1. Introduction
Vine plants are cultivated widely, mainly for the consumption of their berries and the products derived from them, such as wine. Main cultivation practices concerning grape production include fertilizing and pesticide application, winter pruning which regulates the production and shape of the stem, and summer pruning which takes place during the vegetative period and aims to balance the vegetation and improve the quality of the grapes. Leaf removal can be conducted before flowering, during fruit set, and from veraison until fruit ripening. Both leaf removal itself and the plant’s stage during the removal affect grape composition in total soluble sugars, anthocyanins, phenols, and aromatic compounds [
1].
Vine leaves, which are produced as a byproduct of the cultivation practices mentioned above, have been consumed for years as a delicacy in traditional cuisines in the Balkans and the Middle East. They are known for their considerable amount of phenolic and other antioxidant and therapeutic compounds [
2,
3,
4,
5], and in the past few years, their consumption has expanded elsewhere as well. Studies regarding vine leaves have mainly focused on qualitative characteristics. Gülcü et al. [
5] studied the effect of harvest time regarding quality characteristics in ten different grape varieties. Results showed that late harvest led to significantly lower concentrations in total phenolic compounds and flavonoids, while the same lies for antioxidant activity in most of the varieties tested. Lima et al. [
3] focused on the changes caused to the final product by pre-culinary and culinary treatments in grapevine leaves. Güler and Candemir [
2] compared leaves of different varieties based on their qualitative characteristics, such as total phenolic and flavonoid contents, phenolic compositions, and color properties.
According to common practices and based on consumer preferences, young, immature leaves are chosen at the beginning of the vegetation, when leaves are thinner, usually from May until June [
6], and can either be consumed fresh or kept preserved in brine. Freshly harvested leaves seem to outweigh brined leaves concerning antioxidant activity [
7,
8], while the scenery is not the same regarding total phenolic and flavonoid content [
8]. Although freshly harvested leaves are generally preferred, grape cultivation practices require the removal of the tender new shoots, to produce better-quality grapes [
1], leading to a brief leaf harvest period. Furthermore, extreme weather conditions and climate change is making it harder to keep a steady leaf production.
On the other hand, undercover agriculture, and greenhouses especially, can offer crop protection and climate control, which means that temperature, light, and CO
2 can be kept under control offering yearly stable production and accurate prediction of harvest time and yield. In combination with hydroponic cultivation, better resource use efficiency is achieved along with higher yield and higher quality products [
9].
The current level of knowledge in the field of hydroponic viticulture for leaf harvesting in greenhouses is at an early stage, even though grape production in greenhouses has been underway since the 1990s. There are various reports for simple covering of vineyards with nets or plastics, but they mainly concern crops in soil and are not presented in more detail [
10]. The initial reports concerning hydroponic vine cultivation refer to the production of cuttings or grapes. Hydroponic viticulture in greenhouses is found mainly in Mediterranean countries, such as Italy and Turkey, due to the need to overcome problems related to soil-wood diseases, soil fatigue, and water and nutrient savings [
11,
12,
13,
14,
15]. Interest in this method is increasing thanks to the ability of climate control and accurate monitoring of all crop parameters.
The high cost of equipment has made the use of greenhouses prohibitive until recently. Furthermore, greenhouse cultivation is quite demanding in terms of energy consumption. They require significant amounts of energy for heating and cooling, contributing to air pollution from Greenhouse Gases (GHG), such as carbon dioxide (CO
2), methane (CH
4), and nitrous oxide (N
2O). However, with the development of technology, the cost of equipment and energy consumption has decreased, resulting in the expansion of research in this area in recent years. Companies and producers can meet future policies and trade standards (ISO) on fruit and vegetable standardization and marketing, in relation to climate change, by offering consumers products with good, detectable environmental performance [
16]. Carbon Footprint (CF) calculation is the most common method to evaluate the environmental impact of agricultural products based on Life Cycle Assessment (LCA). More specifically CF is defined as the amount of carbon dioxide (CO
2) and other greenhouse gases (GHGs) released into the atmosphere over the course of an activity or product [
17]. Regarding CF on viticulture, research so far has been conducted mostly on wine products [
18,
19], while there are fewer reports concerning table grape varieties [
20].
