Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models
Abstract
:1. Introduction
2. Materials and Methods
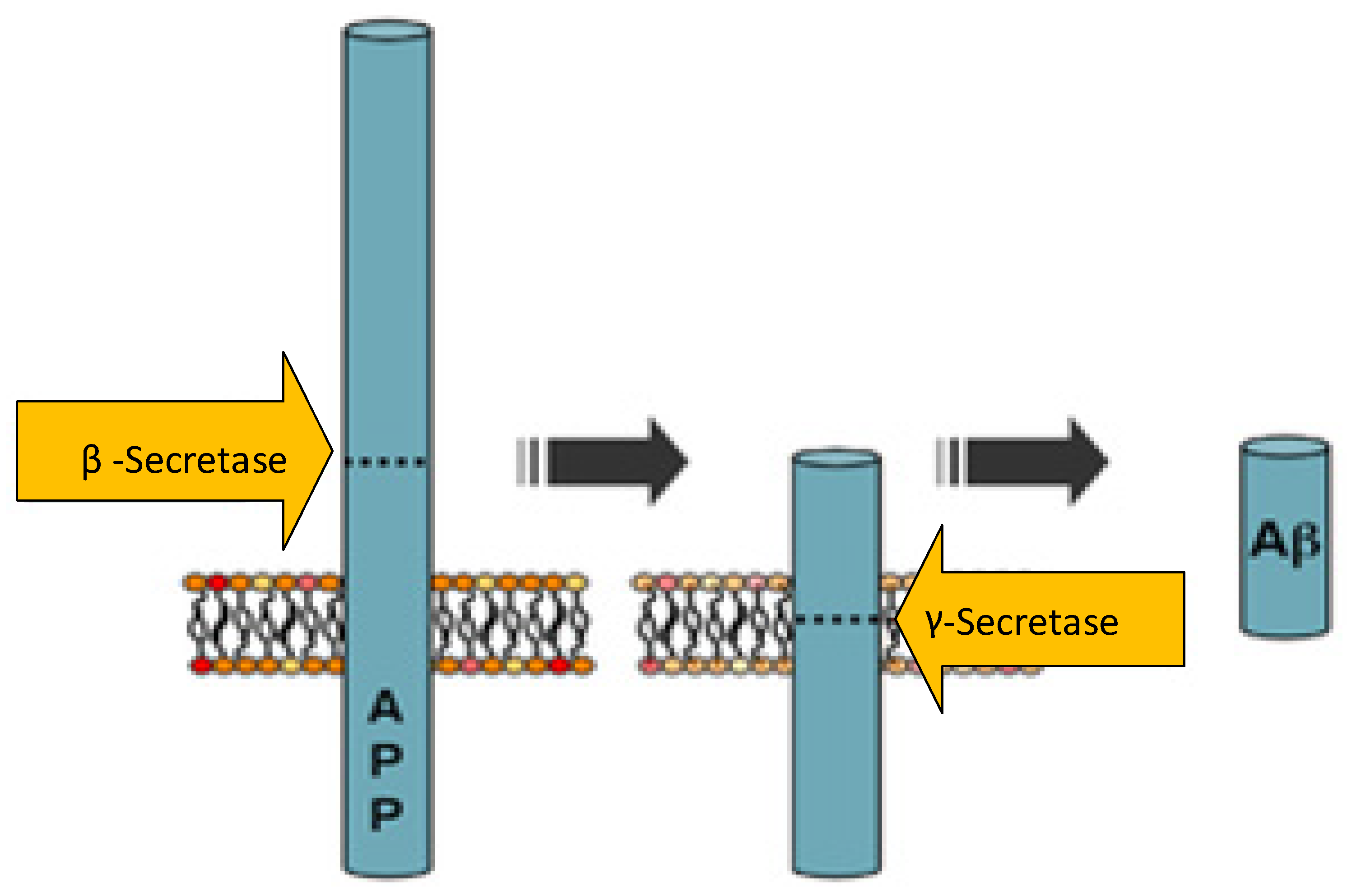
2.1. Molecular Communication and Artificial Neural Network-Based Analysis of Alzheimer’s Disease
2.2. Socioeconomic Status Analysis of Alzheimer’s Disease
2.3. Random Forest Model
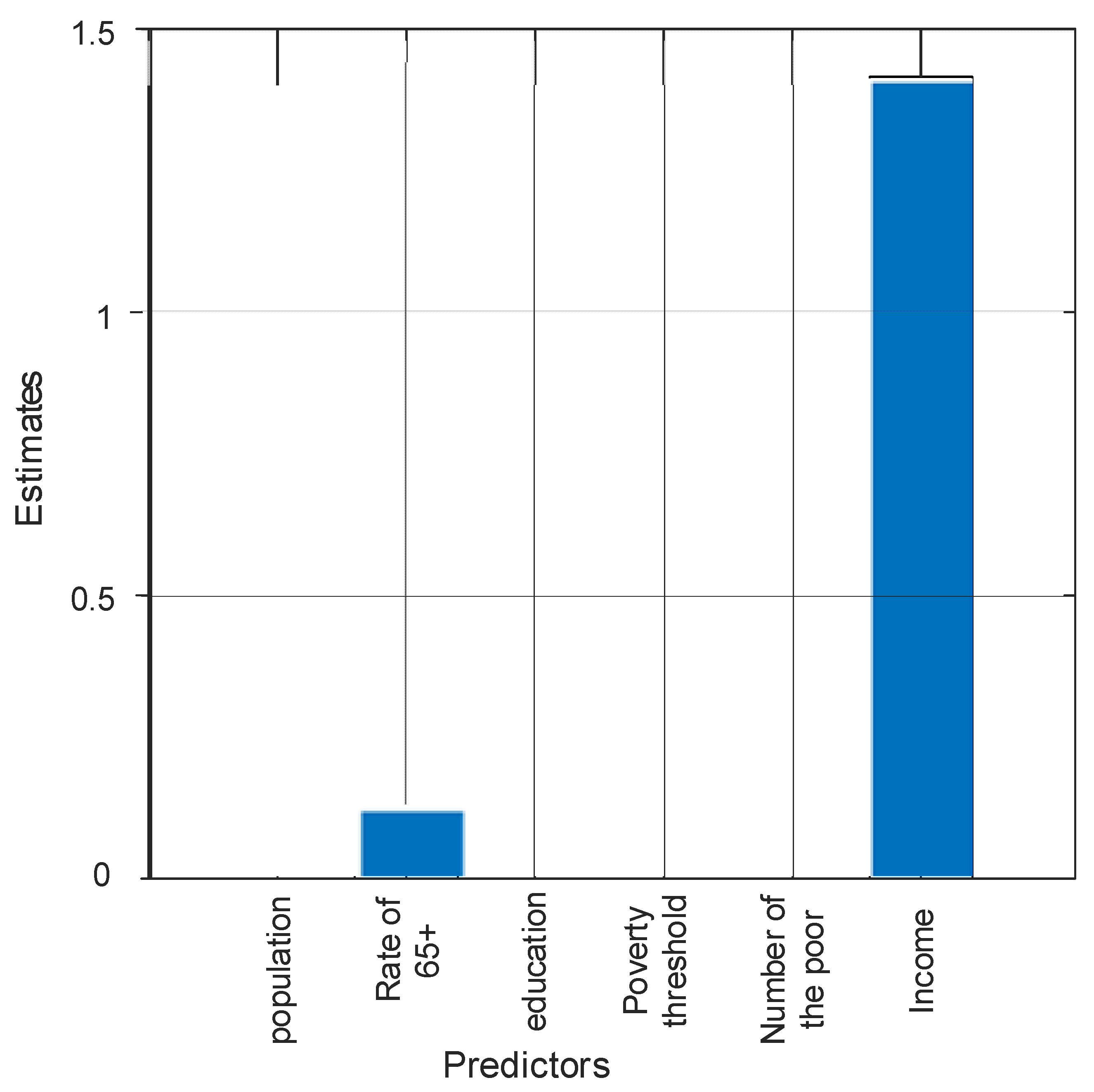
3. Results
4. Conclusions
5. Limitations and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moritani, Y.; Hiyama, S.; Suda, T. Molecular Communication A Biochemically-Engineered Communication System. Proc. Front. Converg. Biosci. Inf. Technol. FBIT 2007, 120, 839–844. [Google Scholar] [CrossRef]
- Barros, M.T.; Silva, W.; Regis, C.D.M. The Multi-Scale Impact of the Alzheimer’s Disease on the Topology Diversity of Astrocytes Molecular Communications Nanonetworks. IEEE Access 2018, 6, 78904–78917. [Google Scholar] [CrossRef]
- Malak, D.; Akan, O.B. Communication theoretical understanding of intra-body nervous nanonetworks. IEEE Commun. Mag. 2014, 52, 129–135. [Google Scholar] [CrossRef]
- Farsad, N.; Eckford, A.W.; Hiyama, S.; Moritani, Y. On-Chip Molecular Communication: Analysis and Design. IEEE Trans. NanoBioscience 2012, 11, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Jonathan Gunn, M.Z. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 8, 284–304. [Google Scholar] [CrossRef] [Green Version]
