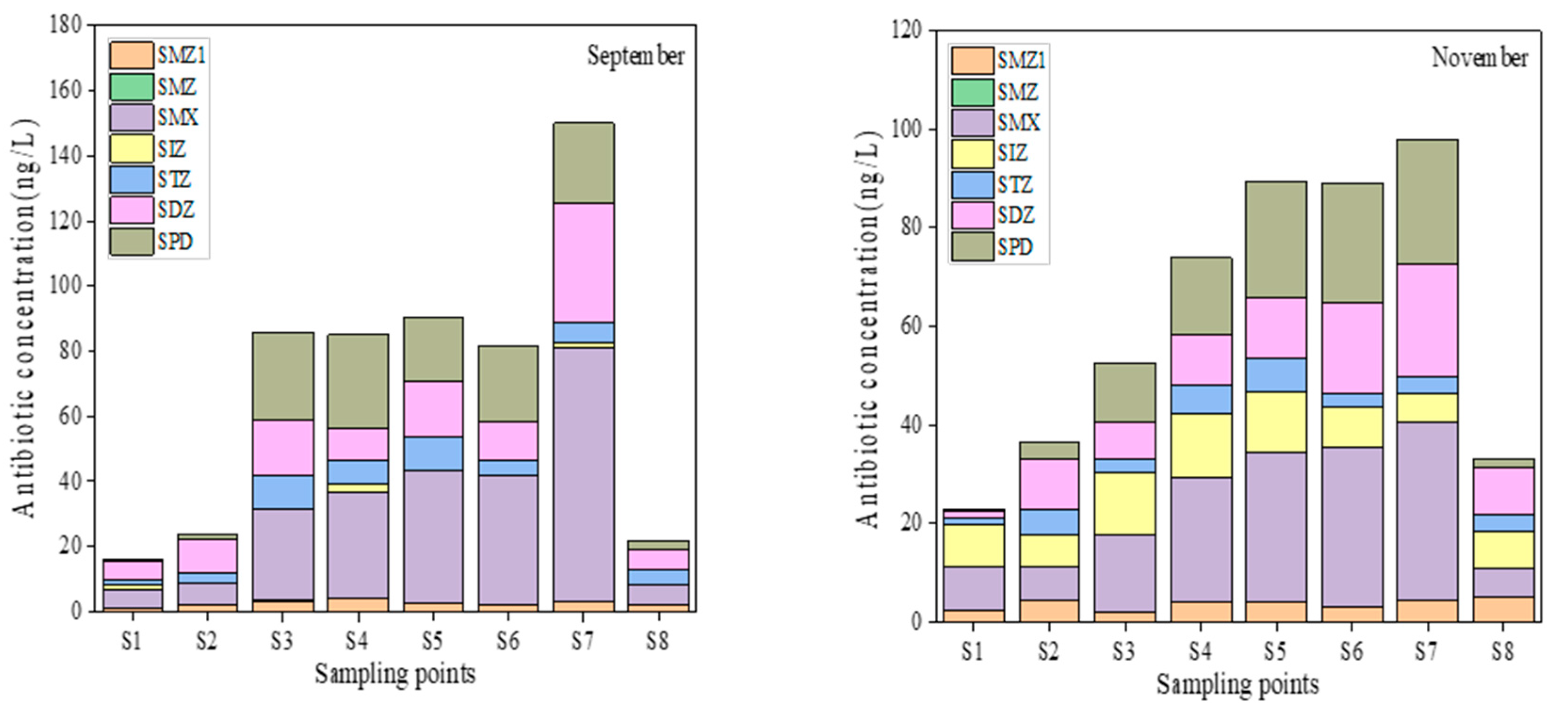
3.1.1. Distribution of Antibiotics in Surface Water
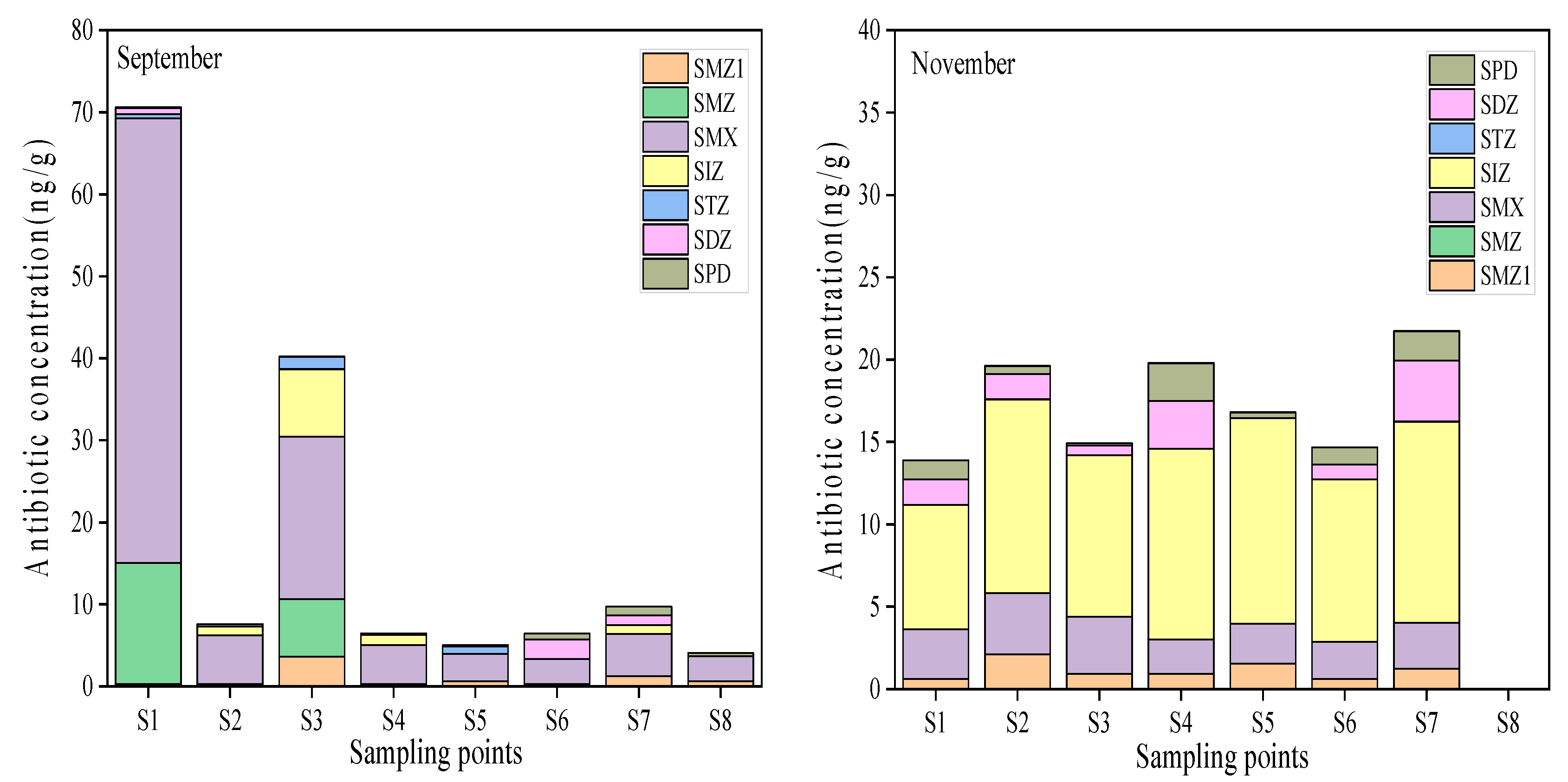
3.1.2. Distribution of Antibiotics in Surface Sediments
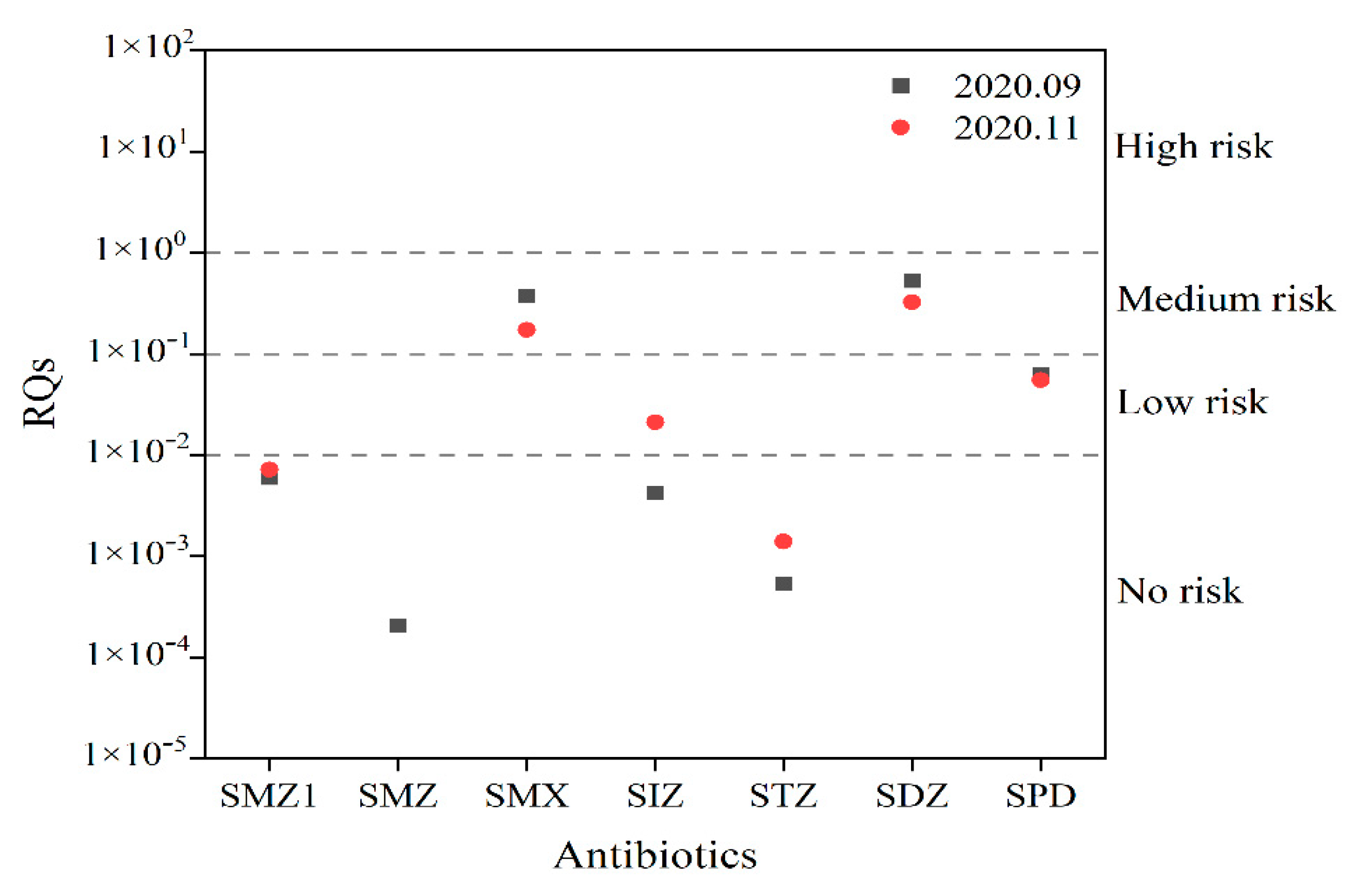
3.1.3. Ecological Risk Assessment
3.2. Distribution Characteristics of Resistance Genes in the Water Environment
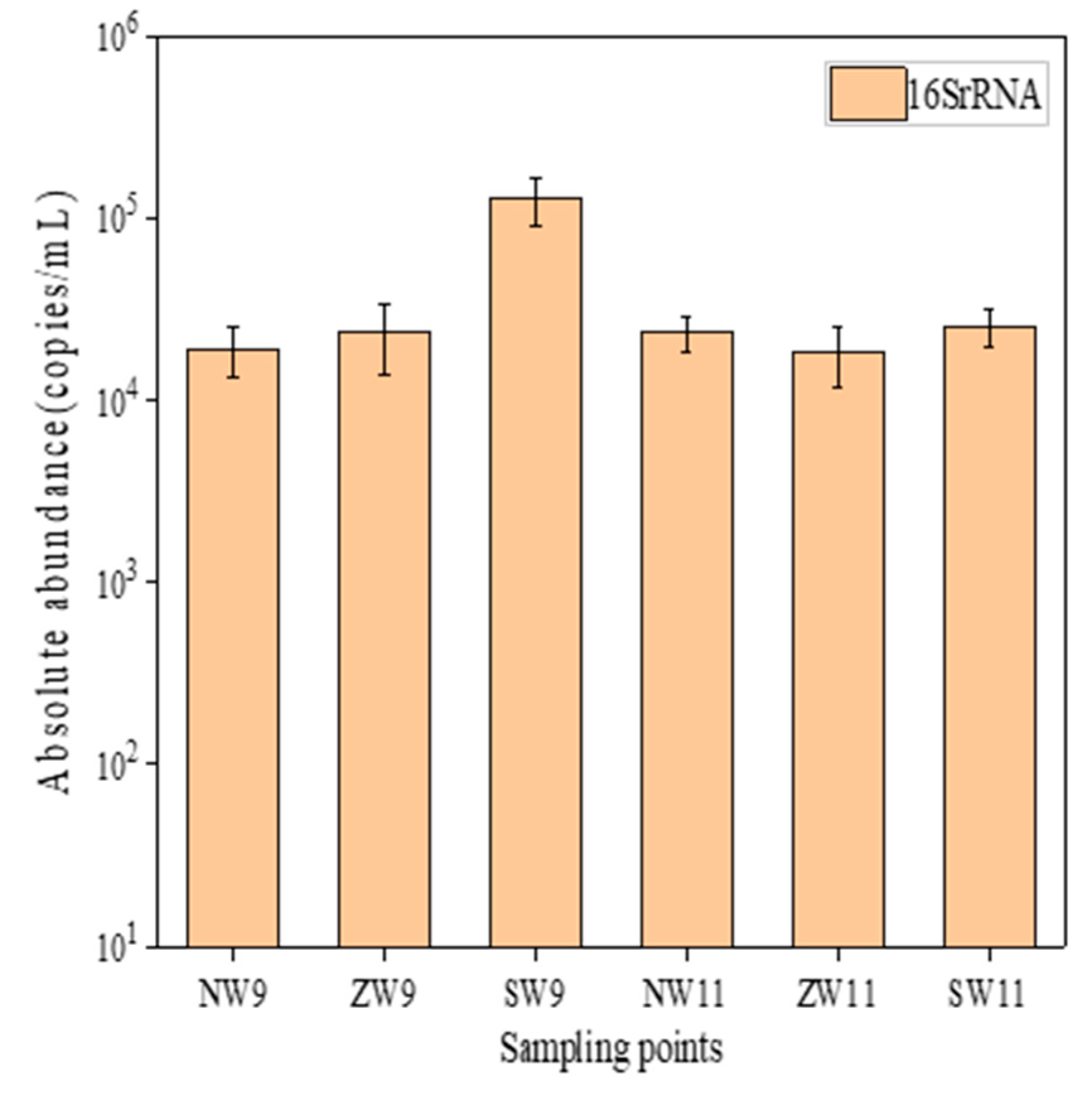
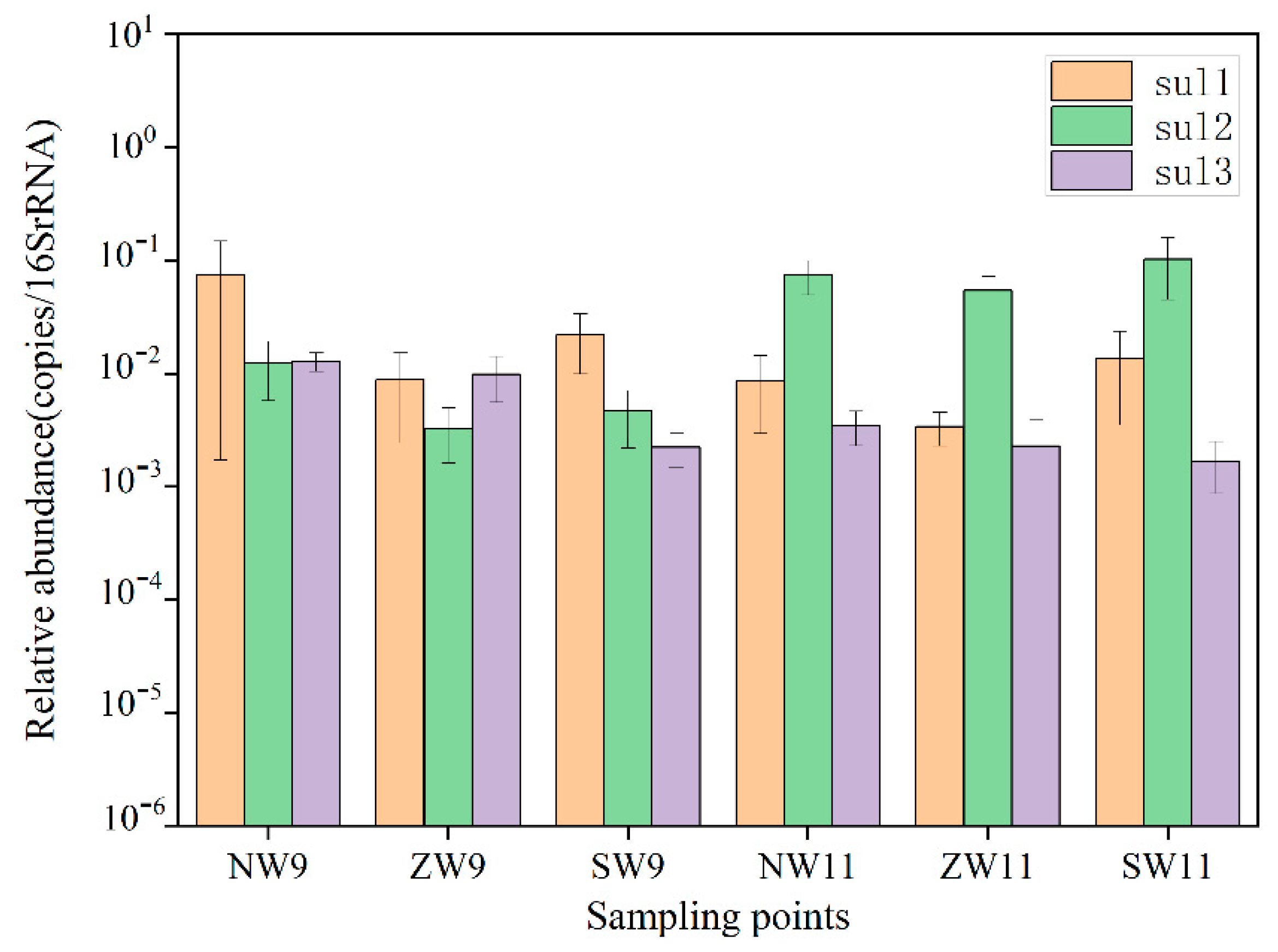
3.2.1. Distribution of Resistance Genes in Surface Water
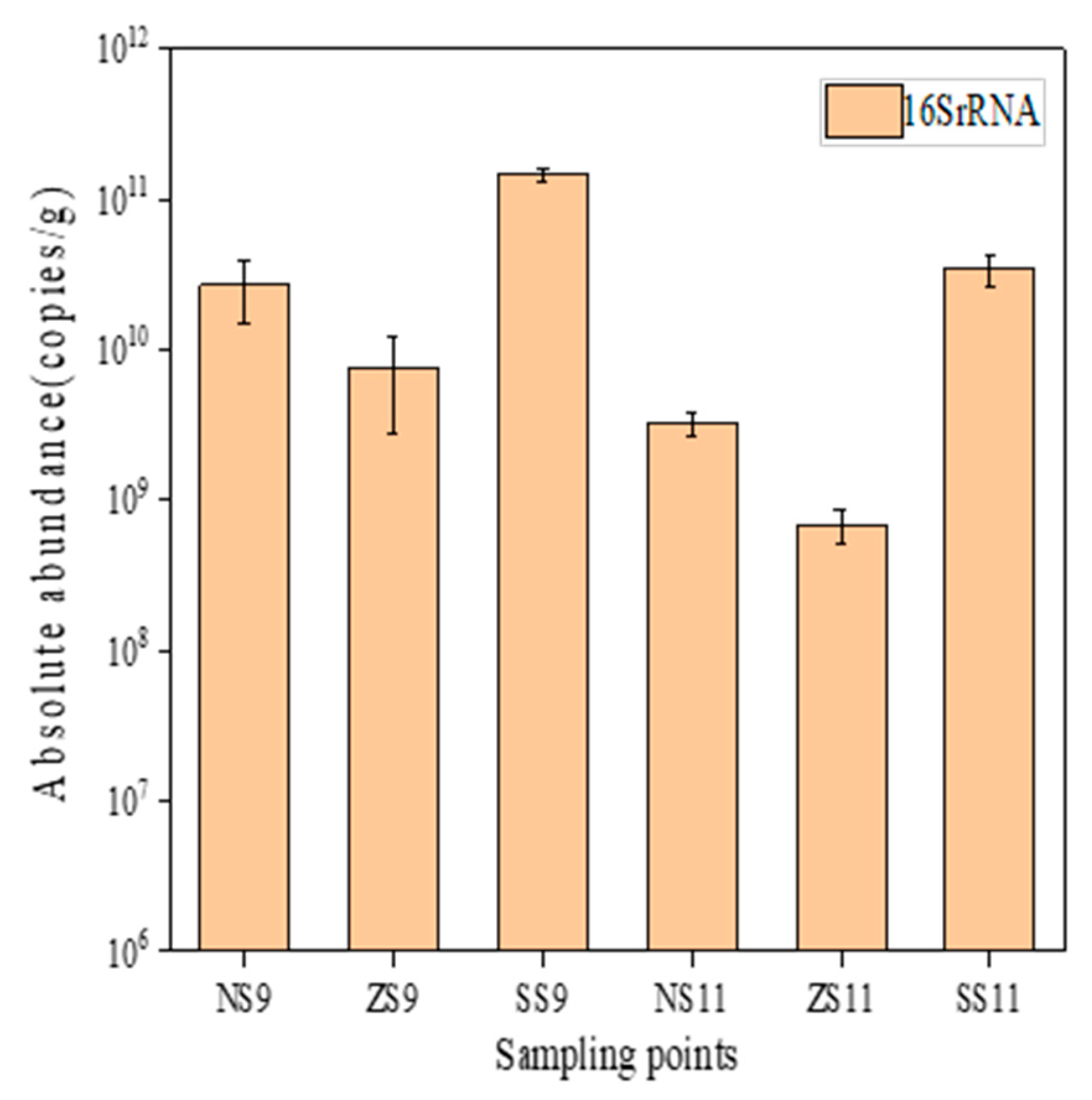
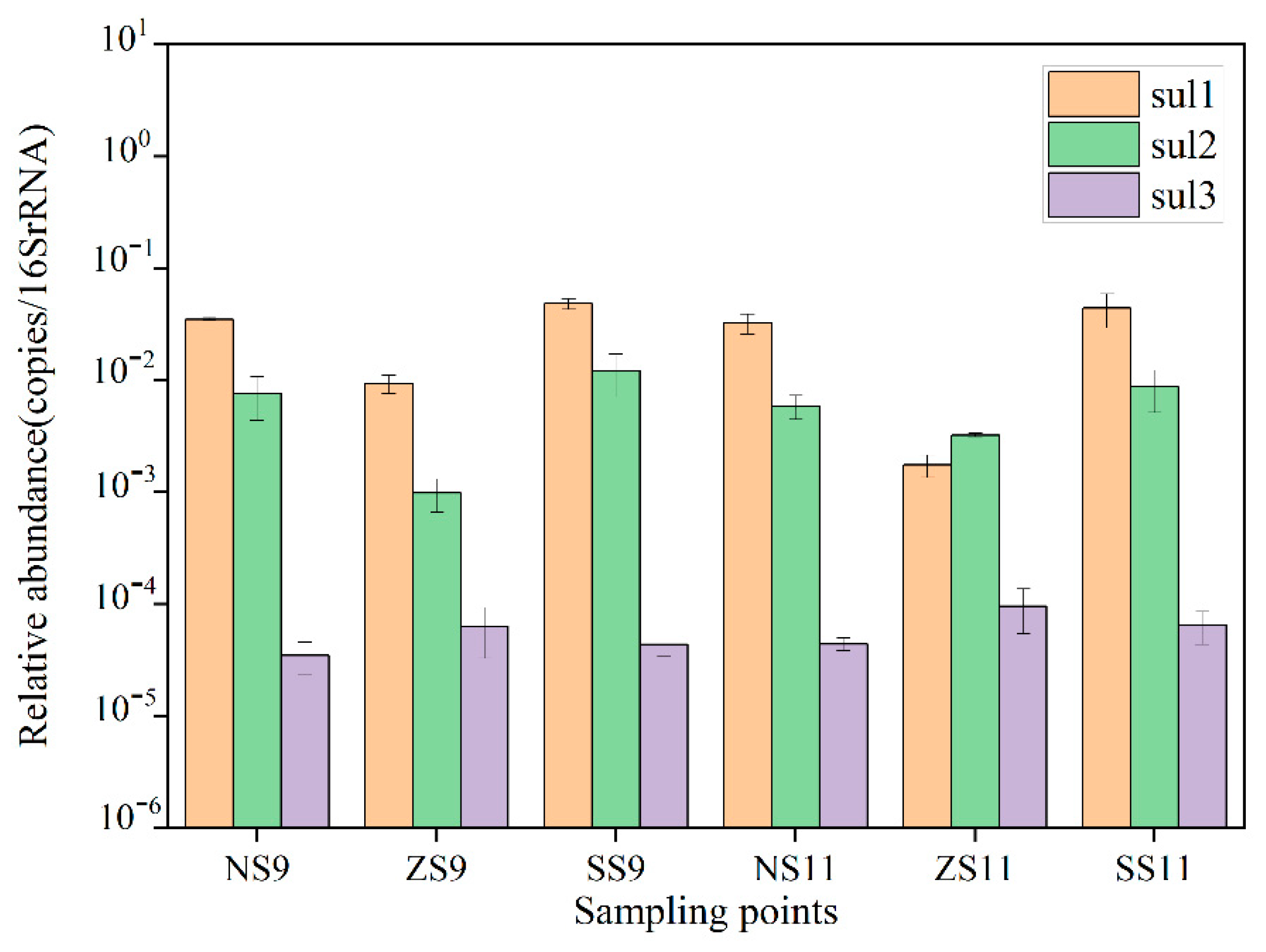
3.2.2. Distribution of Resistance Genes in Surface Sediments
3.3. Correlation Analysis between Antibiotic and Resistance Gene
3.4. Correlation between Antibiotic Resistance Genes and Water Quality Parameter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, W.G.; Sun, Y.X.; Zhang, T.; Ding, X.Y.; Li, Y.F.; Wang, M.Z.; Zeng, Z.L. Antibiotics, Antibiotic Resistance Genes, and Bacterial Community Composition in Fresh Water Aquaculture Environment in China. Environ. Microbiol. 2015, 70, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Duan, Y.J.; Wang, S.P.; Wang, L.T.; Hou, Z.L.; Cui, Y.X.; Hou, J.; Das, R.; Mao, D.Q.; Luo, Y. Occurrence and distribution of clinical and veterinary antibiotics in the faeces of a Chinese population. J. Hazard. Mater. 2020, 383, 121129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.R.; Liu, S.S.; Zhou, G.J.; Sun, K.F.; Zhao, J.L.; Ying, G.G. Antibiotics in typical marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioaccumulation and human dietary exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vazquez, F.M.; Delerue-Matos, C. Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere 2020, 239, 124729. [Google Scholar] [CrossRef]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Liu, J.M.; Cai, Y.Q. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef]