Based on our knowledge, very few studies have been conducted yet regarding the cultivation practices appropriate solely to produce grapevine leaves in greenhouses, even fewer including carbon footprint calculation. To close this gap the current research investigated the potential of cultivating hydroponically vine leaves inside a greenhouse. Four different growing substrates were tested to see whether hydroponic cultivation in combination with higher plant density can prolong the harvest period and lead to higher productivity with no pesticide application. Finally, a primary assessment of the product’s environmental impact is presented through the calculation of the product’s carbon footprint (PCF).
2. Materials and Methods
2.1. Experimental Greenhouse
The experiment was carried out in a pilot greenhouse at the Institute of Plant Breeding and Genetic Resources of the Hellenic Agricultural Organization—Dimitra, Thessaloniki, Greece (40°32′16.9″ N 22°59′57.7″ E). The greenhouse (total area of 150 m2) is a gothic type, with galvanized iron frame, and covered with a flexible polyethylene sheet 180 μm thick on the roof and hard polycarbonate sheets 8 mm thick sideways.
Apart from natural ventilation through roof openings, two fans were used to achieve air recirculation, but no other heating or cooling method was applied. Roof vents were automatically set to open based on the temperature inside the greenhouse. In terms of photosynthetically active radiation, the only shading factor was the greenhouse cover material, as the shading curtains were not put into operation.
2.2. Plant Material and Growing Conditions
A total of 82 grapevine plants (Vitis vinifera Sultana) were planted in stainless steel tanks (6 × 0.5 × 0.5 m) using different substrates, enclosed in a low-density polyethylene (LDPE) sheet. For the purpose of the experiment, three-year-old plants were planted in four different substrates, while soil treatment was used as a control. Perlite was the main substrate; used on its own (P—200 L per tank), in a mix with attapulgite (PA—200 L per tank, 1:1) and zeolite (PZ—200 L per tank, 1:1). Each treatment consisted of six grapevine plants, planted 0.6 m apart (5600 plants ha−1). Furthermore, another perlite treatment was applied (1.7P—340 L per tank), where plants were planted at a higher density (10 plants per tank—9330 plants ha−1—1.7 times the quantity of perlite). The first treatments mentioned (P, PA, PZ, and Soil) were repeated three times, while there was no repetition for the 1.7P treatment. After installation took place in early March, when plants were still in dormancy, plants were pruned and tied to the support wires.
The experiment lasted approximately seven months (from March until the end of October), during which period plants were irrigated with a complete nutrient solution by a drip system automatically controlled by an electronic controller. The number of waterings depended on the amount of draining water and varied from 0.8 to 6.4 L day
−1 plant
−1. The nutrient solution was based on Buttaro et al. [
13] and Di Lorenzo et al. [
15] and adjusted to our needs according to Neokleous [
21]. As far as macronutrients, it contained 12 mmol L
−1 NO
3−, 1.5 mmol L
−1 H
2PO
4−, 1 mmol L
−1 NH
4+, 4.5 mmol L
−1 K
+, 4 mmol L
−1 Ca
2+, 1.5 mmol L
−1 Mg
2+, while micronutrients concentration was 35 mmol L
−1 Fe-DTPA (9% Fe), 20 μmol L
−1 MnSO
4∙H
2O (32%Mn), 3.5 μmol L
−1 ZnSO
4∙7H
2O (23%Zn), 4.5 μmol L
−1 Na
2B
4O
7∙10H
2O (11%B), 0.5 μmol L
−1 CuSO
4∙5H
2O (25%Cu), 0.5 μmol L
−1 Na
2MoO
4∙2H
2O (40% Mo). The water-soluble fertilizers were dissolved in 25 times concentrated stock solution in two separate tanks. Tank-A contained Ca(NO
3)
2, ΝH
4ΝO
3, half the quantity of KNO
3 and Fe-DTPA, while tank-B had the rest of the fertilizers. Considering the desired amounts of fertilizers, electrical conductivity was set at 1.9 mS cm
−1. Finally, a third tank containing HNO
3 was used, to keep pH levels at 5.7.