- Selkoe, D.J.; Yamazaki, T.; Citron, M.; Podlisny, M.B.; Koo, E.H.; Teplow, D.B.; Haass, C. The role of APP processing and trafficking pathways in the formation of amyloid β-protein. Ann. N. Y. Acad. Sci. 1996, 777, 57–64. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Farsad, N.; Yilmaz, H.B.; Eckford, A.; Chae, C.-B.; Guo, W. A Comprehensive Survey of Recent Advancements in Molecular Communication. IEEE Commun. Surv. Tutor. 2016, 18, 1887–1919. [Google Scholar] [CrossRef] [Green Version]
- Pearson, H.A.; Peers, C. Physiological roles for amyloid β peptides. J. Physiol. 2006, 575, 5–10. [Google Scholar] [CrossRef]
- Mordhwaj, S.; Parihar, G.J.B. Amyloid Beta as a Modulator of Synaptic Plasticity. J. Alzheimers Dis. 2010, 22, 741–763. [Google Scholar]
- Wikipedia. Amyloid Precursor Protein Secretase. Available online: http://en.wikipedia.org/wiki/Amyloid_precursor_protein_secretase (accessed on 11 April 2012).
- Gouras, G.K.; Tsai, J.; Naslund, J.; Vincent, B.; Edgar, M.; Checler, F.; Greenfield, J.P.; Haroutunian, V.; Buxbaum, J.D.; Xu, H.; et al. Intraneuronal Aβ42 Accumulation in Human Brain. Am. J. Pathol. 2000, 156, 15–20. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Smith, I.F.; Green, K.N.; LaFerla, F.M. A Dynamic Relationship between Intracellular and Extracellular Pools of Aβ. Am. J. Pathol. 2006, 168, 184–194. [Google Scholar] [CrossRef]
- Ferreiro, E.; Oliveira, C.R.; Pereira, C.M.F. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-β peptide. J. Neurosci. Res. 2004, 76, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, A.; Marchetti, C.; Bianchi, D.; Leinekugel, X.; Poirazi, P.; Migliore, M.; Marie, H. Computational modeling of the effects of amyloid-beta on release probability at hippocampal synapses. Front. Comput. Neurosci. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirrito, J.R.; Yamada, K.A.; Finn, M.B.; Sloviter, R.S.; Bales, K.R.; May, P.C.; Schoepp, D.D.; Paul, S.M.; Mennerick, S.; Holtzman, D.M. Synaptic Activity Regulates Interstitial Fluid Amyloid-β Levels In Vivo. Neuron 2005, 48, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, J.A.; Graves, A.B. Education and other socioeconomic determinants of dementia and alzheimer’ disease. Neurology 1993, 43, S39–S44. [Google Scholar]
- Association, A. 2019 Alzheimer’ s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology. 2022, in press.