- Liang, X.M.; Chen, B.W.; Nie, X.P.; Shi, Z.; Huang, X.P.; Li, X.D. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, G.D.; Liu, Y.; Lu, S.Y.; Qin, P.; Guo, X.C.; Bi, B.; Wang, L.; Xi, B.D.; Wu, F.C.; et al. Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing, China. Environ. Pollut. 2019, 246, 163–173. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.Q.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, D.Q.; Rysz, M.; Zhou, Q.X.; Zhang, H.J.; Xu, L.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sanchez-Melsio, A.; Borrego, C.M.; Barcelo, D.; Balcazar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Yang, J.F.; Ying, G.G.; Zhao, J.L.; Tao, R.; Su, H.C.; Liu, Y.S. Spatial and seasonal distribution of selected antibiotics in surface waters of the Pearl Rivers, China. J. Environ. Sci. Health B 2011, 46, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Younesa, H.A.; Mahmoud, H.M.; Abdelrahman, M.M.; Nassar, H.F. Seasonal occurrence, removal efficiency and associated ecological risk assessment of three antibiotics in a municipal wastewater treatment plant in Egypt. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100239. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.Y.; Xu, J.; Kong, X.J.; Gao, S.X.; Shan, Z.J. Pollution characteristics and environmental risk assessment of typical veterinary antibiotics in livestock farms in Southeastern China. J. Environ. Sci. Health B 2014, 49, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.C.; Zhu, C.J.; He, Z.M.; Luan, T.G.; Xu, W.H.; Zhang, G. Preliminary study on the contamination of antibiotic resistance genes in the water of the Beijiang River. Asian J. Ecotox. 2009, 4, 655–660. [Google Scholar]
