Towards Sustainable Sources of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Northern Australian Tropical Crossbred Beef Steers through Single Nucleotide Polymorphisms in Lipogenic Genes for Meat Eating Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets and Experimental Design
2.2. Loin Eye Muscle Sampling and Chemical Analysis
2.3. Blood Sampling and Genomic DNA Extraction
2.4. Primer Design, Amplification of Target Genes, Clean-Up of PCR Products, Library Preparation, Sequencing and Data Analysis
2.5. Calculations and Statistical Analysis
3. Results
3.1. Genetic Diversity of the Identified Single Nucleotide Polymorphisms
3.2. Correlations between Single Nucleotide Polymorphisms, Intramuscular Fat, Fat Melting Point, and Fatty Acid Composition
3.3. Associations between Single Nucleotide Polymorphisms, Intramuscular Fat, Fat Melting Point, and Fatty Acid Composition
4. Discussion
4.1. Fatty Acid Binding Protein 4 Gene Polymorphisms
4.2. Stearoyl-CoA Desaturase Gene Polymorphisms
4.3. Fatty Acid Synthase Gene Polymorphisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Biodiversity and Sustainable Diets—United against Hunger; FAO: Rome, Italy, 2010. [Google Scholar]
- Hoa, V.-B.; Song, D.-H.; Seol, K.-H.; Kang, S.-M.; Kim, H.-W.; Kim, J.-H.; Cho, S.-H. Coating with chitosan containing lauric acid (C12:0) significantly extends the shelf-life of aerobically—Packaged beef steaks during refrigerated storage. Meat Sci. 2021, 184, 108696. [Google Scholar] [CrossRef]
- Pogorzelski, G.; Pogorzelska-Nowicka, E.; Pogorzelski, P.; Półtorak, A.; Hocquette, J.-F.; Wierzbicka, A. Towards an integration of pre- and post-slaughter factors affecting the eating quality of beef. Livest. Sci. 2021, 255, 104795. [Google Scholar] [CrossRef]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Byrne, C.J.; Fair, S.; Dick, J.R.; Lonergan, P.; Kenny, D.A. Dietary supplementation with fish oil and safflower oil, during the finishing period, alters brisket muscle fatty acid profile and n-6/n-3 ratio but not carcass traits of dairy beef bulls. Appl. Anim. Sci. 2021, 37, 436–444. [Google Scholar] [CrossRef]
- Correa, L.B.; Netto, A.S.; da Silva, J.S.; Cônsolo, N.R.B.; Pugine, S.M.P.; de Melo, M.P.; Santana, R.S.D.S.; Zanetti, M.A. Changes on meat fatty acid profile, cholesterol and hepatic metabolism associated with antioxidants and canola oil supplementation for Nellore cattle. Livest. Sci. 2022, 257, 104850. [Google Scholar] [CrossRef]
- Monteiro, P.; Maciel, I.; Alvarenga, R.; Oliveira, A.; Barbosa, F.; Guimarães, S.; Souza, F.; Lanna, D.; Rodrigues, B.; Lopes, L. Carcass traits, fatty acid profile of beef, and beef quality of Nellore and Angus x Nellore crossbred young bulls finished in a feedlot. Livest. Sci. 2022, 256, 104829. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.; Curran, J.; Gall, H.; Henriksen, E.; O’Connor, A.; Paine, L.; Richardson, B.; van Sliedregt, H.; Smith, L. Genetics and nutrition impacts on herd productivity in the Northern Australian beef cattle production cycle. Vet. Anim. Sci. 2021, 15, 100228. [Google Scholar] [CrossRef]
- Mwangi, F.W.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E.O. Diet and Genetics Influence Beef Cattle Performance and Meat Quality Characteristics. Foods 2019, 8, 648. [Google Scholar] [CrossRef]
- Tume, R.K. The effects of environmental factors on fatty acid composition and the assessment of marbling in beef cattle: A review. Aust. J. Exp. Agric. 2004, 44, 663–668. [Google Scholar] [CrossRef]
- Prache, S.; Adamiec, C.; Astruc, T.; Baéza-Campone, E.; Bouillot, P.; Clinquart, A.; Feidt, C.; Fourat, E.; Gautron, J.; Girard, A.; et al. Review: Quality of animal-source foods. Animal 2021, 16, 100376. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Seal, C.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M.; et al. Composition differences between organic and conventional meat: A systematic literature review and meta-analysis. Br. J. Nutr. 2016, 115, 994–1011. [Google Scholar] [CrossRef]
- Butler, G.; Ali, A.M.; Oladokun, S.; Wang, J.; Davis, H. Forage-fed cattle point the way forward for beef? Futur. Foods 2021, 3, 100012. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Davis, H.; Magistrali, A.; Butler, G.; Stergiadis, S. Nutritional Benefits from Fatty Acids in Organic and Grass-Fed Beef. Foods 2022, 11, 646. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Torres, R.D.N.S.; Bertoco, J.P.A.; Arruda, M.C.G.; Coelho, L.D.M.; Paschoaloto, J.R.; Ezequiel, J.M.B.; Almeida, M.T.C. The effect of dietary inclusion of crude glycerin on performance, ruminal fermentation, meat quality and fatty acid profile of beef cattle: Meta-analysis. Res. Vet. Sci. 2021, 140, 171–184. [Google Scholar] [CrossRef]
- Menci, R.; Coppa, M.; Torrent, A.; Natalello, A.; Valenti, B.; Luciano, G.; Priolo, A.; Niderkorn, V. Effects of two tannin extracts at different doses in interaction with a green or dry forage substrate on in vitro rumen fermentation and biohydrogenation. Anim. Feed Sci. Technol. 2021, 278, 114977. [Google Scholar] [CrossRef]
- Ward, R.E.; Woodward, B.; Otter, N.; Doran, O. Relationship between the expression of key lipogenic enzymes, fatty acid composition, and intramuscular fat content of Limousin and Aberdeen Angus cattle. Livest. Sci. 2010, 127, 22–29. [Google Scholar] [CrossRef]
