Performance of Poly(caprolactone) (PCL) as an Impact Modifier for Polystyrene (PS): Effect of Functionalized Compatibilizers with Maleic Anhydride and Glycidyl Methacrylate
Abstract
:1. Introduction
2. Materials
3. Extrusion and Injection Molding Processing of the Blends
4. Characterization
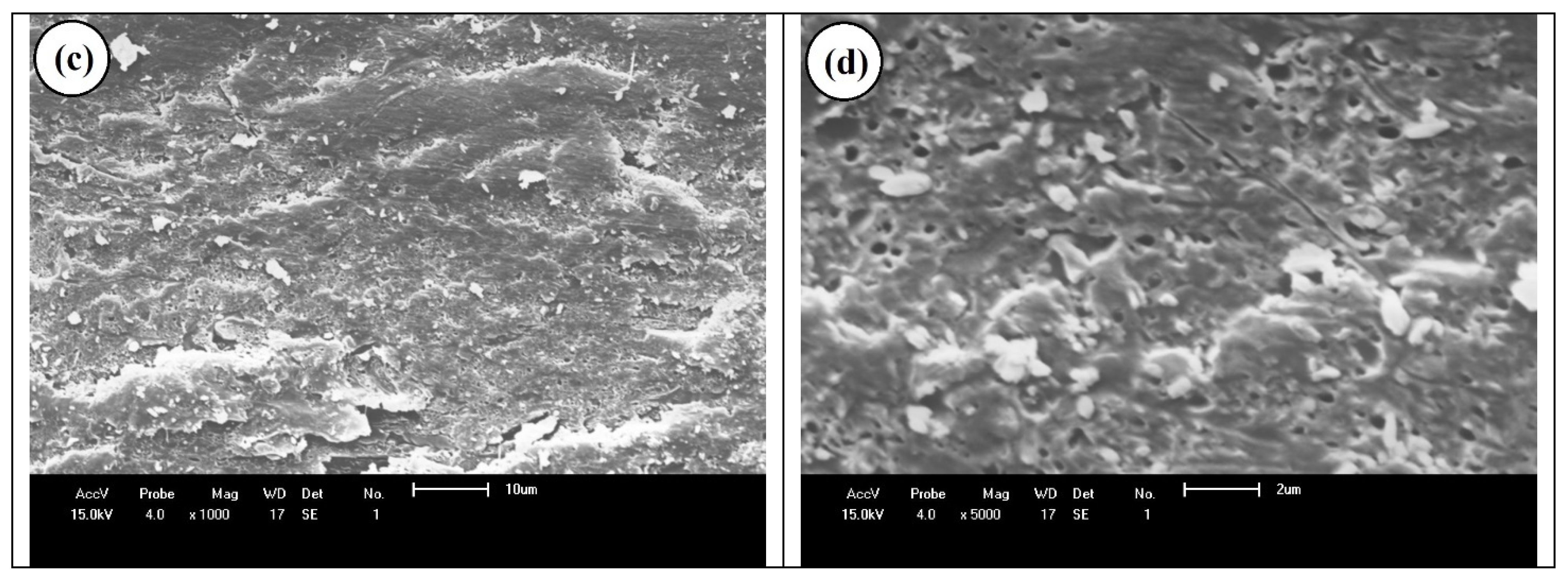
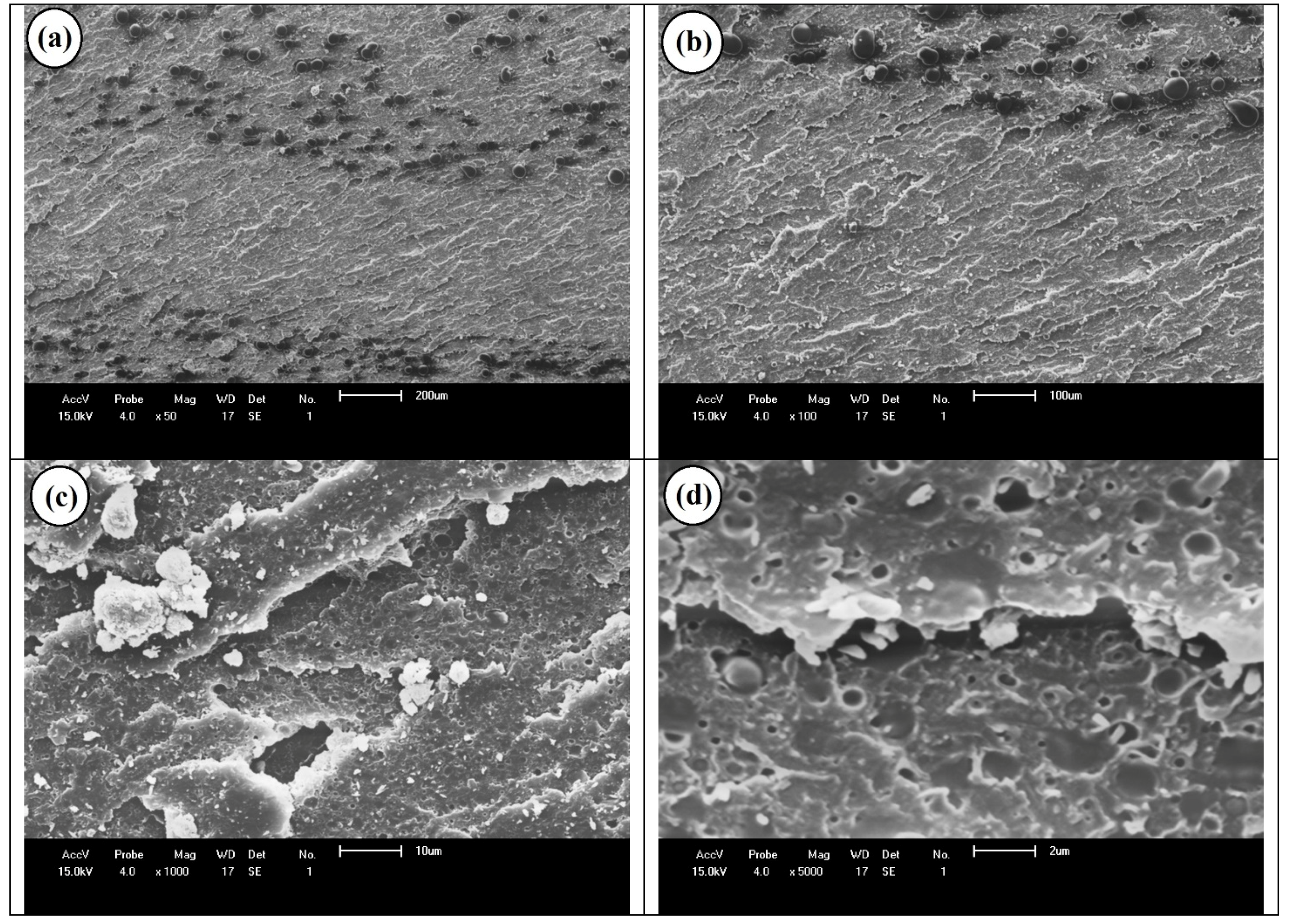
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coutinho, F.M.B.; Costa, M.P.M.; Guimarães, M.J.O.C.; Soares, B.G. Preparation and characterization of high-impact polystyrene using different types of polybutadiene. J. Appl. Polym. Sci. 2007, 108, 406–413. [Google Scholar] [CrossRef]
- Luna, C.; Siqueira, D.; Ferreira, E.; Silva, W.; Nogueira, J.; Araújo, E. From Disposal to Technological Potential: Reuse of Polypropylene Waste from Industrial Containers as A Polystyrene Impact Modifier. Sustainability 2020, 12, 5272. [Google Scholar] [CrossRef]
- Grassi, V.G.; Forte, M.M.C.; Pizzol, M.F.D. Morphologic Aspects and Structure-Properties Relations of High Impact Polystyrene. Polímeros 2001, 11, 158–168. [Google Scholar] [CrossRef]
- Rovere, J.; Correa, C.A.; Grassi, V.G.; Pizzol, M.F.D. Morphological Characterization of High Impact Polystyrene (HIPS). Polímeros 2008, 18, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Libio, I.C.; Grassi, V.G.; Pizzol, M.F.D.; Nachtigall, S.M.B. Toughened polystyrene with improved photoresistance: Effects of the compatibilizers. J. Appl. Polym. Sci. 2012, 126, 179–185. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Feng, X.; Han, K.; Huan, Q.; Song, J.; Ma, Y.; Yu, M. Deformation and toughening mechanism for high impact polystyrene (HIPS) by pressure-induced-flow processing. RSC Adv. 2013, 3, 6879–6887. [Google Scholar] [CrossRef]