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; Di Pellegrino, G. Context-dependent extinction of threat memories: Influences of healthy aging. Sci. Rep. 2018, 8, 12592. [Google Scholar] [CrossRef]
- Tanaka, M.; Török, N.; Vécsei, L. Novel Pharmaceutical Approaches in Dementia. NeuroPsychopharmacotherapy 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Gurland, B.; Tatemichi, T.K.; Tang, M.X.; Wilder, D.; Mayeux, R. Influence of Education and Occupation on the Incidence of Alzheimer’s Disease. JAMA J. Am. Med. Assoc. 1994, 271, 1004–1010. [Google Scholar] [CrossRef]
- Ganguli, M.; Dodge, H.H.; Chen, P.; Belle, S.; DeKosky, S.T. Ten-year incidence of dementia in a rural elderly US community population: The MoVIES Project. Neurology 2000, 54, 1109–1116. [Google Scholar] [CrossRef]
- Qiu, C.; Bäckman, L.; Winblad, B.; Agüero-Torres, H.; Fratiglioni, L. The Influence of Education on Clinically Diagnosed Dementia Incidence and Mortality Data From the Kungsholmen Project. Arch. Neurol. 2001, 58, 2034–2039. [Google Scholar] [CrossRef]
- Ngandu, T.; von Strauss, E.; Helkala, E.L.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Kivipelto, M. Education and dementia: What lies behind the association? Neurology 2007, 69, 1442–1450. [Google Scholar] [CrossRef]
- Katzman, R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology 1993, 43, 13–29. [Google Scholar] [CrossRef]
- Evans, D.A.; Hebert, L.E.; Beckett, L.A.; Scherr, P.A.; Albert, M.S.; Chown, M.J.; Pilgrim, D.M.; Taylor, J.O. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch. Neurol. 1997, 54, 1399–1405. [Google Scholar] [CrossRef]
- Qiu, C.; Karp, A.; von Strauss, E.; Winblad, B.; Fratiglioni, L.; Bellander, T. Lifetime principal occupation and risk of Alzheimer’s disease in the Kungsholmen project. Am. J. Ind. Med. 2003, 43, 204–211. [Google Scholar] [CrossRef]
- Barahona, A.J.; Bursac, Z.; Veledar, E.; Lucchini, R.; Tieu, K.; Richardson, J.R. Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011–2014. Toxics 2022, 10, 191. [Google Scholar] [CrossRef]
- Sini, P.; Dang, T.B.C.; Fais, M.; Galioto, M.; Padedda, B.M.; Lugliè, A.; Iaccarino, C.; Crosio, C. Cyanobacteria, Cyanotoxins, and Neurodegenerative Diseases: Danger. Liaisons Int. J. Mol. Sci. 2021, 22, 8726. [Google Scholar] [CrossRef] [PubMed]
- Carrera-González, M.D.P.; Cantón-Habas, V.; Rich-Ruiz, M. Aging, depression and dementia: The inflammatory process. Adv. Clin. Exp. Med. 2022, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, H.N.; Saadeldin, H.M.; Ezzeldin, A.M.; Tohamy, A.M.; Eltellawy, S.; Bathalath, A.M.; Shehab, M.M. Genetic, clinical, and biochemical aspects of patients with Alzheimer disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 1–9. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Sun, P.; Su, L.; Zhu, H.; Li, X.; Guo, Y.; Du, X.; Zhang, L.; Qin, C. Gut Microbiota Regulation and Their Implication in the Development of Neurodegenerative Disease. Microorganisms 2021, 9, 2281. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12442. [Google Scholar] [CrossRef]
- Orso, B.; Lorenzini, L.; Arnaldi, D.; Girtler, N.; Brugnolo, A.; Doglione, E.; Mattioli, P.; Biassoni, E.; Massa, F.; Peira, E.; et al. The Role of Hub and Spoke Regions in Theory of Mind in Early Alzheimer’s Disease and Frontotemporal Dementia. Biomedicines 2022, 10, 544. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [Green Version]
- Akkaya, A.; Yilmaz, H.B.; Chae, C.-B.; Tugcu, T. Effect of Receptor Density and Size on Signal Reception in Molecular Communication via Diffusion with an Absorbing Receiver. IEEE Commun. Lett. 2014, 19, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Einolghozati, A.; Sardari, M.; Fekri, F. Capacity of diffusion-based molecular communication with ligand receptors. In Proceedings of the 2011 IEEE Information Theory Workshop, Paraty, Brazil, 16–20 October 2011; pp. 85–89. [Google Scholar] [CrossRef] [Green Version]
- Felicetti, L.; Femminella, M.; Reali, G. Directional Receivers for Diffusion-Based Molecular Communications. IEEE Access 2018, 7, 5769–5783. [Google Scholar] [CrossRef]
- Freitas, R.A. Nanomedicine, V. 1. Basic Capabilities; Landes Bioscience: Georgetown, TX, USA, 1999; Volume 1. [Google Scholar]