- Zhang, S.; Knight, T.J.; Reecy, J.M.; Beitz, D.C. DNA polymorphisms in bovine fatty acid synthase are associated with beef fatty acid composition. Anim. Genet. 2008, 39, 62–70. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Edriss, M.A.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. Breed differences and genetic parameters for melting point, marbling score and fatty acid composition of lot-fed cattle. J. Anim. Physiol. Anim. Nutr. 2000, 83, 95–105. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Edriss, M.A.; Siebert, B.D.; Bottema, C.D.K.; Deland, M.P.B.; Pitchford, W.S. Estimates of genetic parameters for triacylglycerol fatty acids in beef castle at weaning and slaughter. J. Anim. Physiol. Anim. Nutr. 2000, 83, 169–180. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Edriss, M.A.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. Breed differences and heterosis in triacylglycerol fatty acid composition of bovine adipose tissue. J. Anim. Physiol. Anim. Nutr. 2000, 83, 106–112. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Siebert, B.D.; Bottema, C.; Pitchford, W. Heterosis, sex and breed differences in the fatty acid composition of muscle phospholipids in beef cattle. J. Anim. Physiol. Anim. Nutr. 2000, 83, 113–120. [Google Scholar] [CrossRef][Green Version]
- Inoue, K.; Kobayashi, M.; Shoji, N.; Kato, K. Genetic parameters for fatty acid composition and feed efficiency traits in Japanese Black cattle. Animal 2011, 5, 987–994. [Google Scholar] [CrossRef]
- Kelly, M.J.; Tume, R.K.; Newman, S.; Thompson, J.M. Genetic variation in fatty acid composition of subcutaneous fat in cattle. Anim. Prod. Sci. 2013, 53, 129–133. [Google Scholar] [CrossRef]
- Nogi, T.; Honda, T.; Mukai, F.; Okagaki, T.; Oyama, K. Heritabilities and genetic correlations of fatty acid compositions in longissimus muscle lipid with carcass traits in Japanese Black cattle. J. Anim. Sci. 2011, 89, 615–621. [Google Scholar] [CrossRef]
- Sugita, H.; Ardiyanti, A.; Yokota, S.; Yonekura, S.; Hirayama, T.; Shoji, N.; Yamauchi, E.; Suzuki, K.; Katoh, K.; Roh, S.-G. Effect of single nucleotide polymorphisms in GH gene promoter region on carcass traits and intramuscular fatty acid compositions in Japanese Black cattle. Livest. Sci. 2014, 165, 15–21. [Google Scholar] [CrossRef]
- Chiaia, H.L.J.; Peripoli, E.; Silva, R.M.D.O.; Aboujaoude, C.; Feitosa, F.L.B.; de Lemos, M.V.A.; Berton, M.P.; Olivieri, B.F.; Espigolan, R.; Tonussi, R.L.; et al. Genomic prediction for beef fatty acid profile in Nellore cattle. Meat Sci. 2017, 128, 60–67. [Google Scholar] [CrossRef]
- Li, X.; Ekerljung, M.; Lundström, K.; Lundén, A. Association of polymorphisms at DGAT1, leptin, SCD1, CAPN1 and CAST genes with color, marbling and water holding capacity in meat from beef cattle populations in Sweden. Meat Sci. 2013, 94, 153–158. [Google Scholar] [CrossRef]
- Gui, L.-S.; Raza, S.H.A.; Memon, S.; Li, Z.; El-Aziz, A.H.A.; Ullah, I.; Jahejo, A.R.; Shoorei, H.; Khan, R.; Quan, G.; et al. Association of hormone-sensitive lipase (HSL) gene polymorphisms with the intramuscular fat content in two Chinese beef cattle breeds. Genomics 2020, 112, 3883–3889. [Google Scholar] [CrossRef]
- Pewan, S.B.; Otto, J.R.; Huerlimann, R.; Budd, A.M.; Mwangi, F.W.; Edmunds, R.C.; Holman, B.W.B.; Henry, M.L.E.; Kinobe, R.T.; Adegboye, O.A.; et al. Next Generation Sequencing of Single Nucleotide Polymorphic DNA-Markers in Selecting for Intramuscular Fat, Fat Melting Point, Omega-3 Long-Chain Polyunsaturated Fatty Acids and Meat Eating Quality in Tattykeel Australian White MARGRA Lamb. Foods 2021, 10, 2288. [Google Scholar] [CrossRef]
- Oh, D.; Lee, Y.; La, B.; Yeo, J.; Chung, E.; Kim, Y.; Lee, C. Fatty acid composition of beef is associated with exonic nucleotide variants of the gene encoding FASN. Mol. Biol. Rep. 2011, 39, 4083–4090. [Google Scholar] [CrossRef]
- Morris, C.A.; Cullen, N.G.; Glass, B.C.; Hyndman, D.L.; Manley, T.R.; Hickey, S.M.; McEwan, J.C.; Pitchford, W.S.; Bottema, C.D.; Lee, M.A. Fatty acid synthase effects on bovine adipose fat and milk fat. Mamm. Genome 2007, 18, 64–74. [Google Scholar] [CrossRef]
- Mannen, H. Genes Associated with Fatty Acid Composition of Beef. Food Sci. Technol. Res. 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Abe, T.; Saburi, J.; Hasebe, H.; Nakagawa, T.; Misumi, S.; Nade, T.; Nakajima, H.; Shoji, N.; Kobayashi, M.; Kobayashi, E. Novel Mutations of the FASN Gene and Their Effect on Fatty Acid Composition in Japanese Black Beef. Biochem. Genet. 2009, 47, 397–411. [Google Scholar] [CrossRef]
- Taniguchi, M.; Utsugi, T.; Oyama, K.; Mannen, H.; Kobayashi, M.; Tanabe, Y.; Ogino, A.; Tsuji, S. Genotype of stearoyl-CoA desaturase is associated with fatty acid composition in Japanese Black cattle. Mamm. Genome 2004, 14, 142–148. [Google Scholar] [CrossRef]
- Jiang, Z.; Michal, J.J.; Tobey, D.J.; Daniels, T.F.; Rule, D.C.; MacNeil, M.D. Significant associations of stearoyl-CoA desaturase (SCD1) gene with fat deposition and composition in skeletal muscle. Int. J. Biol. Sci. 2008, 4, 345–351. [Google Scholar] [CrossRef]
