LED versus HPS Lighting: Effects on Water and Energy Consumption and Yield Quality in Lettuce Greenhouse Production
Abstract
:1. Introduction
2. Materials and Methods
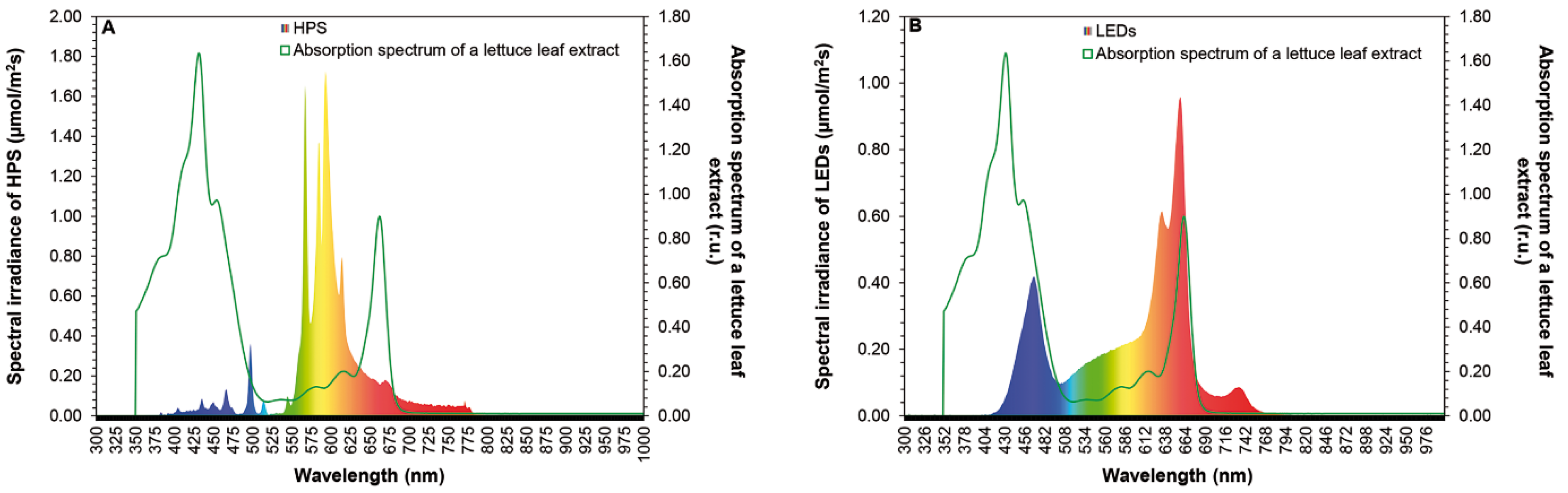
2.1. Overhead-Lighting
2.2. Experimental Set-Up
2.3. Energy and Water Consumption
2.4. Assessment of Plant Growth Characteristics
2.5. Optical Readings of Chlorophyll
2.6. Chemical Analysis
2.7. Data Analysis
3. Results
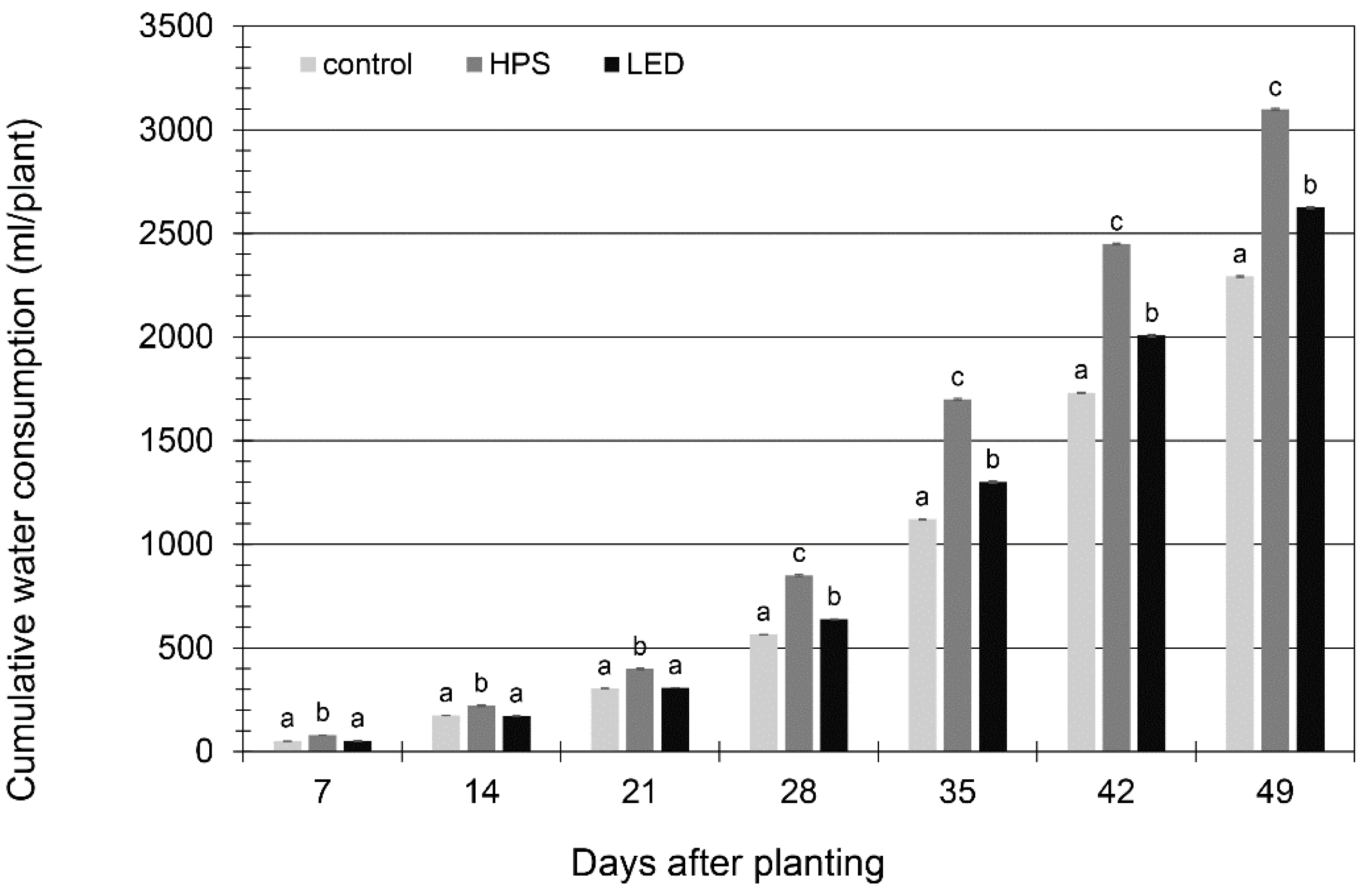
3.1. Energy and Water Savings
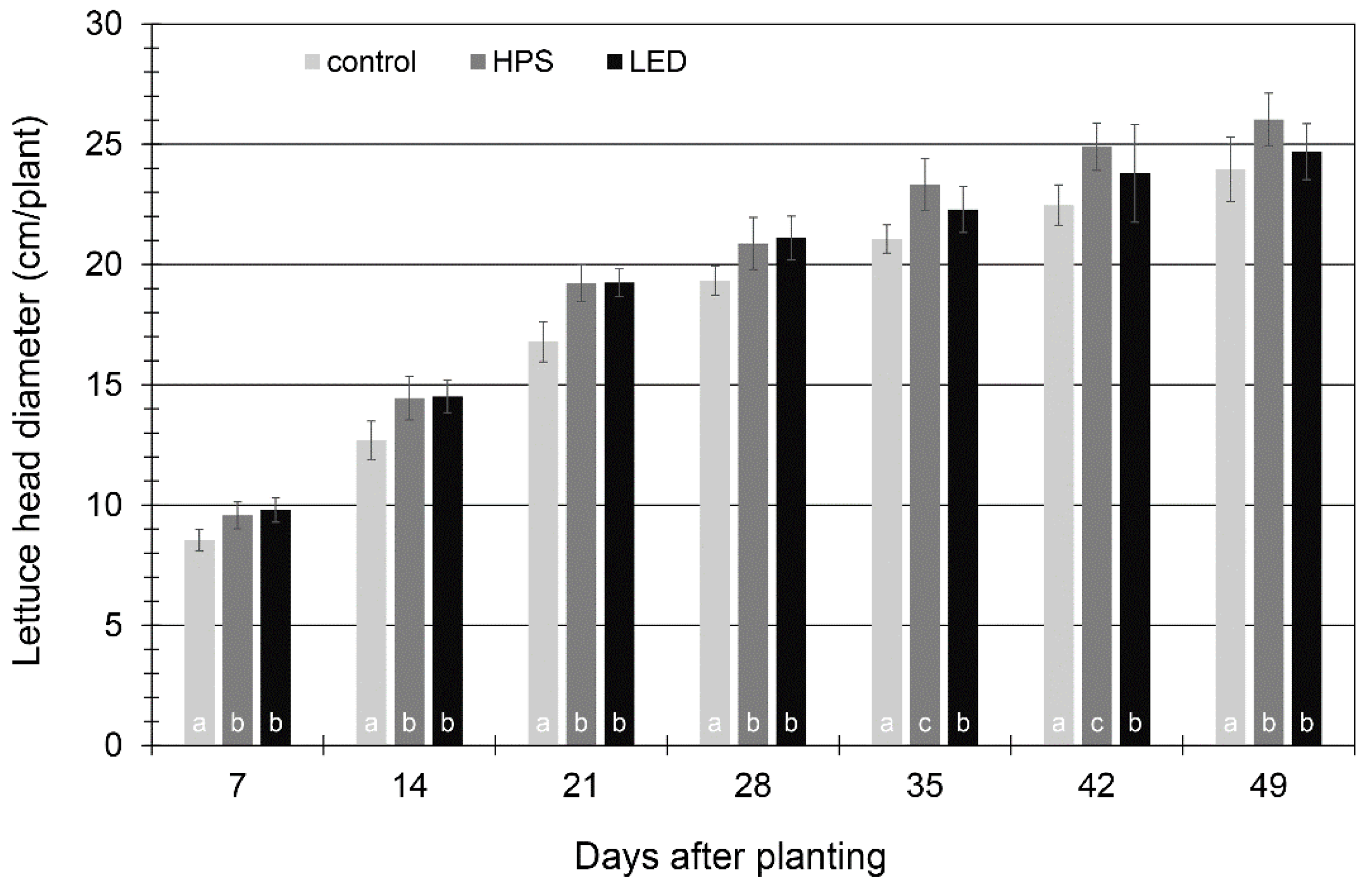
3.2. Plant Growth and Yield
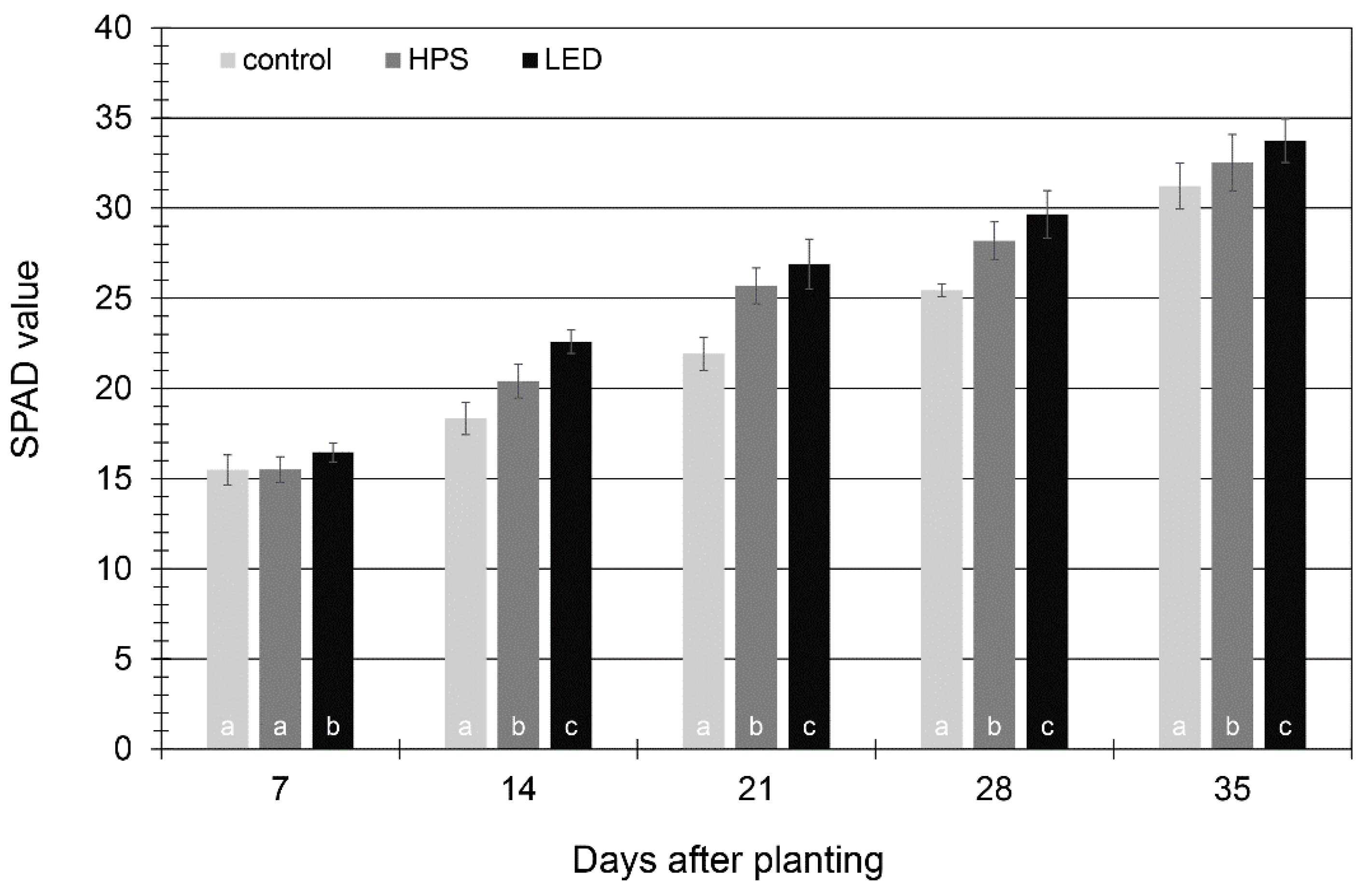
3.3. Phenolic Compounds and Chlorophyll Pigments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, R.M. A historical background of plant lighting: An Introduction to the workshop. Hortscience 2008, 43, 1942–1943. [Google Scholar] [CrossRef] [Green Version]
- Ouzounis, T.; Giday, H.; Kjær, K.H.; Ottosen, C.O. LED or HPS in ornamentals? A case study in roses and campanulas. Eur. J. Hortic. Sci. 2018, 83, 166–172. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Terfa, M.T.; Solhaug, K.A.; Gislerød, H.R.; Olsen, J.E.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa×hybrida but does not affect time to flower opening. Physiol. Plantarum 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.-H.; Lin, C.-C.; Li, T.-H.; Li, C.-J.; Peng, S.-H.; Yang, F.-C.; Thomas, K.; Kumar, D.; Chi, Y.; Hsu, B.-D. Plant growth absorption spectrum mimicking light sources. Materials 2015, 8, 5265–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE 2015, 10, e138930. [Google Scholar] [CrossRef]