- Fowler, M.W.; Padeiro, N. Rubber toughening of polystyrene through reactive blending. Polym. Eng. Sci. 1988, 28, 1427–1433. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, J.; Yang, H.; Zhou, C.; Zhang, H. Deformation mechanism of polystyrene toughened with sub-micrometer monodisperse rubber particles. Polym. Int. 2006, 55, 1215–1221. [Google Scholar] [CrossRef]
- Fang, Z.; Guo, Z.; Zha, L. Toughening of Polystyrene with Ethylene-Propylene-Diene Terpolymer(EPDM) Compatibilized by Styrene-Butadiene-Styrene Block Copolymer(SBS). Macromol. Mater. Eng. 2004, 289, 743–748. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; Costa, M.P.M.; Guimarães, M.J.O.C.; Soares, B.G. Comparative Study of Different Types of Polybutadiene on the Toughening of Polystyrene. Polímeros 2007, 17, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.B.B.; Da Silva, D.F.; Araújo, E.M.; De Mélo, T.J.A.; Bezerra, E.D.O.T.; Siqueira, D.D.; Oliveira, A. Blends of Polystyrene/Shoes Waste (SBRr): Influence of Mixture Sequence and Compatibilizer. Macromol. Symp. 2019, 383, 1800046. [Google Scholar] [CrossRef] [Green Version]
- Tack, H.; Kim, H.R.; Kim, J.K.; Park, J.Y. The morphology and mechanical properties of the blends of syndiotactic polystyrene and polystyrene-block-poly(ethylene-co-butylene)-block-polystyrene copolymers. Korea-Aust. Rheol. J. 2001, 13, 83–87. [Google Scholar]
- Wenguang, C.; Yanlei, G.; Fen, G. Toughening study of fire-retardant high-impact polystyrene. Fire Mater. 2009, 33, 99–108. [Google Scholar] [CrossRef]