- Bartoň, L.; Kott, T.; Bureš, D.; Řehák, D.; Zahrádková, R.; Kottová, B. The polymorphisms of stearoyl-CoA desaturase (SCD1) and sterol regulatory element binding protein-1 (SREBP-1) genes and their association with the fatty acid profile of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 2010, 85, 15–20. [Google Scholar] [CrossRef]
- Li, C.; Aldai, N.; Vinsky, M.; Dugan, M.E.R.; McAllister, T.A. Association analyses of single nucleotide polymorphisms in bovine stearoyl-CoA desaturase and fatty acid synthase genes with fatty acid composition in commercial cross-bred beef steers. Anim. Genet. 2012, 43, 93–97. [Google Scholar] [CrossRef]
- Bartoň, L.; Bureš, D.; Kott, T.; Řehák, D. Associations of polymorphisms in bovine DGAT1, FABP4, FASN, and PPARGC1A genes with intramuscular fat content and the fatty acid composition of muscle and subcutaneous fat in Fleckvieh bulls. Meat Sci. 2016, 114, 18–23. [Google Scholar] [CrossRef]
- Zalewska, M.; Puppel, K.; Sakowski, T. Associations between gene polymorphisms and selected meat traits in cattle—A review. Anim. Biosci. 2021, 34, 1425–1438. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, Y.-S.; La, B.-M.; Yeo, J.-S. Identification of the SNP (Single Nucleotide Polymorphism) for Fatty Acid Composition Associated with Beef Flavor-related FABP4 (Fatty Acid Binding Protein 4) in Korean Cattle. Asian-Australas. J. Anim. Sci. 2012, 25, 913–920. [Google Scholar] [CrossRef][Green Version]
- Tait, R.G.; Shackelford, S.D.; Wheeler, T.L.; King, D.A.; Keele, J.W.; Casas, E.; Smith, T.P.L.; Bennett, G.L. CAPN1, CAST, and DGAT1 genetic effects on preweaning performance, carcass quality traits, and residual variance of tenderness in a beef cattle population selected for haplotype and allele equalization1,2,3,4. J. Anim. Sci. 2014, 92, 5382–5393. [Google Scholar] [CrossRef]
- Ardicli, S.; Samli, H.; Alpay, F.; Dincel, D.; Soyudal, B.; Balci, F. Association of Single Nucleotide Polymorphisms in the FABP4 Gene with Carcass Characteristics and Meat Quality in Holstein Bulls. Ann. Anim. Sci. 2017, 17, 117–130. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013; ISBN 186-496-5-975. [Google Scholar]
- Mwangi, F.W.; Blignaut, D.J.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E. Lipid metabolism, carcass characteristics and longissimus dorsi muscle fatty acid composition of tropical crossbred beef cattle in response to desmanthus spp. forage backgrounding. Metabolites 2021, 11, 804. [Google Scholar] [CrossRef]
- Mwangi, F.W.; Suybeng, B.; Gardiner, C.P.; Kinobe, R.T.; Charmley, E.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Effect of incremental proportions of Desmanthus spp. in isonitrogenous forage diets on growth performance, rumen fermentation and plasma metabolites of pen-fed growing Brahman, Charbray and Droughtmaster crossbred beef steers. PLoS ONE 2022, 17, e0260918. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. Breed comparison of the fatty acid composition of muscle phospholipids in Jersey and Limousin cattle. J. Anim. Sci. 1998, 76, 766–773. [Google Scholar] [CrossRef][Green Version]
- Flakemore, A.R.; Balogun, R.O.; McEvoy, P.D.; Malau-Aduli, B.; Nichols, P.; Malau-Aduli, A.E.O. Genetic Variation in Intramuscular Fat of Prime Lambs Supplemented with Varying Concentrations of Degummed Crude Canola Oil. Int. J. Nutr. Food Sci. 2014, 3, 203. [Google Scholar] [CrossRef]
- Pewan, S.B.; Otto, J.R.; Kinobe, R.T.; Adegboye, O.A.; Malau-Aduli, A.E.O. Margra Lamb Eating Quality and Human Health-Promoting Omega-3 Long-Chain Polyunsaturated Fatty Acid Profiles of Tattykeel Australian White Sheep: Linebreeding and Gender Effects. Antioxidants 2020, 9, 1118. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Holman, B.W.B.; Kashani, A.; Nichols, P.D. Sire breed and sex effects on the fatty acid composition and content of heart, kidney, liver, adipose and muscle tissues of purebred and first-cross prime lambs. Anim. Prod. Sci. 2016, 56, 2122. [Google Scholar] [CrossRef]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Xing, G.; Xing, C. Measuring linkage disequilibrium by the partial correlation coefficient. Heredity 2012, 109, 401–402. [Google Scholar] [CrossRef]
- Pećina, M.; Ivanković, A. Candidate genes and fatty acids in beef meat, a review. Ital. J. Anim. Sci. 2021, 20, 1716–1729. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Oh, D.-Y.; Kim, H.-J.; Jang, G.-S.; Lee, S.-U. Detection of superior genotype of fatty acid synthase in Korean native cattle by an environment-adjusted statistical model. Asian-Australas. J. Anim. Sci. 2017, 30, 765–772. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef]
- Castillo-González, A.; Burrola-Barraza, M.; Domínguez-Viveros, J.; Chávez-Martínez, A. Rumen microorganisms and fermentation. Arch. Med. Vet. 2014, 46, 349–361. [Google Scholar] [CrossRef]
- Alves, S.P.; Francisco, A.; Costa, M.; Santos-Silva, J.; Bessa, R.J. Biohydrogenation patterns in digestive contents and plasma of lambs fed increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Anim. Feed Sci. Technol. 2017, 225, 157–172. [Google Scholar] [CrossRef]
- Sakuma, H.; Saito, K.; Kohira, K.; Ohhashi, F.; Shoji, N.; Uemoto, Y. Estimates of genetic parameters for chemical traits of meat quality in Japanese black cattle. Anim. Sci. J. 2017, 88, 203–212. [Google Scholar] [CrossRef]