- Schewe, J.; Heinke, J.; Gerten, D.; Haddeland, I.; Arnell, N.W.; Clark, D.B.; Dankers, R.; Eisner, S.; Fekete, B.M.; Colón-González, F.J. Multimodel assessment of water scarcity under climate change. Proc. Natl. Acad. Sci. USA 2014, 111, 3245–3250. [Google Scholar] [CrossRef] [Green Version]
- Stanghellini, C.; Kemkes, F.l.K.; Knies, P. Enhancing environmental quality in agricultural systems. Acta Hort. 2003, 609, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C. Unraveling the role of red: Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of yield, lycopene, and lutein content in tomatoes grown under continuous PAR spectrum LED lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Becker, C.; Urlić, B.; Jukić Špika, M.; Kläring, H.-P.; Krumbein, A.; Baldermann, S.; Goreta Ban, S.; Perica, S.; Schwarz, D. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, β-carotene and xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef]
- Becker, C.; Kläring, H.-P. CO2 enrichment can produce high red leaf lettuce yield while increasing most flavonoid glycoside and some caffeic acid derivative concentrations. Food Chem. 2016, 199, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Göhler, F.; Molitor, H.-D. Erdelose Kulturverfahren im Gartenbau; Eugen Ulmer: Stuttgart, Germany, 2002. [Google Scholar]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Hemming, S.; Bakker, J.C.; Campen, J.B.; Kempkes, F.L.K. Sustainable use of energy in greenhouses. In Achieving Sustainable Greenhouse Cultivation; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2019; pp. 445–492. [Google Scholar]
- Gomez, C.; Morrow, R.C.; Bourget, C.M.; Massa, G.D.; Mitchell, C.A. Comparison of intracanopy light-emitting diode towers and overhead high-pressure sodium lamps for supplemental lighting of greenhouse-grown tomatoes. HortTechnology 2013, 23, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Katzin, D.; Marcelis, L.F.; van Mourik, S. Energy savings in greenhouses by transition from high-pressure sodium to LED lighting. Appl. Energy 2021, 281, 116019. [Google Scholar] [CrossRef]
- Ouzounis, T.; Razi Parjikolaei, B.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Żupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F. Adding far-red to red-blue light-emitting diode light promotes yield of lettuce at different planting densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, R.; Jiang, H.; Wu, L.; Zhang, Y.; Song, S.; Su, W.; Liu, H. End-Of-Day LED lightings influence the leaf color, growth and phytochemicals in two cultivars of lettuce. Agronomy 2020, 10, 1475. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Chen, X.-l.; Xue, X.-z.; Guo, W.-z.; Wang, L.-c.; Qiao, X.-j. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