- Barrera, G.M.; Menchaca, C.; Pietkiewicz, D.; Brostow, W. Polystyrene + Styrene-Butadiene Blends: Mechanical and Morphological Properties. Mater. Sci. 2004, 10, 166–172. [Google Scholar]
- Espinosa, E.A.; Escobar-Barrios, V.; Escobedo, G.P.; Mendoza, M.A.W. Thermal and mechanical properties of UHMWPE/HDPE/PCL and bioglass filler: Effect of polycaprolactone. J. Appl. Polym. Sci. 2020, 138, 50374. [Google Scholar] [CrossRef]
- Mandal, D.K.; Bhunia, H.; Bajpai, P.K.; Chaudhari, C.V.; Dubey, K.A.; Varshney, L.; Kumar, A. Preparation and characterization of polypropylene/polylactide blends and nanocomposites and their biodegradation study. J. Thermoplast. Compos. Mater. 2019, 34, 725–744. [Google Scholar] [CrossRef]
- Lekube, B.M.; Burgstaller, C. Study of mechanical and rheological properties, morphology, and miscibility in polylactid acid blends with thermoplastic polymers. J. Appl. Polym. Sci. 2021, 139, 51662. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Khan, F.U.; Ullah, R.; Haq, F.; Iqbal, M.; Ullah, A. Synthesis of Carboxymethyl Starch-Bio-Based Epoxy Resin and their Impact on Mechanical Properties. Z. Für Phys. Chem. 2019, 234, 1759–1769. [Google Scholar] [CrossRef]
- Kumar, D.; Babu, G.; Krishnan, S. Study on mechanical & thermal properties of PCL blended graphene biocomposites. Polímeros 2019, 29, e2019024. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, D.D.; Luna, C.B.B.; Morais, D.D.S.; Araújo, E.M.; França, D.C.; Wellen, R.M.R. Efeito das variáveis reacionais na síntese de um polímero biodegradável funcionalizado: PCL-g-MA. Matéria 2018, 23, e12252. [Google Scholar] [CrossRef] [Green Version]