- Moritani, Y.; Hiyama, S.S.; Suda, T. Molecular communication for health care applications. In Proceedings of the Fourth Annual IEEE International Conference on Pervasive Computing and Communications Workshops (PERCOMW’06), Pisa, Italy, 13–16 March 2006. [Google Scholar] [CrossRef]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H. Computational systems biology. Nature 2002, 420, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Patrick Couvreur, C.V. Nanotechnology: Intelligent Design to Treat Complex Disease. Int. J. Clin. Exp. Pathol. 2006, 23, 3243–3250. [Google Scholar]
- REQUICHA, A.A.G. Nanorobots, NEMS, and Nanoassembly. Proc. IEEE 2003, 91, 1922–1933. [Google Scholar] [CrossRef]
- Işik, I.; Işik, E.; Toktamiş, H. Dose and fading time estimation of glass ceramic by using artificial neural network method. DÜMF Mühendislik Derg. 2020, 12, 47–52. [Google Scholar] [CrossRef]
- Atakan, B.; Akan, O.B.; Balasubramaniam, S. Body area nanonetworks with molecular communications in nanomedicine. IEEE Commun. Mag. 2012, 50, 28–34. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Isik, E. Analyzing of the diffusion constant on the nano-scale systems by using artificial neural networks. AIP Adv. 2021, 11, 105105. [Google Scholar] [CrossRef]
- Işik, İ.; Tağluk, M.E.; Işik, E. Fick difüzyon yasası kullanılarak nano/mikro ölçekli haberleşme sistemlerinde girişim ve molekül alım olasılığı analizi. Gazi Üniversitesi. Mühendislik-Mimar. Fakültesi Derg. 2021, 2, 967–983. [Google Scholar] [CrossRef]
- Isik, I.; Er, M.B.; Isik, E. Analysis and classification of the mobile molecular communication systems with deep learning. J. Ambient Intell. Humaniz. Comput. 2022, 13, 2903–2919. [Google Scholar] [CrossRef]
- Isik, I. How Mobility of Transmitter and Receiver Effect the Communication Quality. AIP Adv. 2017, 12, 025205. [Google Scholar] [CrossRef]
- Moore, M.J.; Suda, T.; Oiwa, K. Molecular Communication: Modeling Noise Effects on Information Rate. IEEE Trans. NanoBioscience 2009, 8, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.B.; Chae, C. Simulation Modelling Practice and Theory Simulation study of molecular communication systems with an absorbing receiver. Simul. Model. Pract. Theory 2014, 49, 136–150. [Google Scholar] [CrossRef]
- Guo, W.; Asyhari, A.T.; Farsad, N.; Yilmaz, H.B.; Li, B.; Eckford, A.; Chae, C.-B. Molecular communications: Channel model and physical layer techniques. IEEE Wirel. Commun. 2016, 23, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, S.; Yang, J.; Nakano, T. A Mathematical Model of Non-Diffusion-Based Mobile Molecular Communication Networks. IEEE Commun. Lett. 2017, 21, 1969–1972. [Google Scholar] [CrossRef]
- Isik, I.; Yilmaz, H.B.; Demirkol, I.; Tagluk, M.E. Effect of receiver shape and volume on the Alzheimer disease for molecular communication via diffusion. IET Nanobiotechnol. 2020, 14, 602–608. [Google Scholar] [CrossRef]
- Ozyilmaz, A.; Bayraktar, Y.; Toprak, M.; Isik, E.; Guloglu, T.; Aydin, S.; Olgun, M.F.; Younis, M. Socio-Economic, Demographic and Health Determinants of the COVID-19 Outbreak. Healthcare 2022, 10, 748. [Google Scholar] [CrossRef]
- Bayraktar, Y.; Özyılmaz, A.; Toprak, M.; Işık, E.; Büyükakın, F.; Olgun, M.F. Role of the Health System in Combating Covid-19: Cross-Section Analysis and Artificial Neural Network Simulation for 124 Country Cases. Soc. Work Public Health 2020, 36, 178–193. [Google Scholar] [CrossRef]
- Boulesteix, A.-L.; Janitza, S.; Kruppa, J.; König, I.R. Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. WIREs Data Min. Knowl. Discov. 2012, 2, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Segal, M.R. Machine Learning Benchmarks and Random Forest Regression Publication Date Machine Learning Benchmarks and Random Forest Regression. Cent. Bioinform. Mol. Biostat. 2004, 15, 1–18. [Google Scholar]
- Auret, L.; Aldrich, C. Empirical comparison of tree ensemble variable importance measures. Chemom. Intell. Lab. Syst. 2011, 105, 157–170. [Google Scholar] [CrossRef]
- Gupta, S.; Matthew, S.; Abreu, P.M.; Aires-De-Sousa, J. QSAR analysis of phenolic antioxidants using MOLMAP descriptors of local properties. Bioorganic Med. Chem. 2006, 14, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Tuik. Data Portal for Statistics. Available online: https://data.tuik.gov.tr (accessed on 11 May 2022).