- Fang, P.; Dai, H.F.; Zhuang, X.; Xie, H.Q.; Luo, W.K.; Ren, M.Z.; Zheng, J. Study on the distribution of antibiotic resistance genes in typical drinking water sources in the lower reaches of Dongjiang River. Asian J. Ecotox. 2019, 28, 548–554. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China. Method of Water and Wastewater Monitoring, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 222–231. (In Chinese)
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.B.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Garnier-Laplace, J.; Copplestone, D.; Gilbin, R.; Alonzo, F.; Ciffroy, P.; Gilek, M.; Agüero, A.; Björk, M.; Oughton, D.H.; Jaworska, A.; et al. Issues and practices in the use of effects data from FREDERICA in the ERICA Integrated Approach. J. Environ. Radioact. 2008, 99, 1474–1483. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Zhang, L.; Fan, W.Y.; Yang, C.; Li, E.H.; Du, Y.; Wang, X.L. Inconsistent seasonal variation of antibiotics between surface water and groundwater in the Jianghan Plain: Risks and linkage to land uses. J. Environ. Sci. 2021, 109, 102–113. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Q.M.; Wan, J.Q.; Yang, S.; Wang, Y.; Fan, H.M. Occurrence and risk assessment of antibiotics in multifunctional reservoirs in Dongguan, China. Environ. Sci. Pollut. Res. 2020, 27, 13565–13574. [Google Scholar] [CrossRef]