- Filho, E.A.S.; Luna, C.B.B.; Silva, A.L.; Ferreira, E.S.B.; Araújo, E.M.; Costa, A.C.F.M. Effect of kaolin waste annealing on the structural and thermal behavior of poly(ε−caprolactone). Momento 2022, 64, 66–84. [Google Scholar] [CrossRef]
- Roa, J.P.B.; Mano, V.; Faustino, P.B.; Felix, E.B.; Silva, M.E.S.R.; Filho, J.D.S. Síntese e Caracterização do Copolímero Poli(3-Hidroxibutirato-co-ε-Caprolactona) a Partir de Poli(3-Hidroxibutirato) e Poli(ε-Caprolactona). Polímeros 2010, 20, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Reul, L.T.A.; Pereira, C.A.B.; Sousa, F.M.; Santos, R.M.; Carvalho, L.H.; Canedo, E.L. Polycaprolactone/babassu compounds: Rheological, thermal, and morphological characteristics. Polym. Compos. 2018, 40, E540–E549. [Google Scholar] [CrossRef]
- Mohamed, A.; Gordon, S.H.; Biresaw, G. Polycaprolactone/polystyrene bioblends characterized by thermogravimetry, modulated differential scanning calorimetry and infrared photoacoustic spectroscopy. Polym. Degrad. Stab. 2007, 92, 1177–1185. [Google Scholar] [CrossRef]
- Biresaw, G.; Carriere, C. Compatibility and mechanical properties of blends of polystyrene with biodegradable polyesters. Compos. Part A Appl. Sci. Manuf. 2004, 35, 313–320. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Luna, C.B.B.; Siqueira, D.D.; Filho, E.A.S.; Araújo, E.M.; Wellen, R.M.R. Production of Eco-Sustainable Materials: Compatibilizing Action in Poly (Lactic Acid)/High-Density Biopolyethylene Bioblends. Sustainability 2021, 13, 12157. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2018, 51, 291–336. [Google Scholar] [CrossRef]