In soil treatment, a simple 12-12-17 (Ν-Ρ-Κ) fertilizer was applied, following the field’s cultivation practices. Plain water was supplied in an amount varying between 4 and 7.5 L week−1 plant−1, depending on greenhouse temperatures.
2.3. Microclimatic Measurements
Air temperature, humidity, and photosynthetic active radiation in the greenhouse were measured by two sensors, one inside and one outside of the greenhouse, which were set in operation in May. The climatic variables were measured every 5 min and values were stored using a computer-based data acquisition system.
2.4. Quantitative Measurements
Leaf harvesting commenced in late April, 26 days after bud burst, and harvests continued approximately every 10 days, until 1 November. Upon each harvest, all leaves bigger than 12 cm in diameter were removed and weighted, separately for each treatment and repetition. A total of nineteen (19) harvests took place.
2.5. Qualitative Measurements
2.5.1. Color
Color was the main feature for the quality evaluation of the leaves. Since lighter leaf color is preferable concerning leaf consumption, the lightest leaves were harvested, and measurements taken were set as a target color. Measurements were taken on the leaf tip from random leaves of four harvests, and average measurements from each treatment were compared to the target. Samples were acquired from all hydroponic treatments (P, PA, PZ) including 1.7P, as well as from the soil treatment inside the greenhouse. Further samples were acquired from vineyards in the field to have a comparison of the field environment. However, due to extreme weather conditions and excessive disease infestations, sampling from the field was not possible to continue along with the samples inside the greenhouse.
Measurements were carried out immediately after harvesting, without prior processing of the leaves. A digital colorimeter (CR-400 Chroma Meter, Konica Minolta Inc., Tokyo, Japan) was used to determine colorimetric parameters such as lightness (L*, 0–100: black to white), and the variables a* and b*. Positive a* indicates a hue of red, negative a*, of green, while positive b* indicates yellow and negative b* blue [
22].
2.5.2. Photosynthetic Parameters
Measurements were taken approximately once every month, the day before harvesting. Records took place in situ in six replications for each treatment, as well as 1.7P.
The net photosynthetic rate (μmol CO2 m−2 s−1), stomatal conductance (mol m−2 s−1), and transpiration rate (mmol m−2 s−1) were measured under complete sunlight conditions, by using an infrared gas analyzer (LCi-SD portable photosynthesis system, ADC BioScientific Ltd., Hoddesdon, UK).
2.5.3. Total Phenolic Compounds
Total phenolic content was determined using the method described by Scalbert et al. [
23]. In a few words, 0.5 mL methanolic plant extract, 2.5 mL of Folin–Ciocalteau reagent, and 2 mL of 7.5% sodium carbonate solution were incubated at 50 °C for 5 min. The blank consisted of 0.5 mL 80% aqueous methanol. The absorbance of the colored product was measured at 760 nm, while results were expressed as mg gallic acid equivalent g
−1. Samples of four random harvests along the cultivation period were used.
2.5.4. Nitrates
Nitrate content was determined chromatographically, according to Cataldo et al. [
24]. Briefly, samples (2.5 g) were extracted with 25 mL H
2O. Aliquot 0.2 mL aqueous plant extract was added in two test tubes along with 0.8 mL H
2SO
4 and 0.8 mL 5% salicylic acid to H
2SO
4 respectively followed by 19 mL 2N NaOH addition. The colored product was placed in a spectrophotometer (UV-VIS, Shimadzu Scientific Instruments, Columbia, MD, USA), and its absorbance was measured at 410 nm, while results were expressed as mg kg
−1 fresh weight. Samples from four different random harvests were used.