- Pitchford, W.S.; Deland, M.P.B.; Siebert, B.D.; Malau-Aduli, A.E.O.; Bottema, C.D.K. Genetic variation in fatness and fatty acid composition of crossbred cattle1. J. Anim. Sci. 2002, 80, 2825–2832. [Google Scholar] [CrossRef]
- Maharani, D.; Jo, C.-R.; Jeon, J.-T.; Lee, J.-H. Quantitative Trait Loci and Candidate Genes Affecting Fatty Acid Composition in Cattle and Pig. Korean J. Food Sci. Anim. Resour. 2011, 31, 325–338. [Google Scholar] [CrossRef][Green Version]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E. Main regulatory factors of marbling level in beef cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, A.M.; Cardoso, D.F.; Carvalheiro, R.; Júnior, G.A.F.; de Albuquerque, L.G.; de Oliveira, H.N. Signatures of selection in Nelore cattle revealed by whole-genome sequencing data. Genomics 2022, 114, 110304. [Google Scholar] [CrossRef] [PubMed]
- Das, D.N.; Paul, D.; Mondal, S. Role of biotechnology on animal breeding and genetic improvement. In Emerging Issues in Climate Smart Livestock Production. Biological Tools and Techniques; Mondal, S., Singh, R.L., Eds.; Academic Press: Amsterdam, The Netherlands, 2022; pp. 317–337. ISBN 9780128222652. [Google Scholar]
- Yan, W.; Zhou, H.; Hu, J.; Luo, Y.; Hickford, J.G. Variation in the FABP4 gene affects carcass and growth traits in sheep. Meat Sci. 2018, 145, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.-Z.; Fang, J.-C.; Zhang, J.-S.; Zhang, L.-M.; Xu, C.; Xu, H.-Y.; Shao, J.; Xia, G.-J. Correlations between single nucleotide polymorphisms in FABP4 and meat quality and lipid metabolism gene expression in Yanbian yellow cattle. PLoS ONE 2020, 15, e0234328. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-A.; Park, T.S.; Yoon, D.-H.; Cheong, H.S.; Namgoong, S.; Park, B.L.; Lee, H.W.; Han, C.S.; Kim, E.M.; Cheong, I.-C.; et al. Identification of genetic polymorphisms in FABP3 and FABP4 and putative association with back fat thickness in Korean native cattle. BMB Rep. 2008, 41, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, S.; Provenza, F.D.; Kronberg, S.L. Health-Promoting Phytonutrients Are Higher in Grass-Fed Meat and Milk. Front. Sustain. Food Syst. 2021, 4, 555426. [Google Scholar] [CrossRef]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef]
- Hoashi, S.; Hinenoya, T.; Tanaka, A.; Ohsaki, H.; Sasazaki, S.; Taniguchi, M.; Oyama, K.; Mukai, F.; Mannen, H. Association between fatty acid compositions and genotypes of FABP4 and LXR-alpha in Japanese Black cattle. BMC Genet. 2008, 9, 84. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Cai, C.; Su, W.; Xie, J.; Zhang, Z.; Yang, P.; Lyu, S.; Li, Z.; Lei, C.; et al. Two cSNPs sites in the fatty acid-binding protein 4 (FABP4) gene and their association analysis with body measurement data in five Chinese cattle breeds. Anim. Biotechnol. 2021, 1–8. [Google Scholar] [CrossRef]
- Zembayashi, M.; Nishimura, K.; Lunt, D.K.; Smith, S.B. Effect of breed type and sex on the fatty acid composition of subcutaneous and intramuscular lipids of finishing steers and heifers. J. Anim. Sci. 1995, 73, 3325–3332. [Google Scholar] [CrossRef]
- Gotoh, T.; Takahashi, H.; Nishimura, T.; Kuchida, K.; Mannen, H. Meat produced by Japanese Black cattle and Wagyu. Anim. Front. 2014, 4, 46–54. [Google Scholar] [CrossRef]
- Van Tran, L.; Malla, B.A.; Kumar, S.; Tyagi, A.K. Polyunsaturated Fatty Acids in Male Ruminant Reproduction—A Review. Asian-Australas. J. Anim. Sci. 2016, 30, 622–637. [Google Scholar] [CrossRef]
- Taniguchi, M.; Mannen, H.; Oyama, K.; Shimakura, Y.; Oka, A.; Watanabe, H.; Kojima, T.; Komatsu, M.; Harper, G.S.; Tsuji, S. Differences in stearoyl-CoA desaturase mRNA levels between Japanese Black and Holstein cattle. Livest. Prod. Sci. 2004, 87, 215–220. [Google Scholar] [CrossRef]
- Kim, Y.C.; Ntambi, J.M. Regulation of Stearoyl-CoA desaturase gene: Role in cellular metabolism and preadipocyte differentiation. Biochem. Biophys. Res. Commun. 1999, 266, 1–4. [Google Scholar] [CrossRef]
- Ohsaki, H.; Tanaka, A.; Hoashi, S.; Sasazaki, S.; Oyama, K.; Taniguchi, M.; Mukai, F.; Mannen, H. Effect of SCD and SREBP genotypes on fatty acid composition in adipose tissue of Japanese Black cattle herds. Anim. Sci. J. 2009, 80, 225–232. [Google Scholar] [CrossRef]
- Wu, X.X.; Yang, Z.P.; Shi, X.K.; Li, J.Y.; Ji, D.J.; Mao, Y.J.; Chang, L.L.; Gao, H.J. Association of SCD1 and DGAT1 SNPs with the intramuscular fat traits in Chinese Simmental cattle and their distribution in eight Chinese cattle breeds. Mol. Biol. Rep. 2011, 39, 1065–1071. [Google Scholar] [CrossRef]
- Dujková, R.; Ranganathan, Y.; Dufek, A.; Macák, J.; Bezdíček, J. Polymorphic effects of FABP4 and SCD genes on intramuscular fatty acid profiles in longissimus muscle from two cattle breeds. Acta Vet. Brno 2015, 84, 327–336. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Yokota, S.; Sugita, H.; Ardiyanti, A.; Shoji, N.; Nakajima, H.; Hosono, M.; Otomo, Y.; Suda, Y.; Katoh, K.; Suzuki, K. Contributions of FASN and SCD gene polymorphisms on fatty acid composition in muscle from Japanese Black cattle. Anim. Genet. 2012, 43, 790–792. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Understanding Omega-3 Polyunsaturated Fatty Acids. Postgrad. Med. 2009, 121, 148–157. [Google Scholar] [CrossRef]