- Park, S.; Choi, K. Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 2008, 17, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Białk-Bielinska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity evaluation of selected sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Cao, X.H.; Lin, H.; Wang, J. Antibiotics and Antibiotic Resistance Genes in Sediment of Honghu Lake and East Dongting Lake, China. Microb. Ecol. 2016, 72, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Xu, C.; Cao, X.H.; Lin, H.; Wang, J. Antibiotic resistance genes in surface water of eutrophic urban lakes are related to heavy metals, antibiotics, lake morphology and anthropic impact. Ecotoxicology 2017, 26, 831–840. [Google Scholar] [CrossRef]
- Dang, B.J.; Mao, D.Q.; Xu, Y.; Luo, Y. Conjugative multi-resistant plasmids in Haihe River and their impacts on the abundance and spatial distribution of antibiotic resistance genes. Water Res. 2017, 111, 81–91. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Liu, W.Z.; Xu, C.; Wei, B.Q.; Wang, J. Antibiotic resistance genes in lakes from middle and lower reaches of the Yangtze River, China: Effect of land use and sediment characteristics. Chemosphere 2017, 178, 19–25. [Google Scholar] [CrossRef]
- Guo, X.P.; Yang, Y.; Lu, D.P.; Niu, Z.S.; Feng, J.N.; Chen, Y.R.; Tou, F.Y.; Garner, E.; Xu, J.; Liu, M.; et al. Biofilms as a sink for antibiotic resistance genes (ARGs) in the Yangtze Estuary. Water Res. 2018, 129, 277–286. [Google Scholar] [CrossRef]
- Li, A.L.; Chen, L.J.; Zhang, Y.; Tao, Y.L.; Xie, H.; Li, S.; Sun, W.L.; Pan, J.G.; He, Z.D.; Mai, C.A.; et al. Occurrence and distribution of antibiotic resistance genes in the sediments of drinking water sources, urban rivers, and coastal areas in Zhuhai, China. Environ. Sci. Pollut. Res. 2018, 25, 26209–26217. [Google Scholar] [CrossRef]