2.5.5. Determination of Plant Nutrient Concentrations
An equal number of leaves of four random harvests were washed with tapped and distilled water and dried for 48 h at 68 °C; afterward, they were ground to a fine powder. A portion of 1 g of the fine powder of each sample was ash-dried in a muffle furnace, at 550 °C for 5 h. Then, the ash was dissolved with 5 mL of 6 N HCl and diluted with distilled water up to 50 mL. The concentrations of P, K, Ca, Mg, B, Fe, Mn, Zn, and Cu were determined by ICP (Perkin Elmer-Optical Emission Spectrometer, OPTIMA 2100 DV) [
25]. Nitrogen was determined by the Kjeldahl method. Macronutrient concentrations were expressed as percentage dry weight, while those of micronutrients were expressed in ppm.
2.6. Carbon Footprint
According to the LCA method, to determine carbon footprint, it is necessary to define the boundaries of the system and the functional unit. In our case scenario, calculations concern the cultivation phase so the other input categories such as transportation, processing, and the final disposal to the consumers were omitted (
Figure 1). Greenhouse construction materials, electric energy consumption, and water, along with fertilizers used in hydroponics throughout the experiment, were taken into consideration.
All data acquired from the categories mentioned above along with the produced grape leaves were recorded per hectare and per year, and the materials’ life span is shown in
Table 1. To quantify the CF of vine leaves, 1 kg of vine leaves was used as a functional unit. Calculations were conducted by using a commercial LCA software, SimaPro 9.2.0.1 (PRé Sustainability, Amersfoort, The Netherlands), and the Ecoinvent 3.7 database (Zurich, Switzerland).
2.7. Statistical Analysis
Three cultivation rows of six plants per row were applied for Soil, Perlite, Perlite-Attapulgite, and Perlite-Zeolite treatment, considering each row a replication, and samples were acquired evenly from all three replications. Regarding 1.7Perlite, since no repetition was applied, statistical analysis concerning qualitative characteristics was based on collecting the same sample size as the rest of the treatments from the one and only available cultivation row. Finally, regarding Soil-Field samples, data could not undergo any statistical analysis, although recorded values are presented without being further interpreted. Average measurements of all acquired data per treatment were analyzed by applying a one-way analysis of variance (ANOVA). Comparisons of the means were performed using Tukey’s post-hoc test at the 0.05 level.
3. Results and Discussion
Results from microclimate parameters along with quantitative and qualitative characteristics of the grape leaves in the greenhouse are presented below. Specifically, these are air temperature, illuminance (Photosynthetically Active Radiation—PAR), relative humidity, total yield, color parameters, total phenolic compounds, transpiration rate, stomatal conductance, photosynthetic rate, nutrient concentration, and carbon footprint.
3.1. Microlimatic Conditions
Microclimatic parameters can highly affect many biochemical and physiological functions such as photosynthesis, transpiration, and enzymatic activities. Temperature is the premier environmental factor affecting all the above, while humidity, solar radiation, and carbon dioxide concentration are also critical parameters [
26].
The average air temperature inside the greenhouse ranged from 15 to 30 °C, during the whole cultivation period. The lowest temperatures were noted in November, with a minimum of 6.4 °C, while the highest temperatures were in August, with a maximum of 42.9 °C. Data showed important variation both in the whole cultivation period and during the twenty-four-hour period, especially during the autumn months, due to the lack of any heating or cooling method.
Relative humidity during the cultivation period ranged between 50 and 80%, on average per month, with the highest average value noted in November and the lowest in July.
Average, minimum, and maximum temperatures and relative humidity inside and outside the greenhouse are shown per month in
Figure 2 and
Figure 3, respectively.
As far as photosynthetically active radiation, differences in recorded data are exclusively due to covering material, resulting in approximately 30 ± 5% less photosynthetically active radiation inside the greenhouse.
3.2. Quantitative Characteristics—Yield
During the cultivation period, 19 harvests took place, starting in May and ending in November, and the total number of produced leaves along with their weight was evaluated.
The highest yield was achieved on Perlite in comparison to the other hydroponic treatments, as well as the soil. However, results showed that there was no significant difference between the yield achieved on different substrates (P, PA, PZ). On the other hand, between soil and every hydroponic treatment, differences were statistically significant. Soil yield was remarkably lower in comparison to all three hydroponic treatments, probably as a result of the poorest fertilization.