- Cherfaoui, M.; Durand, D.; Bonnet, M.; Cassar-Malek, I.; Bauchart, D.; Thomas, A.; Gruffat, D. Expression of Enzymes and Transcription Factors Involved in n-3 Long Chain PUFA Biosynthesis in Limousin Bull Tissues. Lipids 2012, 47, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B. Marbling and Its Nutritional Impact on Risk Factors for Cardiovascular Disease. Korean J. Food Sci. Anim. Resour. 2016, 36, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n−3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-Chain Omega-3 Oils–An Update on Sustainable Sources. Nutrients 2010, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.T.; Luo, H.; Ausman, L.M. Cost Implications of Alternative Sources of (n-3) Fatty Acid Consumption in the United States. J. Nutr. 2012, 142, 605S–609S. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 41–54. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Meat Consumption (Indicator). Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 21 April 2020).
- Zhu, B.; Niu, H.; Zhang, W.; Wang, Z.; Liang, Y.; Guan, L.; Guo, P.; Chen, Y.; Zhang, L.; Guo, Y.; et al. Genome wide association study and genomic prediction for fatty acid composition in Chinese Simmental beef cattle using high density SNP array. BMC Genom. 2017, 18, 464. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. The fate and intermediary metabolism of stearic acid. Lipids 2005, 40, 1187–1191. [Google Scholar] [CrossRef]
- Scollan, N.; Hocquette, J.-F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Clarke, S.D.; Nakamura, M.T. Fatty Acid Structure and Synthesis. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 285–289. ISBN 9780123786319. [Google Scholar]
- Uemoto, Y.; Abe, T.; Tameoka, N.; Hasebe, H.; Inoue, K.; Nakajima, H.; Shoji, N.; Kobayashi, M.; Kobayashi, E. Whole-genome association study for fatty acid composition of oleic acid in Japanese Black cattle. Anim. Genet. 2011, 42, 141–148. [Google Scholar] [CrossRef]
- Dawood, M.; Kramer, L.M.; Shabbir, M.I.; Reecy, J.M. Genome-Wide Association Study for Fatty Acid Composition in American Angus Cattle. Animals 2021, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.S.; Kim, Y.K.; Kim, H.J.; Lee, D.H.; Lee, S.H.; Yoon, H.B.; Lee, S.H. Genome-wide association study and prediction of genomic breeding values for fatty-acid composition in Korean Hanwoo cattle using a high-density single-nucleotide polymorphism array. J. Anim. Sci. 2018, 96, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, D.; Zhang, S.; Yang, S.; Alim, M.A.; Zhang, Q.; Li, Y.; Liu, L. Genetic effects of FASN, PPARGC1A, ABCG2 and IGF1 revealing the association with milk fatty acids in a Chinese Holstein cattle population based on a post genome-wide association study. BMC Genet. 2016, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, T.; Maruyama, S.; Uemoto, Y.; Kobayashi, N.; Mannen, H.; Abe, T.; Sakaguchi, S.; Kobayashi, E. Effects of bovine fatty acid synthase, stearoyl-coenzyme A desaturase, sterol regulatory element-binding protein 1, and growth hormone gene polymorphisms on fatty acid composition and carcass traits in Japanese Black cattle1. J. Anim. Sci. 2011, 89, 12–22. [Google Scholar] [CrossRef]
- Yeon, S.; Lee, S.; Choi, B.; Lee, H.; Jang, G.; Lee, K.; Kim, K.; Lee, J.; Chung, H. Genetic variation of FASN is associated with fatty acid composition of Hanwoo. Meat Sci. 2013, 94, 133–138. [Google Scholar] [CrossRef]
- Lee, J.; Jin, M.; Lee, Y.; Ha, J.; Yeo, J.; Oh, D. Gene–gene interactions of fatty acid synthase (FASN) using multifactor-dimensionality reduction method in Korean cattle. Mol. Biol. Rep. 2014, 41, 2021–2027. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.A.; Yu, S.L.; Jeon, J.T.; Yoon, D.; Cho, Y.M.; Park, E.W.; Kim, N.K.; Kim, K.S.; Lee, J.H. DNA Polymorphisms in SREBF1 and FASN Genes Affect Fatty Acid Composition in Korean Cattle (Hanwoo). Asian-Australas. J. Anim. Sci. 2009, 22, 765–773. [Google Scholar] [CrossRef]
- De Smet, S.; Raes, K.; Demeyer, D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Anim. Res. 2004, 53, 81–98. [Google Scholar] [CrossRef]
- Mosley, E.E.; Shafii, B.; Moate, P.; McGuire, M.A. cis-9, trans-11 Conjugated Linoleic Acid Is Synthesized Directly from Vaccenic Acid in Lactating Dairy Cattle. J. Nutr. 2006, 136, 570–575. [Google Scholar] [CrossRef]
- Guo, M. Lipids and lipid related functional foods. In Functional Foods: Principles and Technology; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 161–196. [Google Scholar]
- Dervishi, E.; Serrano, C.; Joy, M.; Serrano, M.; Rodellar, C.; Calvo, J.H. Effect of the feeding system on the fatty acid composition, expression of the Δ9-desaturase, Peroxisome Proliferator-Activated Receptor Alpha, Gamma, and Sterol Regulatory Element Binding Protein 1 genes in the semitendinous muscle of light lambs of the R. BMC Vet. Res. 2010, 6, 40. [Google Scholar] [CrossRef]
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.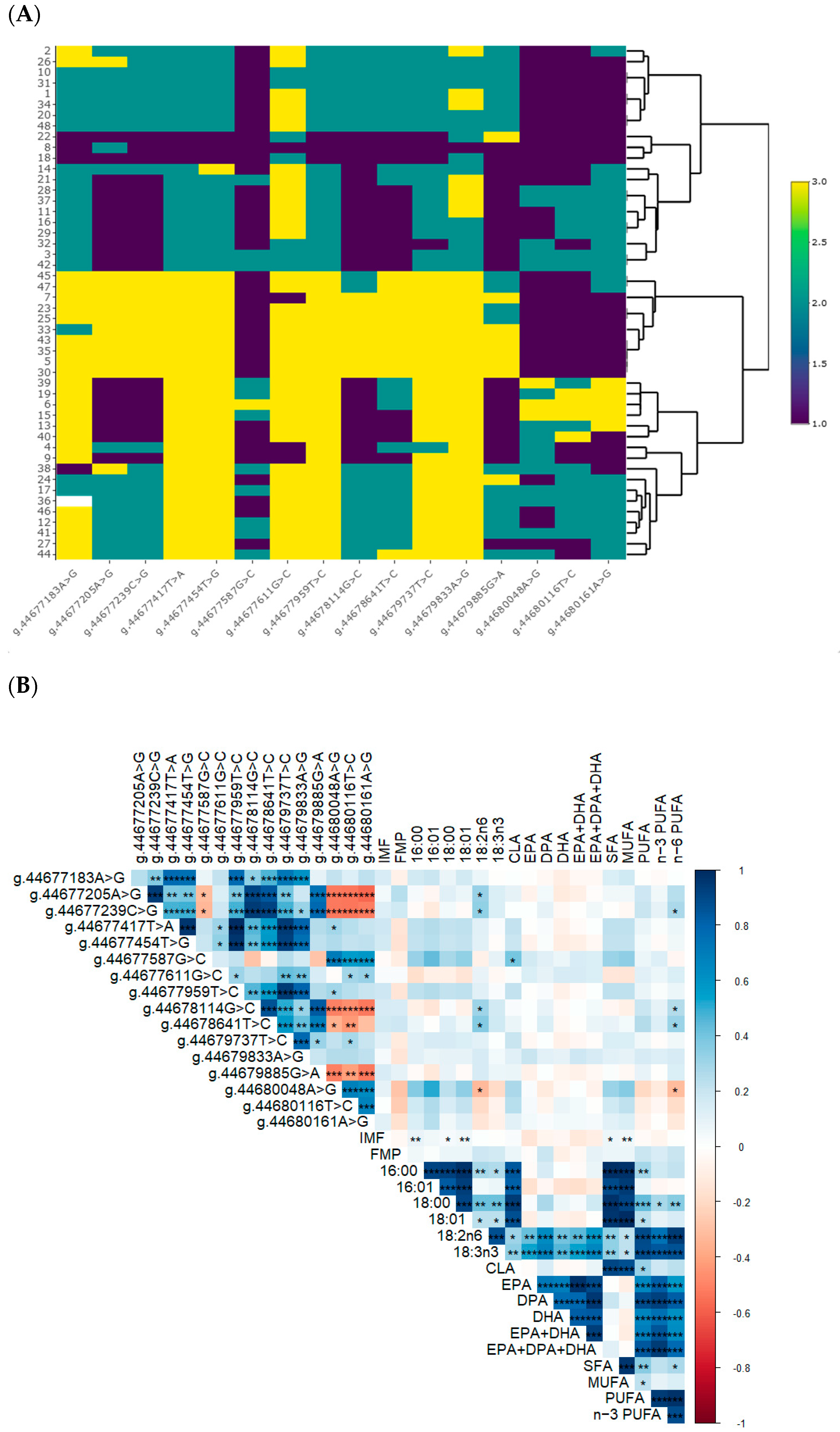
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
 homozygotes similar to the reference sequence genotype (Hereford),
homozygotes similar to the reference sequence genotype (Hereford),  heterozygotes and
heterozygotes and  alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.