| Parameters | HPS Lighting | LED Fixture |
|---|---|---|
| Number of lamps | 1 | 1 |
| Number of plants | 16 | 16 |
| Application time per day (h) | 15 | 15 |
| Total hours of application (h) | 735 | 735 |
| Energy consumption per hour (kJ) | 1558.08 | 378.36 |
| Total energy consumption (MJ) | 1145.19 | 278.10 |
| * Energy saving in comparison to HPS lighting (%) | 75.72 | |
| ** Water saving in comparison to HPS lighting (%) | 15.27 |
| Plant Characteristics | ||||
|---|---|---|---|---|
| Treatment | Number of Leaves (Leaves/Plant) | Fresh Weight (g/Plant) | Dry Matter (%) | WUE (g FW/L H2O) |
| Control | 161.25 ± 7.76 a | 131.20 ± 7.08 a | 4.78 ± 0.12 a | 57.36 ± 1.31 a |
| HPS | 250.42 ± 28.68 b | 154.23 ± 12.37 b | 5.30 ± 0.48 b | 49.63 ± 3.32 b |
| LEDs | 253.50 ± 17.24 b | 160.02 ± 5.52 b | 5.36 ± 0.17 b | 60.83 ± 1.23 a |
| Treatment | |||
|---|---|---|---|
| Phenolic Compounds | Control (µmol/g DW) | HPS (µmol/g DW) | LEDs (µmol/g DW) |
| Caffeoyltartaric acid | 1.92 ± 0.04 a | 2.09 ± 0.11 a | 2.38 ± 0.13 b |
| Caffeoylquinic acid | 1.14 ± 0.06 a | 1.57 ± 0.16 b | 1.84 ± 0.29 b |
| Caffeoylmalic acid | 1.07 ± 0.19 a | 1.06 ± 0.17 a | 1.12 ± 0.05 a |
| Dicaffeoyltartaric acid | 6.12 ± 0.32 a | 8.26 ± 0.62 b | 8.49 ± 0.63 b |
| Dicaffeoylquinic acid | 0.08 ± 0.01 a | 0.13 ± 0.03 ab | 0.21 ± 0.11 b |