Regarding 1.7P, the higher density applied led to a significantly higher yield per hectare in comparison to the rest of the hydroponic treatments. More specifically, 1.7P yield was 39% higher than Perlite and approximately 50% higher than Perlite—Attapulgite and Perlite—Zeolite.
Overall, the yield per hectare in all hydroponic treatments outweighed reported yields of field cultivation which ranged from 2233 to 4221 kg ha
−1, depending on cultivation practices at ten-year-old vine cultivars on soil [
27]. Furthermore, in our study, hydroponic leaves were quite thin and light, resulting in approximately 58.8 to 62.3 leaves per 100 g. Our results in leaves per 100 g were importantly higher compared to results reported in Cangi and Kılıç [
27], which ranged from 33.3 to 39.9 leaves per 100 g in Narince cultivars and 34.0 to 49.0 in Sultana cultivars.
All results concerning the total yield calculated per hectare are presented in
Table 2.
3.3. Qualitative Characteristics
3.3.1. Color
Good quality leaves should have a fresh appearance with a bright green color, and no decay or discoloration. Bright green leaves result in the preferred golden color when blanched or processed in any other way before consumption, while darker green leaves develop an undesirable brownish color [
28].
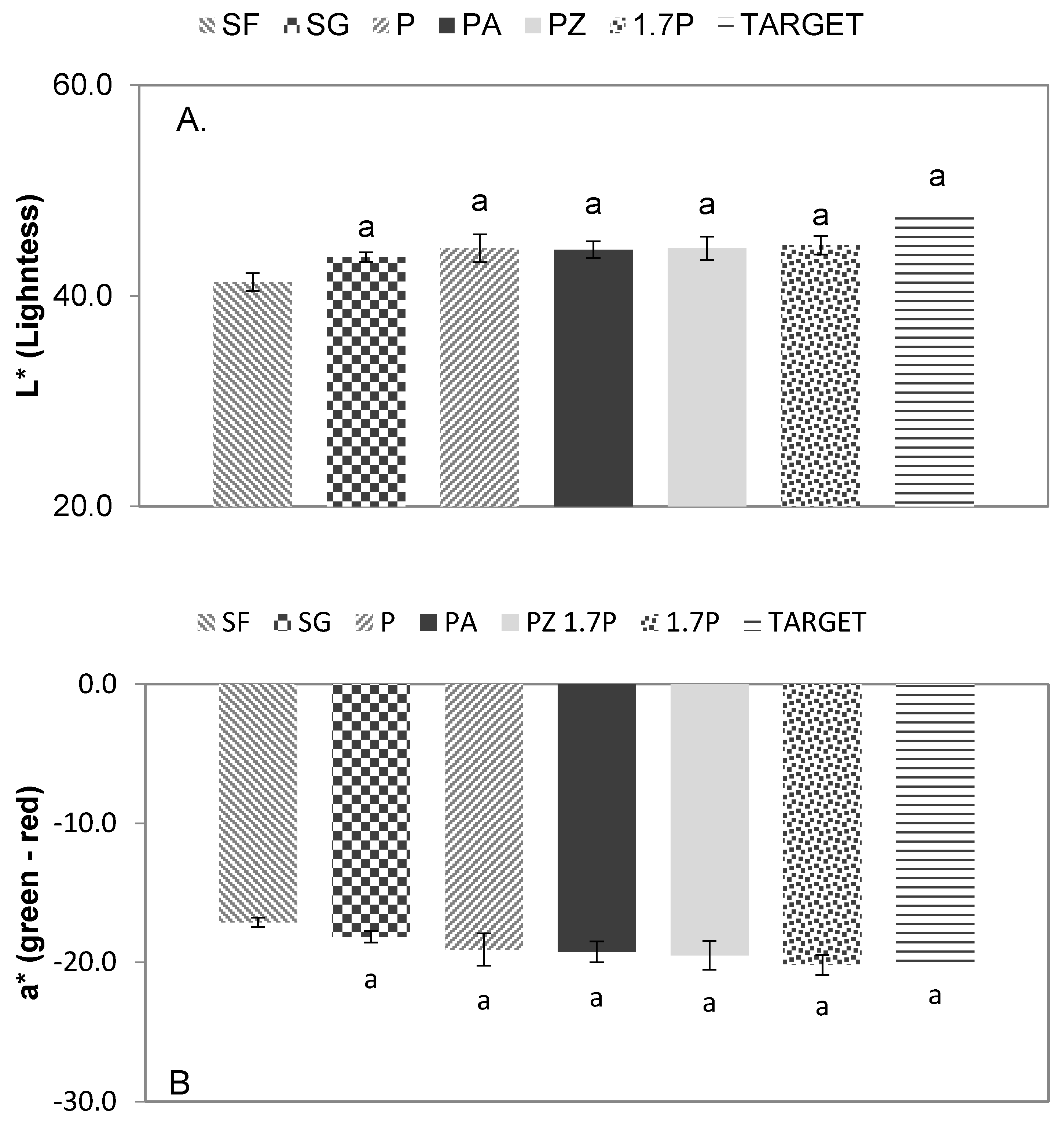
Comparison between the applied treatments (SF—Soil-Field; SG—Soil-Greenhouse; P—Perlite; PA—Perlite-Attapulgite; PZ—Perlite-Zeolite and 1.7P—1.7 times Perlite, in relation to the set Target) showed no significant differences between any of the evaluated color parameters. Average measurements regarding lightness ranged from 43.67 in the Soil-Greenhouse sample, to 44.79 in the 1.7Perlite sample, while the target was set at 47.79. Recorded data comes in agreement with previous studies concerning grape leaf color, mentioning lightness of around 43.9 on the brightest and less mature leaf stage [
6], and ranging from 40.26 to 44.91 on other Sultana cultivars [
2].
Regarding parameters a* (green-red) and b* (blue-yellow), the values varied between −20.19 and −18.16 and between 26.87 and 29.18 respectively. The average values of our samples are closer to the brighter and less mature leaves mentioned by Cantwell et al. [
6], which is the desired leaf color. Sultana cultivars in Güler and Candemir [