- Yang, S.L.; Wang, X.M.; Wang, W.H.; Hu, X.Y.; Gao, L.W.; Sun, X.B. Distribution and Ecological Risk Assessment of Antibiotics in the Songhua River Basin of the Harbin Section and Ashe River. Environ. Sci. 2021, 42, 136–146. (In Chinese) [Google Scholar]

| ARGs | Primer | Primer Sequence (5′ to 3′) [14,15] | Primer Length | Annealing Temperature |
|---|---|---|---|---|
| 16S rRNA | 16s-F | ATGGCTGTCGTCAGCT | 337 | 60 °C |
| 16s-R | ACGGGCGGTGTGTAC | |||
| sul1 | sul1-F | CGCACCGGAAACATCGCTGCAC | 163 | 65 °C |
| sul1-R | TGAAGTTCCGCCGCAAGGCTCG | |||
| sul2 | sul2-F | TCCGGTGGAGGCCGGTATCTGG | 191 | 57.5 °C |
| sul2-R | CGGGAATGCCATCTGCCTTGAG | |||
| sul3 | sul3-F | TCCGTTCAGCGAATTGGTGCAG | 143 | 61 °C |
| sul3-R | TTCGTTCACGCCTTACACCAGC |
| Water Quality Index | Detection Method |
|---|---|
| pH | Portable pH meter |
| WT | Thermometer |
| DO | Portable DO instrument |
| SD | Seidel disk method |
| CODCr | Rapid determination of COD |
| TN | Potassium persulfate oxidation ultraviolet spectrophotometry |
| NH3-N | Nessler reagent spectrophotometry |
| TP | Molybdenum antimony resistance spectrophotometry |
| Chl-a | Ultrasonic extraction |
| Antibiotics | Range (ng/L) | Mean (ng/L) | Freq (%) | |
|---|---|---|---|---|
| September (n = 8, ng/L) | SMZ1 | 1.10–4.03 | 2.50 | 100% |
| SMZ | ND–0.36 | 0.05 | 13% | |
| SMX | 5.50–77.76 | 29.67 | 100% | |
| SIZ | ND–2.64 | 0.73 | 38% | |
| STZ | 1.38–10.32 | 5.89 | 100% | |
| SDZ | 5.78–36.73 | 14.50 | 100% | |
| SPD | 0.69–28.76 | 15.92 | 100% | |
| November (n = 8, ng/L) | SMZ1 | 1.91–4.88 | 3.54 | 100% |
| SMZ | ND–ND | ND | 0% | |
| SMX | 5.98–36.31 | 20.28 | 100% | |
| SIZ | ND–13.16 | 9.27 | 100% | |
| STZ | 1.49–6.78 | 3.90 | 100% | |
| SDZ | 1.36–22.80 | 11.61 | 100% | |
| SPD | 0.34–25.41 | 13.24 | 100% |