| Quercetin-3-O-(6″-malonylglucoside) | 0.07 ± 0.01 a | 0.06 ± 0.02 a | 0.08 ± 0.02 a |
| Total phenolic compounds | 10.42 ± 0.58 a | 13.33 ± 0.77 b | 14.41 ± 0.81 b |
| Phenolic Compounds | Chlorophyll (r) |
|---|---|
| Caffeoyltartaric acid | 0.986 * |
| Caffeoylquinic acid | 0.994 * |
| Caffeoylmalic acid | 0.587 |
| Dicaffeoyltartaric acid | 0.914 * |
| Dicaffeoylquinic acid | 0.989 * |
| Quercetin-3-O-(6″-malonylglucoside) | 0.484 |
| Total phenolic compounds | 0.971 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. LED versus HPS Lighting: Effects on Water and Energy Consumption and Yield Quality in Lettuce Greenhouse Production. Sustainability 2021, 13, 8651. https://doi.org/10.3390/su13158651
Dannehl D, Schwend T, Veit D, Schmidt U. LED versus HPS Lighting: Effects on Water and Energy Consumption and Yield Quality in Lettuce Greenhouse Production. Sustainability. 2021; 13(15):8651. https://doi.org/10.3390/su13158651
Chicago/Turabian StyleDannehl, Dennis, Thomas Schwend, Daniel Veit, and Uwe Schmidt. 2021. "LED versus HPS Lighting: Effects on Water and Energy Consumption and Yield Quality in Lettuce Greenhouse Production" Sustainability 13, no. 15: 8651. https://doi.org/10.3390/su13158651
APA StyleDannehl, D., Schwend, T., Veit, D., & Schmidt, U. (2021). LED versus HPS Lighting: Effects on Water and Energy Consumption and Yield Quality in Lettuce Greenhouse Production. Sustainability, 13(15), 8651. https://doi.org/10.3390/su13158651