2] recorded values around −5.6 regarding a* and around 10.85 regarding b*, which is quite different than our records. Average values of all three parameters are presented in
Figure 4A–C.
3.3.2. Photosynthetic Parameters
Measurements concerning the transpiration rate, stomatal conductance, and photosynthetic rate, were taken nine times across the cultivation period, approximately once per month, before harvesting. Average measurements of all takings showed no statistically important difference (in significance level of α = 0.05) between the hydroponic substrates and soil treatment in the greenhouse.
Results concerning all photosynthetic parameters mentioned above, as well as Photosynthetically Active Radiation on leaf surface during the measurements, are presented in
Figure 5A–D.
3.3.3. Total Phenolic Compounds
It has been reported in numerous studies that grapevine leaves are a good source of phenolic compounds [
3,
4,
5]. Güler and Candemir [
2] tested total phenolic content on the leaves of various grape cultivars, among which three Sultana cultivars. Total phenolic compounds of their samples were determined between 9.72 and 12.80 mg GAE g
−1 f.w. In agreement with the results mentioned above, in our experiment, total phenolic compounds ranged between 10.42 and 13.52 mg GAE g
−1 f.w., in Perlite-Attapulgite and Soil-Greenhouse respectively. Gülcü et al. [
5] have previously studied the effect of harvest time on bioactive compounds, concluding that leaves harvested after blooming have slightly fewer total phenolic compounds than those harvested before blooming. In our case that was not confirmed, as no correlation between harvest time and total phenolic compounds was noticed. Average measurements showed no statistically important difference in the significance level of α = 0.05, between all applied greenhouse treatments. Results regarding the average values of all four samples taken during the experiment are presented in
Figure 6.
3.3.4. Nitrates
High nitrate accumulation in plants has been proven to have harmful consequences both for plants and for human health [
29,
30]. Accumulation of nitrates is often caused in plants due to excessive nitrogenous fertilizing, while environmental factors and more importantly light intensity also play a very important role [
31]. The European Union has prescribed the maximum limits of nitrates in various leafy plants, such as spinach and lettuce, though no limits were set for the consumption of grape leaves.