- Prince, M. World Alzheimer Report. Available online: https://www.alzint.org/u/WorldAlzheimerReport2015.pdf (accessed on 4 May 2015).
- Wang, R.-Z.; Yang, Y.-X.; Li, H.-Q.; Shen, X.-N.; Chen, S.-D.; Cui, M.; Wang, Y.; Dong, Q.; Yu, J.-T. Genetically determined low income modifies Alzheimer’s disease risk. Ann. Transl. Med. 2021, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Deckers, K.; Cadar, D.; van Boxtel, M.P.; Verhey, F.R.; Steptoe, A.; Köhler, S. Modifiable risk factors explain socioeconomic inequalities in dementia risk. Nature 2018, 388, 539–547. [Google Scholar]

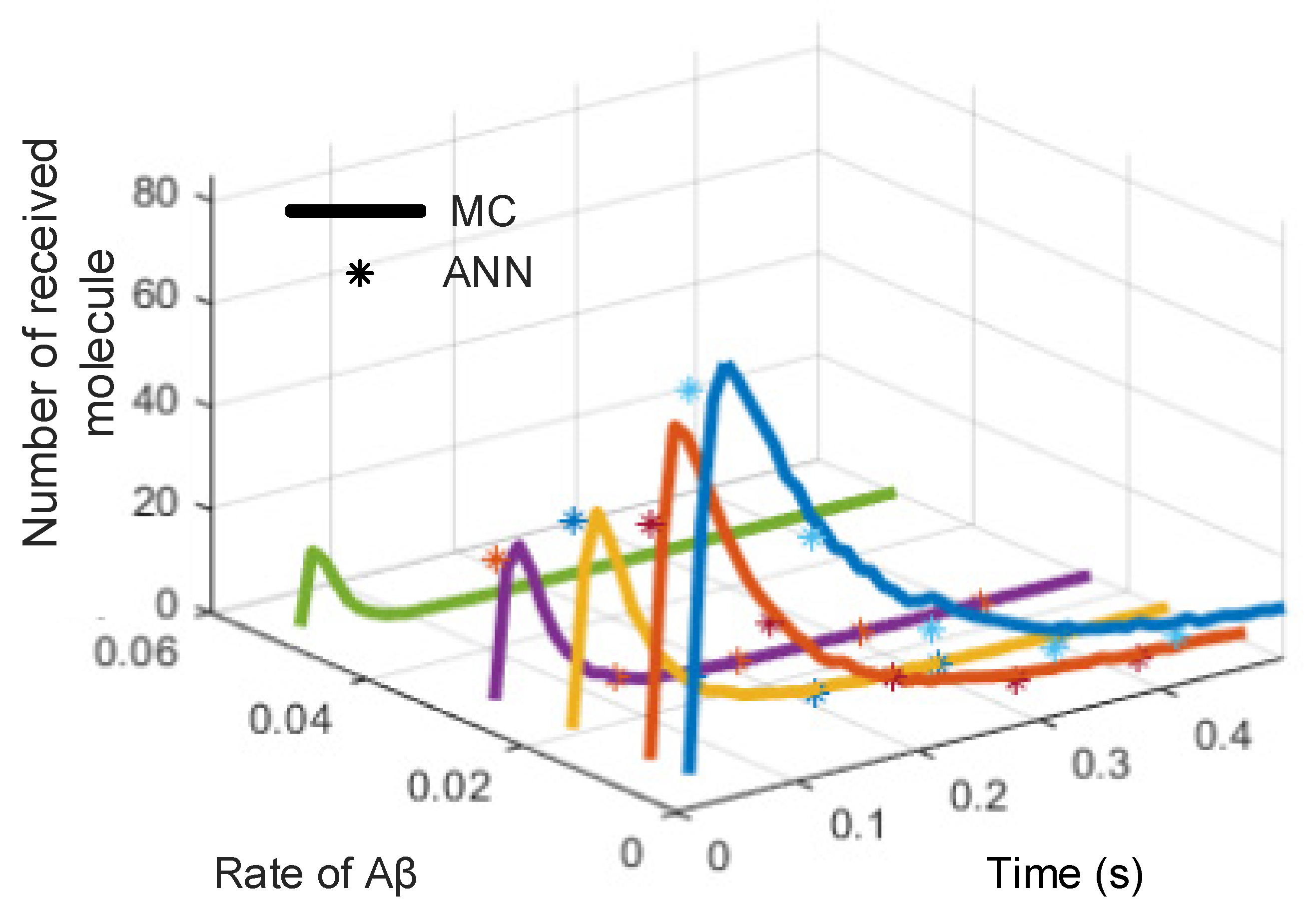
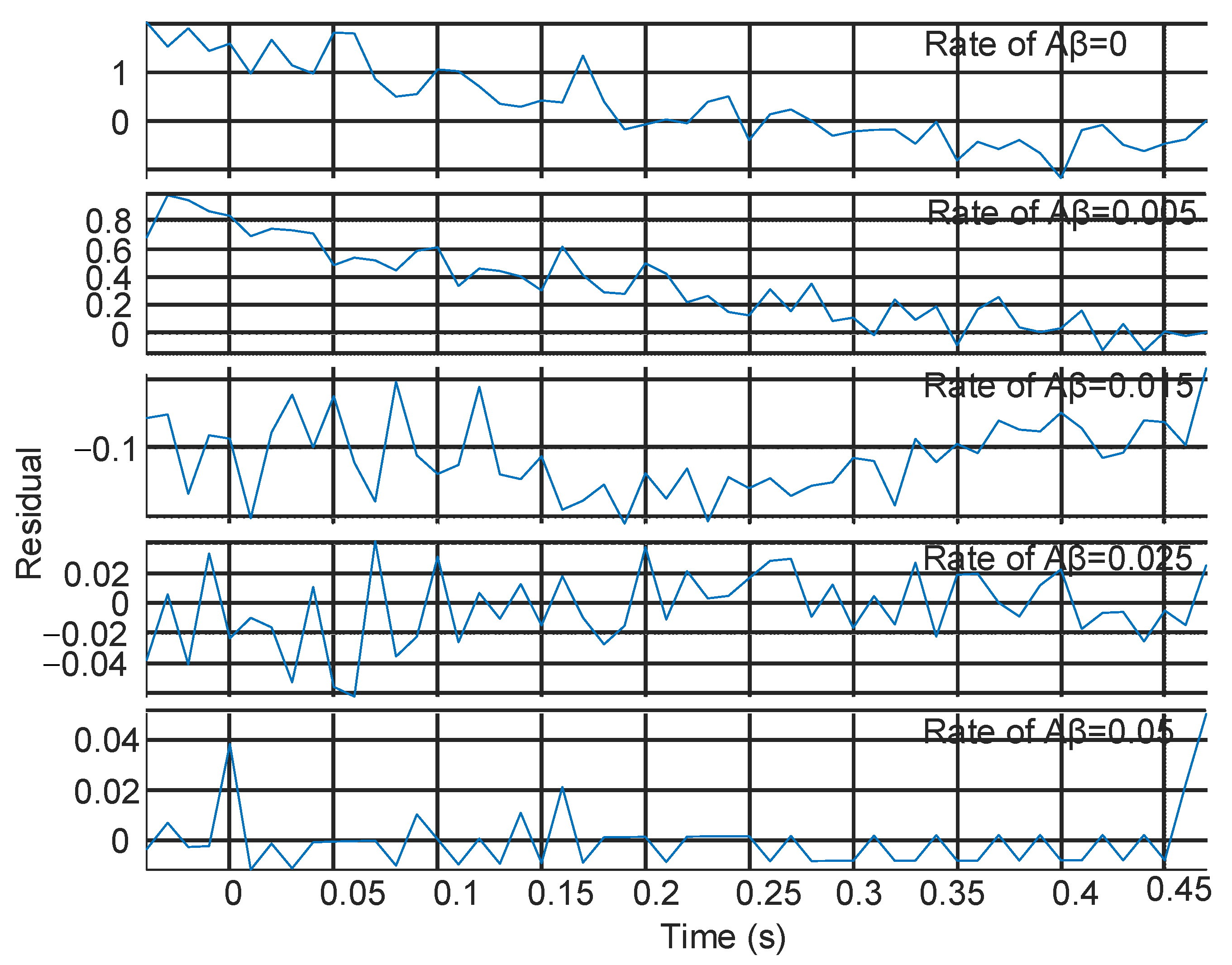
| Time (s) | Number of Received Molecules | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rate of Aβ = 0 | Rate of Aβ = 0.005 | Rate of Aβ = 0.015 | Rate of Aβ = 0.025 | Rate of Aβ = 0.05 | ||||||
| MC | ANN | MC | ANN | MC | ANN | MC | ANN | MC | ANN | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.1 | 65 | 63 | 45 | 46 | 24 | 23 | 20 | 19 | 0 | 0 |