- Shamsuri, A.; Jamil, S. Application of Quaternary Ammonium Compounds as Compatibilizers for Polymer Blends and Polymer Composites—A Concise Review. Appl. Sci. 2021, 11, 3167. [Google Scholar] [CrossRef]
- Lima, J.C.C.; Araújo, J.P.; Agrawal, P.; Mélo, T.J.A. Efeito do teor do copolímero SEBS no comportamento reológico da blenda PLA/SEBS. Remap 2016, 11, 10–17. [Google Scholar]
- Bezerra, E.B.; De França, D.C.; Morais, D.D.D.S.; Silva, I.D.D.S.; Siqueira, D.D.; Araújo, E.M.; Wellen, R.M.R. Compatibility and characterization of Bio-PE/PCL blends. Polímeros 2019, 29, e2019022. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Mélo, T.J.A.; Pinto, J.C.; Balaban, R. Mechanical and Morphological Properties of PLA/BioPE Blend Compatibilized with E-GMA and EMA-GMA Copolymers. Macromol. Symp. 2016, 367, 176–182. [Google Scholar] [CrossRef]
- Filho, E.A.D.S.; Luna, C.B.B.; Siqueira, D.D.; Ferreira, E.D.S.B.; Araújo, E.M. Tailoring Poly(lactic acid) (PLA) Properties: Effect of the Impact Modifiers EE-g-GMA and POE-g-GMA. Polymers 2021, 14, 136. [Google Scholar] [CrossRef]
- Yoo, T.W.; Yoon, H.G.; Choi, S.J.; Kim, M.S.; Kim, Y.H.; Kim, W.N. Effects of compatibilizers on the mechanical properties and interfacial tension of polypropylene and poly(lactic acid) blends. Macromol. Res. 2010, 18, 583–588. [Google Scholar] [CrossRef]
- Chevallier, C.; Becquart, F.; Taha, M. Polystyrene/polycarbonate blends compatibilization: Morphology, rheological and mechanical properties. Mater. Chem. Phys. 2013, 139, 616–622. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araújo, E.M.; De Melo, T.J.A. Polylactide/Biopolyethylene bioblends. Polímeros 2012, 22, 427–429. [Google Scholar] [CrossRef] [Green Version]