alternative allele homozygotes. (B) Correlations between SNP and IMF, FMP and fatty acids. * p < 0.05, ** p < 0.01, and *** p < 0.001.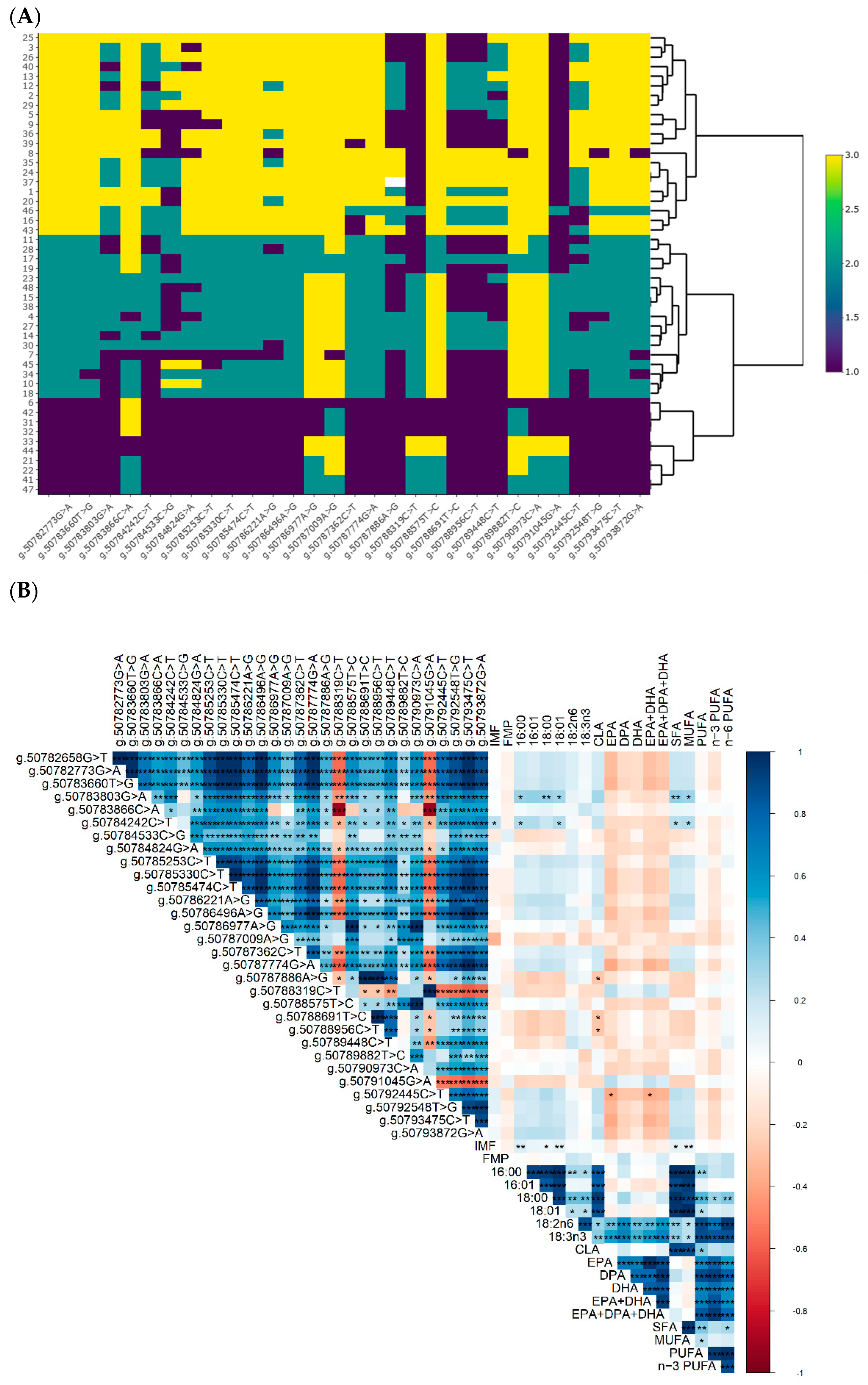
 ), CG (
), CG ( ), and GG (
), and GG ( ).
).
 ), CG (
), CG ( ), and GG (
), and GG ( ).
).
 ), GT (
), GT ( ) and TT (
) and TT ( ).
).
 ), GT (
), GT ( ) and TT (
) and TT ( ).
).
| Gene 1 | SNP (Variant ID) 2 | PCS Position 4 | Amino Acid Substitution |
|---|---|---|---|
| FABP4 | g.44677959 T>C (rs110757796) | 220 | Isoleucine to Valine |
| SCD | g.21272422 C>T (rs41255693) | 878 | Alanine to Valine |
| FASN | g.50782773 G>A (rs715140536) | 1243 | Alanine to Threonine |
| g.50784533 C>G (rs481622676) | 2066 | Alanine to Glycine | |
| g.50784824 G>A (rs209227647) | 2252 | Arginine to Histidine | |
| g.50786496A>G 3 | 3145 | Serine to Glycine | |
| g.50788575T>C (rs41919993) | 4168 | Tyrosine to Histidine | |
| g.50789448C>T (rs516607144) | 4693 | Leucine to Phenylalanine | |
| g.50790973C>A (rs109149276) | 5572 | Leucine to Isoleucine |
| Gene/SNP 1 | p-Value 2 | ||||
|---|---|---|---|---|---|
| FABP4 g.44677239C>G | Total (n = 48) | CC (n = 19) | CG (n = 19) | GG (n = 10) | |
| IMF | 2.3 ± 0.75 | 2.1 ± 0.62 | 2.5 ± 0.9 | 2.2 ± 0.67 | 0.38 |
| FMP | 43.9 ± 4.79 | 42.7 ± 4.58 | 44.6 ± 4.83 | 44.9 ± 5.13 | 0.53 |
| 16:0 (Palmitic acid) | 209.1 ± 149.68 | 194.3 ± 113.24 | 238.7 ± 185.99 | 179.5 ± 133.59 | 0.72 |
| 16:1 (Palmitoleic acid) | 34.6 ± 34.72 | 40.5 ± 47.35 | 34.5 ± 25.6 | 23.3 ± 16.87 | 0.52 |
| 18:0 (Stearic acid) | 128.6 ± 78.22 | 119.2 ± 66.31 | 139.4 ± 83.68 | 125.1 ± 92.35 | 0.61 |
| 18:1 (Oleic acid) | 263.1 ± 195.31 | 244.6 ± 143.89 | 302.4 ± 245.96 | 221.4 ± 170.23 | 0.74 |
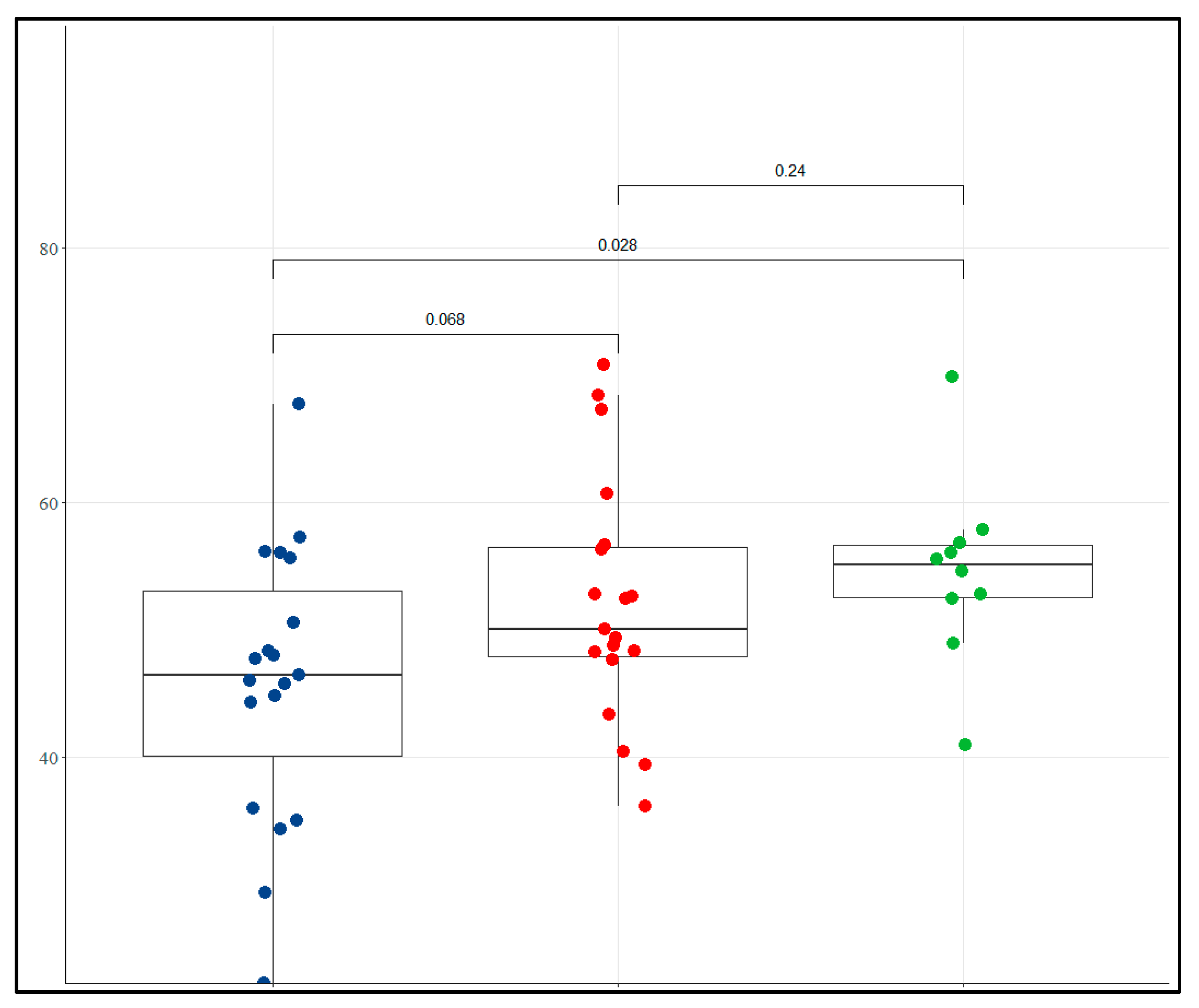
| 18:2ω6 (Linoleic acid) | 50.1 ± 10.2 | 45.8 ± 10.88a | 52.0 ± 9.62 ab | 54.5 ± 7.3b | 0.03 |
| 18:3ω3 (α-linolenic acid) | 16.3 ± 3.14 | 15.7 ± 3.69 | 16.4 ± 2.85 | 16.9 ± 2.73 | 0.73 |
| CLA | 4.3 ± 3.33 | 4.2 ± 3.11 | 4.4 ± 3.49 | 4.1 ± 3.77 | 0.79 |
| EPA | 9.4 ± 2.18 | 9.2 ± 2.13 | 9.8 ± 2.31 | 9.1 ± 2.12 | 0.58 |
| DPA | 14.0 ± 3.37 | 13.0 ± 3.61 | 15.0 ± 2.64 | 13.9 ± 3.92 | 0.12 |
| DHA | 2.3 ± 0.8 | 2.3 ± 0.93 | 2.5 ± 0.69 | 2.2 ± 0.79 | 0.28 |
| EPA+DHA | 11.8 ± 2.77 | 11.5 ± 2.81 | 12.3 ± 2.76 | 11.4 ± 2.86 | 0.33 |
| EPA+DPA+DHA | 25.8 ± 5.79 | 24.6 ± 5.88 | 27.4 ± 5.23 | 25.3 ± 6.53 | 0.14 |
| SFA | 376.9 ± 254.18 | 351.5 ± 201.61 | 420.0 ± 298.96 | 340.8 ± 260.83 | 0.75 |
| MUFA | 313.4 ± 228.69 | 294.7 ± 173.9 | 358.2 ± 285.22 | 261.9 ± 199.01 | 0.69 |
| PUFA | 142.8 ± 26.51 | 134.2 ± 28.71 | 149.6 ± 23.23 | 146.3 ± 25.98 | 0.41 |