| Antibiotics | Range (ng/g) | Mean (ng/g) | Freq (%) | |
|---|---|---|---|---|
| September (n = 8, ng/g) | SMZ1 | 0.31–3.62 | 0.92 | 100% |
| SMZ | ND–14.73 | 2.72 | 25% | |
| SMX | 3.01–54.20 | 12.41 | 100% | |
| SIZ | ND–8.23 | 1.46 | 50% | |
| STZ | ND–1.49 | 0.37 | 38% | |
| SDZ | ND–2.38 | 0.54 | 38% | |
| SPD | 0.04–1.03 | 0.36 | 100% | |
| November (n = 8, ng/g) | SMZ1 | ND–2.12 | 1.00 | 88% |
| SMZ | ND–ND | 0.00 | 0% | |
| SMX | ND–3.70 | 2.46 | 88% | |
| SIZ | ND–12.48 | 9.41 | 88% | |
| STZ | ND–ND | 0.00 | 0% | |
| SDZ | ND–3.71 | 1.39 | 75% | |
| SPD | ND–2.30 | 0.91 | 88% |
| Antibiotics | Species | Toxic Type | Evaluation Factors | EC50 (mg/L) | PNEC (ng/L) |
|---|---|---|---|---|---|
| SMZ1 | Lemna minor | acute | 1000 | 0.68 | 680 |
| SMZ | Lemna minor | acute | 1000 | 1.74 | 1740 |
| SMX | Lemna minor | acute | 1000 | 0.21 | 210 |
| SIZ | Lemna minor | acute | 1000 | 0.62 | 620 |
| STZ | Lemna minor | acute | 1000 | 4.89 | 4890 |
| SDZ | Lemna minor | acute | 1000 | 0.07 | 70 |
| SPD | Lemna minor | acute | 1000 | 0.46 | 460 |
| Sul1 | Sul2 | Sul3 | SMZ1 | SMZ | SMX | SIZ | STZ | SDZ | SPD | |
|---|---|---|---|---|---|---|---|---|---|---|
| sul1 | 1 | |||||||||
| sul2 | −0.401 | 1 | ||||||||
| sul3 | 0.711 | −0.605 | 1 | |||||||
| SMZ1 | 0.037 | 0.333 | −0.35 | 1 | ||||||
| SMZ | 0.972 ** | −0.347 | 0.77 | 0.025 | 1 | |||||
| SMX | −0.046 | −0.501 | −0.237 | 0.196 | −0.241 | 1 | ||||
| SIZ | −0.565 | 0.703 | −0.639 | 0.037 | −0.451 | −0.521 | 1 | |||
| STZ | 0.523 | −0.827 * | 0.836 * | −0.097 | 0.568 | 0.122 | −0.688 | 1 | ||
| SDZ | 0.133 | −0.365 | −0.206 | 0.338 | −0.08 | 0.949 ** | −0.588 | 0.055 | 1 | |
| SPD | 0.497 | −0.217 | 0.151 | 0.799 | 0.434 | 0.427 | −0.52 | 0.397 | 0.578 | 1 |