| 0.2 | 40 | 42 | 25 | 27 | 15 | 16 | 7 | 8 | 2 | 1 |
| 0.3 | 20 | 21 | 8 | 7 | 3 | 4 | 2 | 2 | 1 | 1 |
| 0.4 | 8 | 7 | 3 | 3 | 1 | 1 | 0 | 0 | 0 | 0 |
| Gender | |||||
|---|---|---|---|---|---|
| Year | Total Population | Rate of 65+ (%) | Death from Alzheimer (65+) | Male | Female |
| 2015 | 78,741,053 | 8.2 | 12,059 | 4786 | 7273 |
| 2016 | 79,814,871 | 7.9 | 13,051 | 5061 | 7990 |
| 2017 | 80,810,525 | 8.1 | 13,642 | 5252 | 8390 |
| 2018 | 82,003,882 | 8.8 | 13,859 | 5257 | 8602 |
| 2019 | 83,154,997 | 8.9 | 13,498 | 5049 | 8449 |
| Educational Level | 2015 (%) | 2016 (%) | 2017 (%) | 2018 (%) | 2019 (%) |
|---|---|---|---|---|---|
| Illiterate | 21.9 | 20.8 | 19.6 | 18.3 | 16.9 |
| No school completed | 18.9 | 18.2 | 17.5 | 16.8 | 15.9 |
| Primary school | 43.0 | 43.7 | 44.5 | 45.0 | 45.5 |
| Junior high school or equivalent/primary education | 5.2 | 5.6 | 6.0 | 6.5 | 7.3 |
| High school or equivalent | 5.6 | 5.9 | 6.3 | 6.8 | 7.5 |