| ω3 PUFA | 48.2 ± 8.92 | 47.0 ± 10.06 | 49.9 ± 7.84 | 47.5 ± 8.96 | 0.55 |
| ω6 PUFA | 79.6 ± 16.18 | 73.0 ± 18.67 | 83.6 ± 13.12 | 84.5 ± 13.17 | 0.11 |
| SCD g.21266629 G>T | Total (n = 48) | GG (n = 11) | GT (n = 22) | TT (n = 15) | |
| IMF | 2.3 ± 0.75 | 2.2 ± 0.55 | 2.2 ± 0.62 | 2.5 ± 1.03 | 0.64 |
| FMP | 43.9 ± 4.79 | 42.9 ± 3.64 | 44.1 ± 6.17 | 44.3 ± 3.26 | 0.78 |
| 16:0 (Palmitic acid) | 209.1 ± 149.68 | 182.2 ± 111.02 | 201.0 ± 116.16 | 240.2 ± 209.36 | 0.86 |
| 16:1 (Palmitoleic acid) | 34.6 ± 34.72 | 27.4 ± 16.93 | 38.4 ± 44.8 | 34.2 ± 27.7 | 0.91 |
| 18:0 (Stearic acid) | 128.6 ± 78.22 | 125.6 ± 76.49 | 122.6 ± 60.52 | 139.2 ± 102.52 | 0.82 |
| 18:1 (Oleic acid) | 263.1 ± 195.31 | 241.6 ± 164.92 | 255.0 ± 152.87 | 290.1 ± 266.99 | 0.82 |
| 18:2ω6 (Linoleic acid) | 50.1 ± 10.2 | 50.0 ± 5.35 | 48.0 ± 10.93 | 53.2 ± 11.52 | 0.66 |
| 18:3ω3 (α-linolenic acid) | 16.3 ± 3.14 | 16.5 ± 2.66 | 15.5 ± 3.24 | 17.1 ± 3.28 | 0.49 |
| CLA | 4.3 ± 3.33 | 4.7 ± 4.46 | 4.3 ± 2.71 | 4.0 ± 3.4 | 0.57 |
| EPA | 9.4 ± 2.18 | 8.6 ± 1.61 a | 9.2 ± 2.43 ab | 10.3 ± 1.93 b | 0.08 |
| DPA | 14.0 ± 3.37 | 12.9 ± 1.78 a | 13.4 ± 3.75 ab | 15.7 ± 3.2 b | 0.03 |
| DHA | 2.3 ± 0.8 | 2.1 ± 0.51 a | 2.2 ± 0.88 a | 2.8 ± 0.74 b | 0.02 |
| EPA+DHA | 11.8 ± 2.77 | 10.8 ± 1.89 a | 11.4 ± 3.07 ab | 13.1 ± 2.48 b | 0.03 |
| EPA+DPA+DHA | 25.8 ± 5.79 | 23.7 ± 3.21 a | 24.8 ± 6.31 ab | 28.9 ± 5.51 b | 0.02 |
| SFA | 376.9 ± 254.18 | 345.6 ± 210.17 | 360.6 ± 197.48 | 422.7 ± 348.72 | 0.89 |
| MUFA | 313.4 ± 228.69 | 287.2 ± 191.76 | 303.9 ± 181.66 | 345.7 ± 310.88 | 0.85 |
| PUFA | 142.8 ± 26.51 | 138.0 ± 15.98 | 137.8 ± 29.03 | 153.7 ± 26.98 | 0.31 |
| ω3 PUFA | 48.2 ± 8.92 | 45.5 ± 5.37 | 47.2 ± 10.49 | 51.8 ± 7.77 | 0.12 |
| ω6 PUFA | 79.6 ± 16.18 | 78.5 ± 7.52 | 75.9 ± 19.07 | 85.9 ± 15.07 | 0.40 |
| FASN g.50783803G>A | Total (n = 48) | GG (n = 20) | GA (n = 20) | AA (n = 8) | |
| IMF | 2.3 ± 0.75 | 2.2 ± 0.71 | 2.3 ± 0.60 | 2.5 ± 1.14 | 0.49 |
| FMP | 43.9 ± 4.79 | 44.8 ± 3.56 | 43.1 ± 6.01 | 43.8 ± 3.62 | 0.40 |
| 16:0 (Palmitic acid) | 209.1 ± 149.68 | 161.3 ± 84.44 | 211.4 ± 131.91 | 323.2 ± 248.45 | 0.24 |
| 16:1 (Palmitoleic acid) | 34.6 ± 34.72 | 26.0 ± 13.60 | 41.8 ± 46.84 | 45.3 ± 31.24 | 0.17 |
| 18:0 (Stearic acid) | 128.6 ± 78.22 | 103.2 ± 38.01 | 129.8 ± 76.27 | 189.3 ± 123.57 | 0.28 |
| 18:1 (Oleic acid) | 263.1 ± 195.31 | 196.9 ± 114.57 | 279.5 ± 185.13 | 389.2 ± 309.04 | 0.16 |
| 18:2ω6 (Linoleic acid) | 50.1 ± 10.20 | 50.5 ± 10.29 | 48.9 ± 9.88 | 52.1 ± 11.72 | 0.95 |
| 18:3ω3 (α-linolenic acid) | 16.3 ± 3.14 | 16.3 ± 3.00 | 16.1 ± 3.34 | 16.8 ± 3.32 | 0.87 |