- Reis, M.M.S.; Santos, Z.I.G.; Ueki, M.M.; Brito, G.F. Avaliação do Tipo de Plastificante nas Propriedades de Blendas de Polietileno/Amido Termoplástico. Remap 2016, 11, 130–135. [Google Scholar]
- Reul, L.T.; Carvalho, L.H.; Canedo, E.L. Características Reológicas e Térmicas de Compósitos Policaprolactona/Babaçu. Remap 2018, 12, 174–182. [Google Scholar]
- Ali, A.; Muhammad, N.; Hussain, S.; Jamil, M.; Uddin, A.; Aziz, T.; Tufail, M.; Guo, Y.; Wei, T.; Rasool, G.; et al. Kinetic and Thermal Study of Ethylene and Propylene Homo Polymerization Catalyzed by ansa-Zirconocene Activated with Alkylaluminum/Borate: Effects of Alkylaluminum on Polymerization Kinetics and Polymer Structure. Polymers 2021, 13, 268. [Google Scholar] [CrossRef]
- Alves, T.S.; Neto, J.E.S.; Silva, S.M.; Carvalho, L.H.; Canedo, E.L. Process simulation of laboratory internal mixers. Polym. Test. 2016, 50, 94–100. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Filho, E.A.D.S.; Siqueira, D.D.; Araújo, E.M.; Nascimento, E.P.D.; de Mélo, T.J.A. Influence of Small Amounts of ABS and ABS-MA on PA6 Properties: Evaluation of Torque Rheometry, Mechanical, Thermomechanical, Thermal, Morphological, and Water Absorption Kinetics Characteristics. Materials 2022, 15, 2502. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Liu, B.; Estep, G.; Zhang, J. Super Toughened Poly(lactic acid) Ternary Blends by Simultaneous Dynamic Vulcanization and Interfacial Compatibilization. Macromolecules 2010, 43, 6058–6066. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araújo, E.M.; Mélo, T.J. Effect of combining ethylene/methyl acrylate/glycidyl methacrylate terpolymer and an organoclay on the toughening of poly(lactic acid). Polym. Eng. Sci. 2013, 54, 1922–1930. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araújo, E.M.; de Melo, T.J. Toughening of Polylactide by Melt Blending with an (Ethylene/Methyl Acrylate/Glycidyl Methacrylate) Terpolymer. Polímeros 2012, 22, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Chevallier, C.; Becquart, F.; Majeste, J.C.; Taha, M. Solvent-free preparation, characterization, and properties of SEBS–g–polycarbonate copolymers. Des. Monomers Polym. 2013, 16, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Mosab, K.; Fawaz, D. Preparation and characterization of binary and ternary blends with poly(lactic acid), polystyrene, and acrylonitrile-butadiene-styrene. J. Biomater. Nanobiotechnol. 2012, 3, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.F.; Luna, C.B.B.; Silva, G.; Araújo, E.M.; Melo, T.J.A. Avaliação das propriedades mecânicas de blendas de poliestireno/composto de borracha reciclada (SBRr). Remap 2014, 9, 92–97. [Google Scholar]
- Coutinho, F.M.B.; Costa, M.P.M.; Guimarães, M.J.O.C.; Soares, B.G. Estudo comparativo de diferentes tipos de polibutadieno na tenacificação de poliestireno. Polímeros 2007, 17, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Paz, R.A.; Leite, A.M.D.; Araújo, E.M.; Melo, T.J.A.; Barbosa, R.; Ito, E.N. Nanocompósitos de poliamida 6/Argila organofílica: Efeito do peso molecular da matriz na estrutura e propriedades mecânicas e termomecânicas. Polímeros 2008, 18, 341–347. [Google Scholar] [CrossRef]
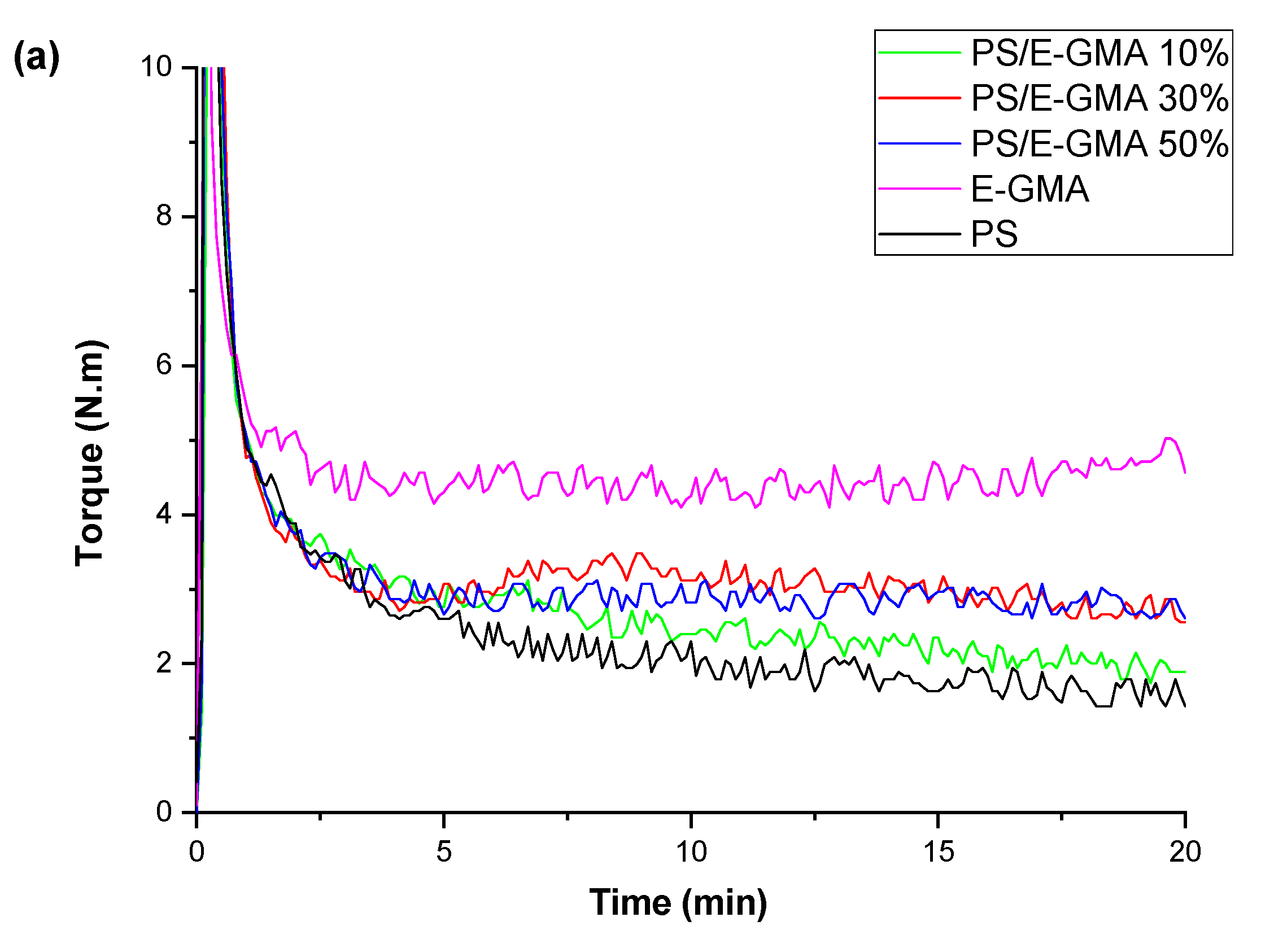
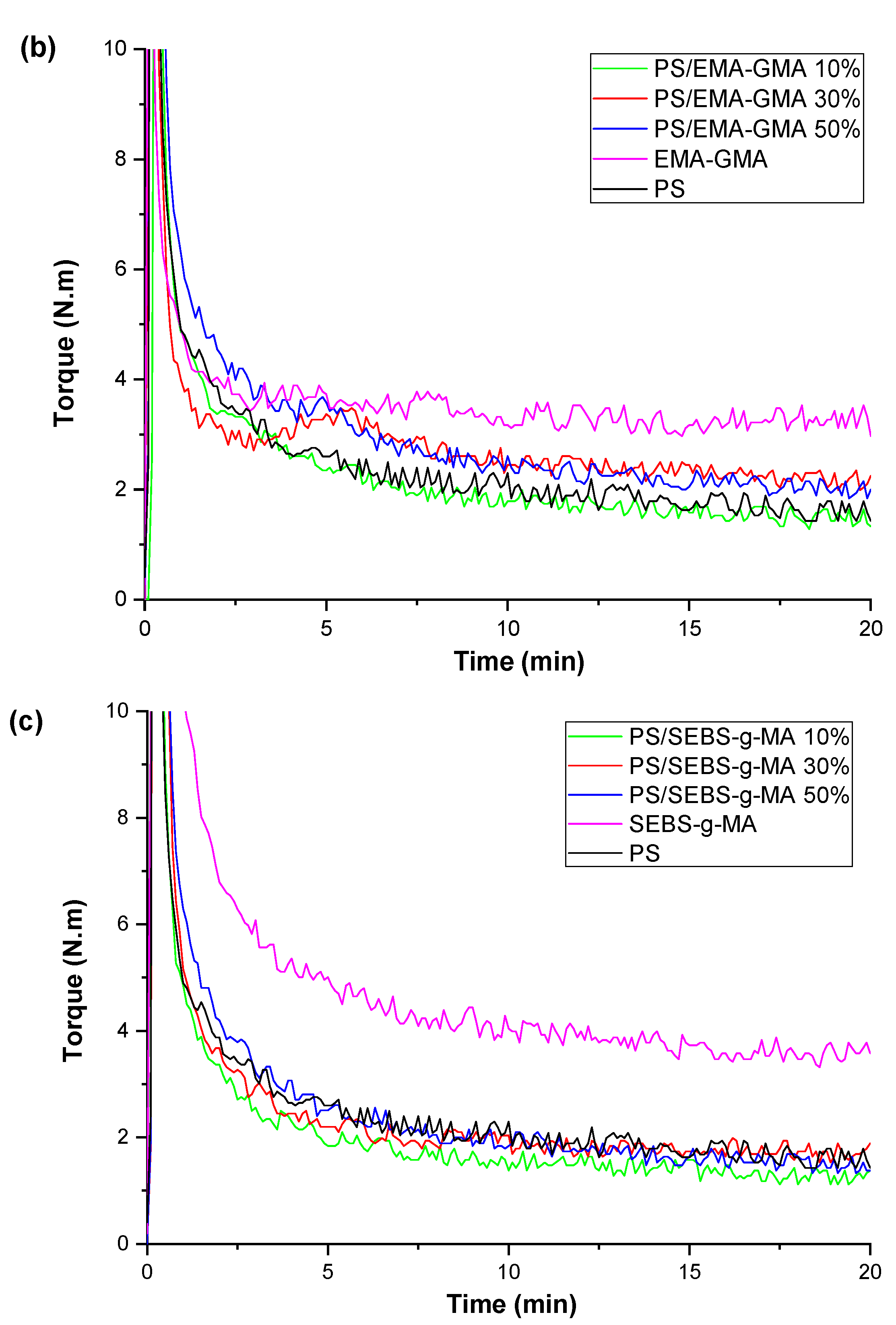

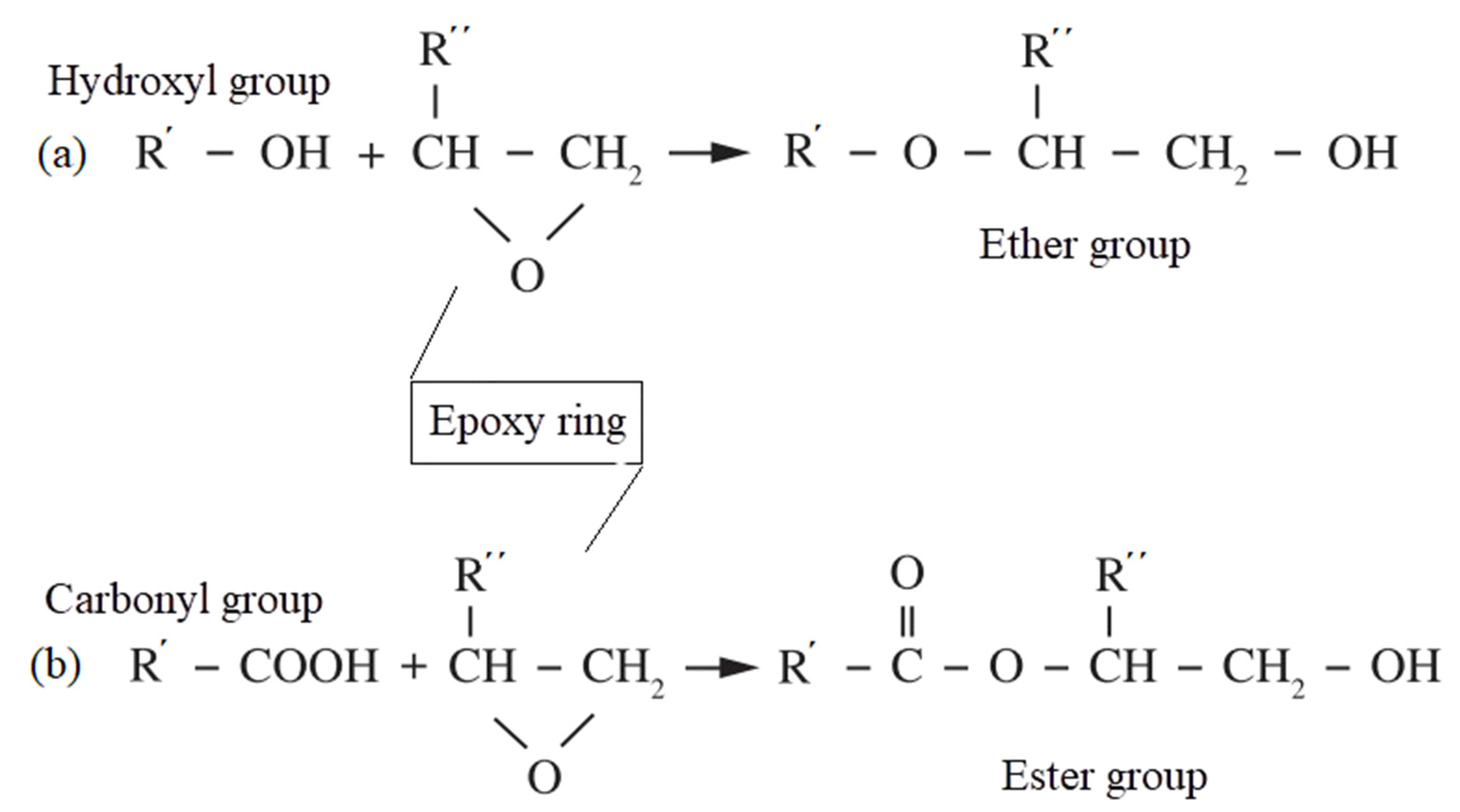

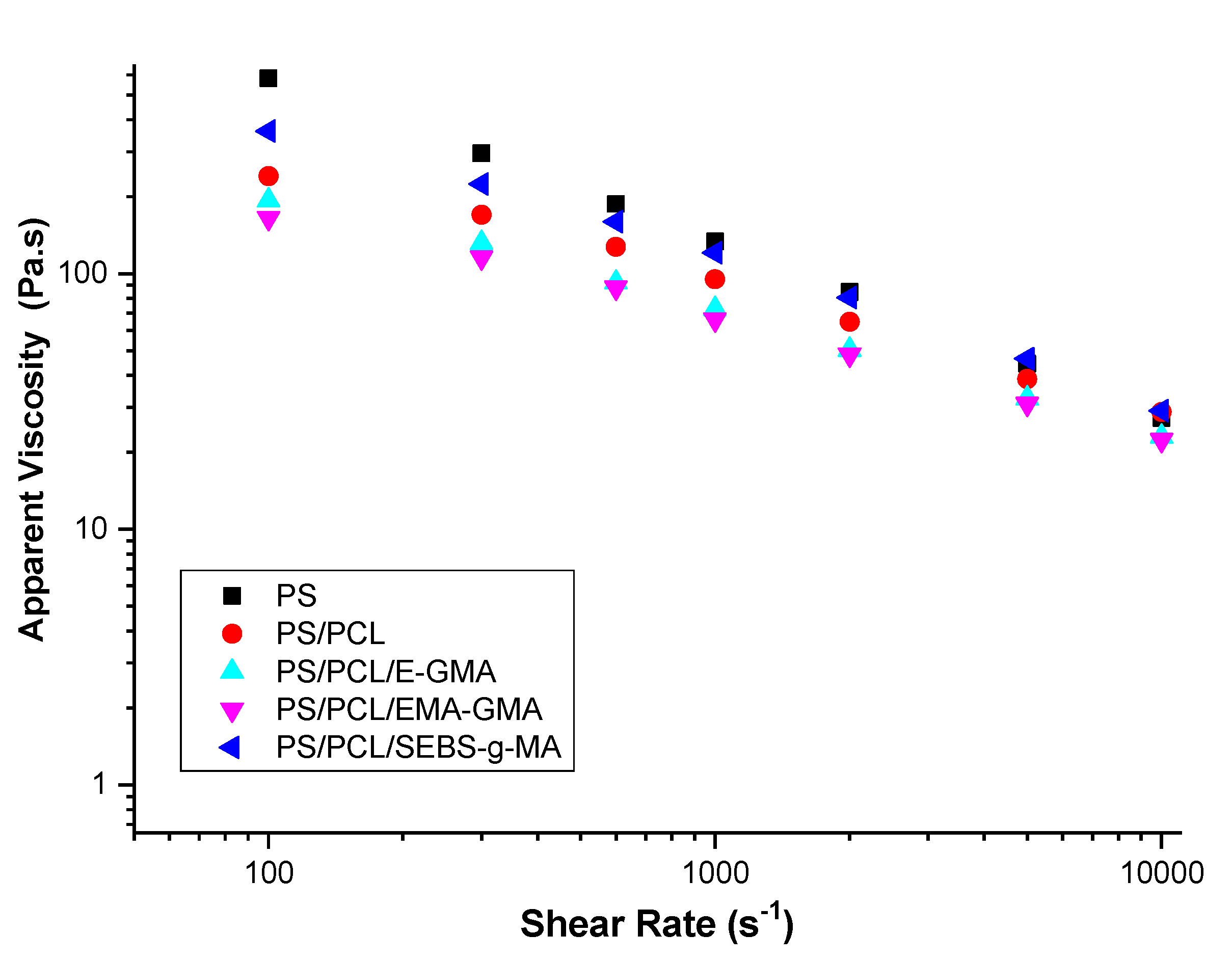