| Higher education | 5.4 | 5.8 | 6.2 | 6.6 | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayraktar, Y.; Isik, E.; Isik, I.; Ozyilmaz, A.; Toprak, M.; Kahraman Guloglu, F.; Aydin, S. Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models. Sustainability 2022, 14, 7901. https://doi.org/10.3390/su14137901
Bayraktar Y, Isik E, Isik I, Ozyilmaz A, Toprak M, Kahraman Guloglu F, Aydin S. Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models. Sustainability. 2022; 14(13):7901. https://doi.org/10.3390/su14137901
Chicago/Turabian StyleBayraktar, Yuksel, Esme Isik, Ibrahim Isik, Ayfer Ozyilmaz, Metin Toprak, Fatma Kahraman Guloglu, and Serdar Aydin. 2022. "Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models" Sustainability 14, no. 13: 7901. https://doi.org/10.3390/su14137901
APA StyleBayraktar, Y., Isik, E., Isik, I., Ozyilmaz, A., Toprak, M., Kahraman Guloglu, F., & Aydin, S. (2022). Analyzing of Alzheimer’s Disease Based on Biomedical and Socio-Economic Approach Using Molecular Communication, Artificial Neural Network, and Random Forest Models. Sustainability, 14(13), 7901. https://doi.org/10.3390/su14137901