| CLA | 4.3 ± 3.33 | 3.4 ± 1.70 | 4.8 ± 4.01 | 5.6 ± 4.49 | 0.35 |
| EPA | 9.4 ± 2.18 | 9.9 ± 2.25 | 9.0 ± 2.05 | 9.3 ± 2.40 | 0.52 |
| DPA | 14.0 ± 3.37 | 14.7 ± 2.78 | 13.1 ± 3.45 | 14.5 ± 4.40 | 0.53 |
| DHA | 2.3 ± 0.80 | 2.4 ± 0.78 | 2.3 ± 0.8 | 2.1 ± 0.91 | 0.48 |
| EPA+DHA | 11.8 ± 2.77 | 12.3 ± 2.89 | 11.4 ± 2.5 | 11.4 ± 3.25 | 0.44 |
| EPA+DPA+DHA | 25.8 ± 5.79 | 27.1 ± 5.48 | 24.6 ± 5.34 | 26.0 ± 7.57 | 0.48 |
| SFA | 376.9 ± 254.18 | 294.0 ± 135.9 | 381.0 ± 229.59 | 574.6 ± 417.76 | 0.28 |
| MUFA | 313.4 ± 228.69 | 235.3 ± 132.97 | 332.1 ± 217.83 | 464.1 ± 359.61 | 0.20 |
| PUFA | 142.8 ± 26.51 | 143.9 ± 22.70 | 139.2 ± 25.97 | 148.9 ± 37.51 | 0.90 |
| ω3 PUFA | 48.2 ± 8.92 | 48.9 ± 8.18 | 47.4 ± 9.19 | 48.9 ± 10.91 | 0.82 |
| ω6 PUFA | 79.6 ± 16.18 | 81.5 ± 14.45 | 76.8 ± 16.70 | 79.6 ± 19.88 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwangi, F.W.; Pewan, S.B.; Otto, J.R.; Adegboye, O.A.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E.O. Towards Sustainable Sources of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Northern Australian Tropical Crossbred Beef Steers through Single Nucleotide Polymorphisms in Lipogenic Genes for Meat Eating Quality. Sustainability 2022, 14, 8409. https://doi.org/10.3390/su14148409
Mwangi FW, Pewan SB, Otto JR, Adegboye OA, Charmley E, Gardiner CP, Malau-Aduli BS, Kinobe RT, Malau-Aduli AEO. Towards Sustainable Sources of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Northern Australian Tropical Crossbred Beef Steers through Single Nucleotide Polymorphisms in Lipogenic Genes for Meat Eating Quality. Sustainability. 2022; 14(14):8409. https://doi.org/10.3390/su14148409
Chicago/Turabian StyleMwangi, Felista W., Shedrach B. Pewan, John R. Otto, Oyelola A. Adegboye, Edward Charmley, Christopher P. Gardiner, Bunmi S. Malau-Aduli, Robert T. Kinobe, and Aduli E. O. Malau-Aduli. 2022. "Towards Sustainable Sources of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Northern Australian Tropical Crossbred Beef Steers through Single Nucleotide Polymorphisms in Lipogenic Genes for Meat Eating Quality" Sustainability 14, no. 14: 8409. https://doi.org/10.3390/su14148409
APA StyleMwangi, F. W., Pewan, S. B., Otto, J. R., Adegboye, O. A., Charmley, E., Gardiner, C. P., Malau-Aduli, B. S., Kinobe, R. T., & Malau-Aduli, A. E. O. (2022). Towards Sustainable Sources of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Northern Australian Tropical Crossbred Beef Steers through Single Nucleotide Polymorphisms in Lipogenic Genes for Meat Eating Quality. Sustainability, 14(14), 8409. https://doi.org/10.3390/su14148409