Analysis conducted in our samples showed a maximum value at 255 mg kg
−1 f.w. in Perlite-Zeolite in April, while average values during the whole cultivation period ranged between 71.48 and 137.38 mg kg
−1 f.w. (4.29 to 13.80 ppm) in Perlite-Attapulgite and Perlite-Zeolite respectively. Despite the fact that the quantity of fertilizers increased across the cultivation period along with the applied irrigations, nitrate concentration appeared to have a decreasing tendency. Samples collected in August showed on average up to 80% less nitrate concentration in comparison to those collected in April, agreeing to the fact that higher light intensity leads to a reduction of the accumulative nitrates. All data collected are presented in
Figure 7.
Previous studies on nitrate content of edible fresh grapevine leaves on soil cultivation, showed results varying from 199.50 to 3441.70 ppm [
32], remarkably higher than our findings. That excessive difference can be explained to some degree by the differences in cultivar species, plant age, and other cultivation practices, or by the differences in environmental factors, due to different locations and time periods. Nonetheless, more research needs to take place regarding nitrates on grape leaves, as the available references in English are quite limited to extract a safe conclusion.
3.3.5. Plant Nutrient Concentrations
Based on our results, substrate did not seem to affect the average intake of the nutrients by the plants (
Table 3). Results regarding the macronutrients come to an agreement with previous reports as mentioned in Cangi and Kılıç [
27], and no deficiency was noticed either on average or on separate records taken. Although, more research needs to take place concerning the properties of the substrates and how those properties affect plants’ nutrient concentration, in combination with the amount and rate of run-offs.
3.3.6. Product Carbon Footprint
The product carbon footprint (PCF) for Sultana grape leaves ranged between 10.35 kg CO
2−eq kg
−1 f.w. in 1.7P and 79.2 kg CO
2−eq kg
−1 f.w. in S treatments. The 1.7P treatment resulted in 1.4 to 7.6 times lower PCF compared to the other treatments, as a result of the higher yield. Energy consumption accounted for the highest percentage of the total carbon footprint, in S, P, PA, and 1.7P treatments, followed by construction materials. Regarding PZ treatment, the growing substrate affected the PCF to a greater degree, followed by energy consumption. Results regarding all applied treatments are presented in
Table 4. Based on our knowledge and research in the international literature, this is the first time that PCF is calculated for Sultana grape leaves produced either in an open field or inside a greenhouse. Scarce studies concerning the Sultana variety referred solely to the production of table grapes, following the corresponding cultivation practices which took place in open field vineyards. Litskas et al. [
20] reported PCF values of 0.8 kg CO
2−eq kg
−1 for the table grape variety Sultana regarding table grapes production, therefore this result cannot be compared to PCF regarding grape-leaves production.
4. Conclusions
Vine plants were successfully cultivated hydroponically in a greenhouse, exclusively to produce vine leaves, in order to extend the cultivation period, and along with high plant density, maximize grape leaves yield. During the cultivation period, 19 pesticide-free harvests took place, starting in May and ending in November, and the total number of produced leaves in Perlite treatment with high-density planting (1.7P) reached a maximum of 8,623,067 per hectare with a maximum weight of 13,874.80 kg per hectare, which is 3–6 times higher than values reported in the literature. Moreover, the 1.7P treatment produced 1.6-8.7 times more leaves per hectare, than the rest of the hydroponic and soil treatments. Consequently, the 1.7P treatment resulted in a 1.4 to 7.6 times lower product carbon footprint compared to the other treatments. Intensive and different hydroponic substrates did not negatively affect vine leaves’ qualitative characteristics, as no statistically significant differences were found regarding color, nitrates, total phenols, or photosynthetic parameters, among the different treatments of this work. In future research, the substrates’ properties will be further studied to assess water and nutrient retention and how those amounts can be attributed to the plants. Finally, it would be of great interest to see whether a year-round production is possible, with heated greenhouses and high climate control so that plants will stay evergreen.