| Sul1 | Sul2 | Sul3 | SMZ1 | SMZ | SMX | SIZ | STZ | SDZ | SPD | |
|---|---|---|---|---|---|---|---|---|---|---|
| sul1 | 1 | |||||||||
| sul2 | 0.917 * | 1 | ||||||||
| sul3 | −0.587 | −0.58 | 1 | |||||||
| SMZ1 | 0.178 | 0.242 | −0.406 | 1 | ||||||
| SMZ | 0.171 | 0.136 | −0.447 | 0.955 ** | 1 | |||||
| SMX | 0.252 | 0.225 | −0.537 | 0.939 ** | 0.990 ** | 1 | ||||
| SIZ | −0.057 | −0.015 | 0.185 | 0.258 | 0.083 | −0.018 | 1 | |||
| STZ | −0.128 | −0.259 | −0.161 | 0.655 | 0.823 * | 0.811 | −0.3 | 1 | ||
| SDZ | 0.614 | 0.524 | 0.243 | −0.231 | −0.309 | −0.313 | 0.306 | −0.469 | 1 | |
| SPD | 0.562 | 0.582 | 0.29 | −0.26 | −0.385 | −0.37 | 0.177 | −0.535 | 0.947 ** | 1 |
| Index | Min | Max | Mean | |
|---|---|---|---|---|
| September | pH | 7.239 | 8.404 | 7.765 |
| WT (°C) | 11.9 | 19.2 | 15.6 | |
| DO (mg/L) | 6.20 | 12.60 | 9.86 | |
| SD (m) | 0.15 | 1.02 | 0.48 | |
| CODCr (mg/L) | 8.28 | 48.91 | 24.46 | |
| TN (mg/L) | 1.52 | 6.71 | 3.52 | |
| NH3-N (mg/L) | 0.60 | 3.58 | 1.04 | |
| TP (mg/L) | 0.12 | 0.78 | 0.31 | |
| Chl-a (mg/L) | 0.18 | 4.42 | 2.20 | |
| November | pH | 7.791 | 9.116 | 8.125 |
| WT (°C) | 0.0 | 4.0 | 1.8 | |
| DO (mg/L) | 4.41 | 13.16 | 9.38 | |
| SD (m) | 0.10 | 0.75 | 0.34 | |
| CODCr (mg/L) | 24.83 | 74.50 | 46.18 | |
| TN (mg/L) | 0.47 | 2.32 | 0.94 | |
| NH3-N (mg/L) | 0.09 | 0.33 | 0.15 | |
| TP (mg/L) | 0.15 | 0.51 | 0.27 | |
| Chl-a (mg/L) | 0.09 | 0.57 | 0.35 |
| SMZ1 | SMZ | SMX | SIZ | STZ | SDZ | SPD | |
|---|---|---|---|---|---|---|---|
| pH | 0.106 | −0.256 | −0.071 | 0.448 | −0.349 | −0.343 | −0.041 |
| WT (°C) | −0.323 | 0.301 | 0.335 | −0.862 ** | 0.541 * | 0.222 | 0.288 |
| DO (mg/L) | 0.477 | 0.300 | 0.382 | 0.067 | 0.433 | 0.364 | 0.590 * |
| SD (m) | 0.285 | 0.066 | 0.554 * | −0.228 | 0.778 ** | 0.465 | 0.551 * |
| CODCr (mg/L) | 0.418 | −0.188 | 0.391 | 0.596 * | 0.010 | 0.420 | 0.430 |
| TN (mg/L) | −0.061 | 0.313 | 0.717 ** | −0.679 ** | 0.622 * | 0.659 ** | 0.481 |
| NH3-N (mg/L) | −0.103 | 0.049 | 0.697 ** | −0.464 | 0.239 | 0.729 ** | 0.236 |
| TP (mg/L) | 0.045 | 0.045 | 0.707 ** | −0.076 | 0.169 | 0.788 ** | 0.394 |
| Chl-a (mg/L) | −0.165 | −0.003 | 0.765 ** | −0.579 * | 0.532 * | 0.588 * | 0.478 |
| Sul1 | Sul2 | Sul3 | |
|---|---|---|---|
| pH | −0.524 | 0.827 * | −0.920 ** |
| WT (℃) | 0.551 | −0.950 ** | 0.734 |
| DO (mg/L) | 0.394 | −0.021 | −0.176 |
| SD (m) | −0.262 | −0.553 | 0.250 |
| CODCr (mg/L) | −0.684 | 0.869 * | −0.870 * |
| TN (mg/L) | 0.430 | −0.850 * | 0.403 |
| NH3-N (mg/L) | 0.145 | −0.594 | −0.123 |
| TP (mg/L) | −0.026 | −0.129 | −0.361 |
| Chl-a (mg/L) | −0.015 | −0.784 | 0.228 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Li, C.; Wang, Q.; Lu, H. Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter. Sustainability 2022, 14, 7301. https://doi.org/10.3390/su14127301
Zhao K, Li C, Wang Q, Lu H. Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter. Sustainability. 2022; 14(12):7301. https://doi.org/10.3390/su14127301
Chicago/Turabian StyleZhao, Ke, Chunming Li, Qian Wang, and Hai Lu. 2022. "Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter" Sustainability 14, no. 12: 7301. https://doi.org/10.3390/su14127301
APA StyleZhao, K., Li, C., Wang, Q., & Lu, H. (2022). Distribution of Sulfonamide Antibiotics and Resistance Genes and Their Correlation with Water Quality in Urban Rivers (Changchun City, China) in Autumn and Winter. Sustainability, 14(12), 7301. https://doi.org/10.3390/su14127301