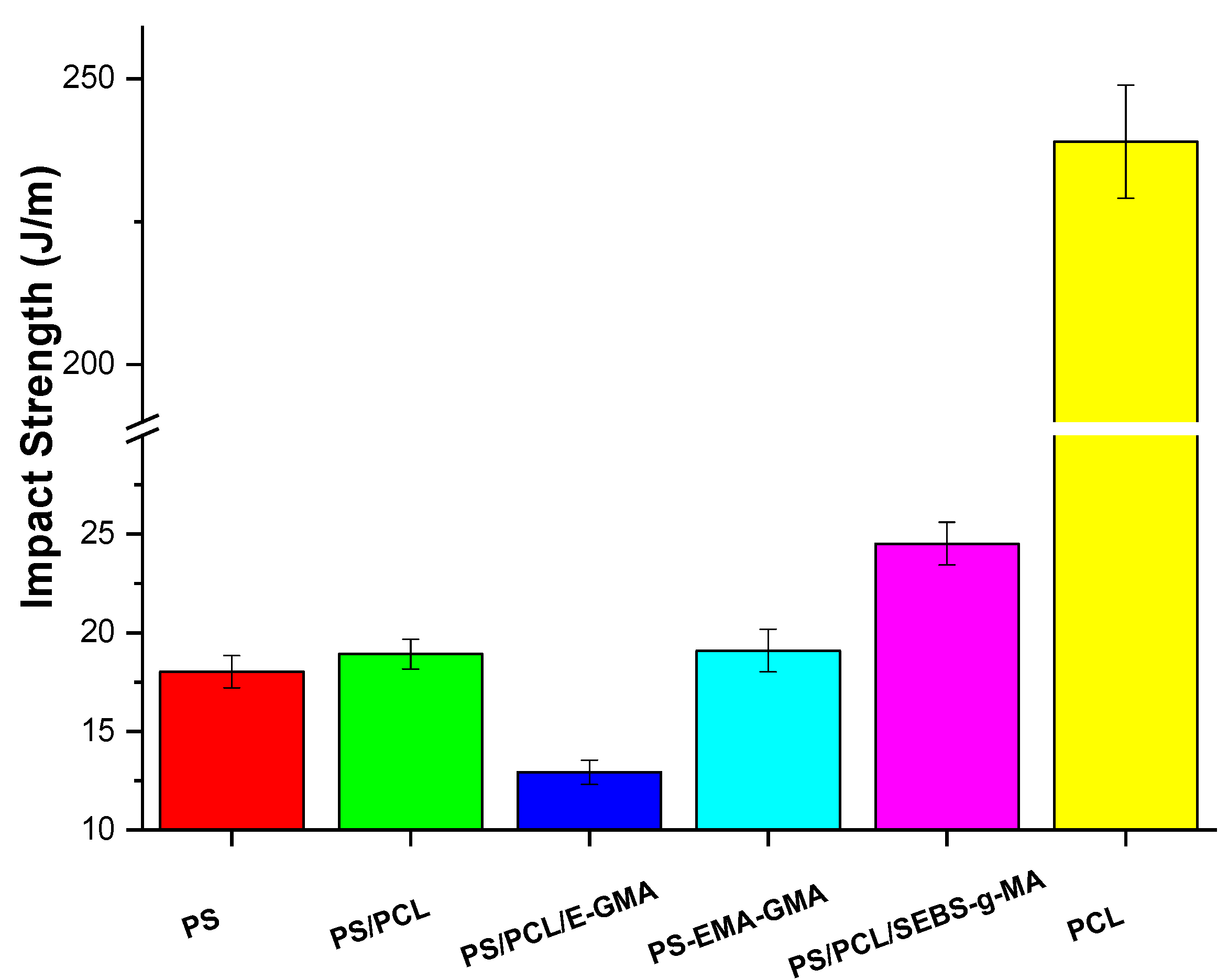

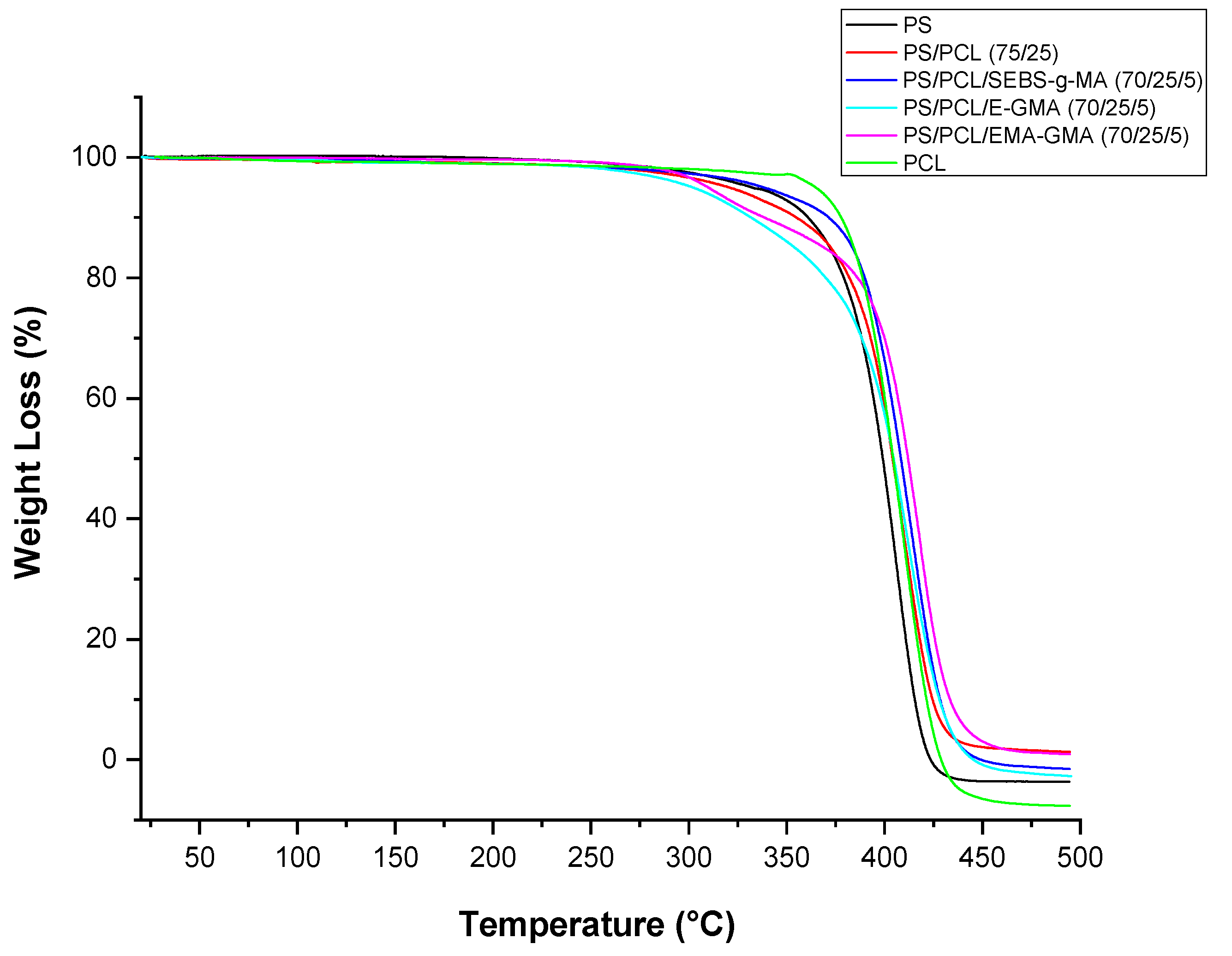

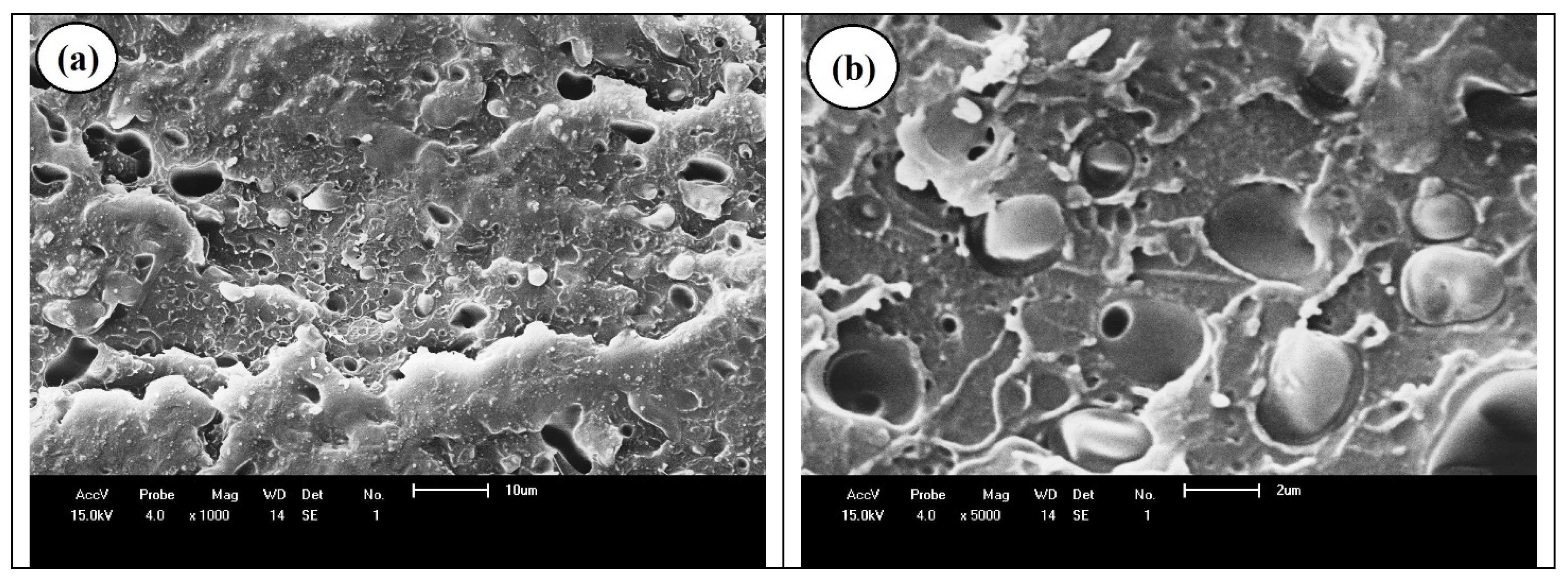
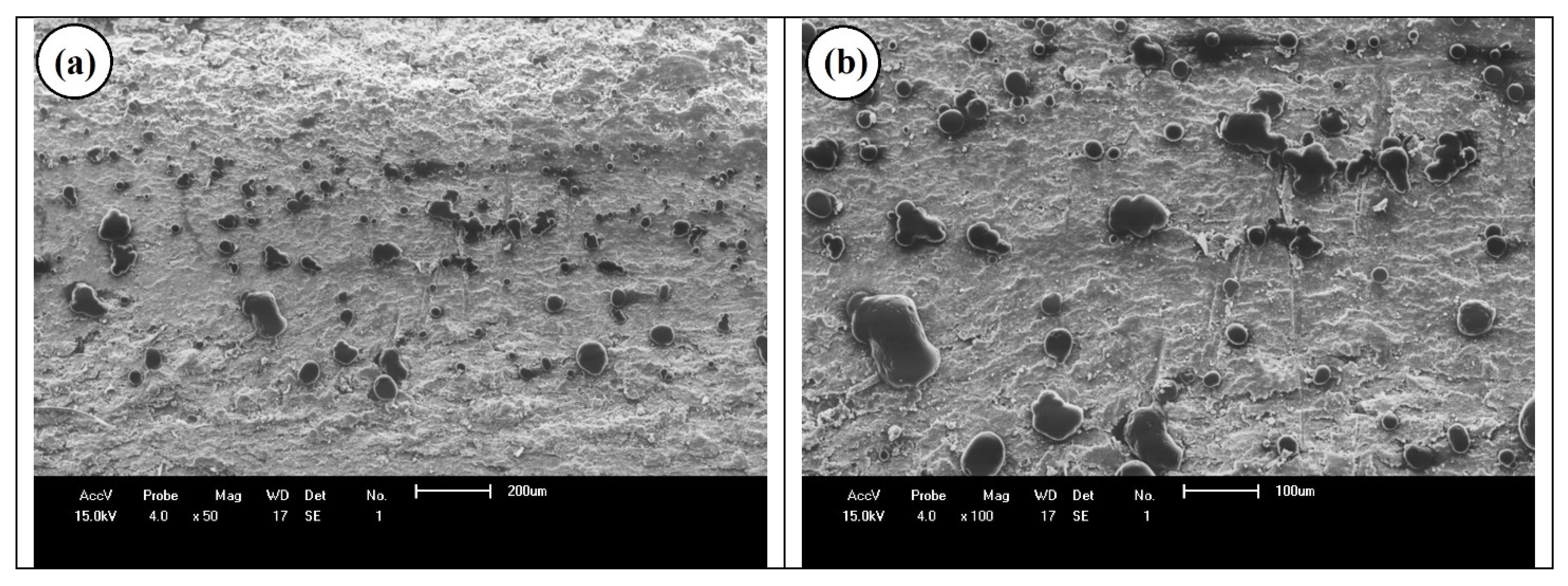

| Samples | PS (wt%) | PCL (wt%) | Compatibilizer (wt%) |
|---|---|---|---|
| PS | 100 | - | - |
| PS/PCL | 75 | 25 | - |
| PS/PCL/E-GMA | 70 | 25 | 5 |
| PS/PCL/EMA-GMA | 70 | 25 | 5 |
| PS/PCL/SEBS-g-MA | 70 | 25 | 5 |
| PCL | - | 100 | - |
| Average Torque (N·m) | ||||||
|---|---|---|---|---|---|---|
| Copolymer Contentin the Blend | 0% | 10% | 30% | 50% | 100% | |
| Composition | ||||||
| PS | 1.62 ± 0.14 | |||||
| PS/E-GMA | 2.18 ± 0.20 | 2.94 ± 0.19 | 2.84 ± 0.14 | |||
| PS/EMA-GMA | 1.61 ± 0.15 | 2.33 ± 0.15 | 2.17 ± 0.17 | |||
| PS/SEBS-g-MA | 1.40 ± 0.14 | 1.78 ± 0.11 | 1.66 ± 0.18 | |||
| PCL/E-GMA | 1.12 ± 0.12 | 1.46 ± 0.11 | 2.10 ± 0.12 | |||
| PCL/EMA-GMA | 1.12 ± 0.12 | 1.86 ± 0.12 | 2.40 ± 0.13 | |||
| PCL/SEBS-g-MA | 1.33 ± 0.12 | 1.74 ± 0.11 | 3.55 ± 0.13 | |||
| E-GMA | 4.64 ± 0.18 | |||||
| EMA-GMA | 3.26 ± 0.14 | |||||
| SEBS-g-MA | 3.58 ± 0.13 | |||||
| PCL | 0.92 ± 0.13 | |||||
| Compositions | Consistency Index K (Pa·sn) | Power Index (n) | Correlation Coefficient (R2) |
|---|---|---|---|
| PS | 12,983.7 | 0.33 | 0.99657 |
| PS/PCL | 2514.1 | 0.51 | 0.98902 |
| PS/PCL/E-GMA (70/25/5%) | 1841.3 | 0.53 | 0.99646 |
| PS/PCL/EMA-GMA (70/25/5%) | 1403.5 | 0.55 | 0.99523 |
| PS/PCL/SEBS-g-MA (70/25/5%) | 5034.2 | 0.45 | 0.98852 |
| Compositions | Elastic Modulus (GPa) | Rupture Strength (MPa) | Yield Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|
| PS | 0.95 ± 0.05 | 34.7 ± 1.06 | - | 5.11 ± 0.4 |
| PS/PCL | 0.51 ± 0.05 | 18.5 ± 1.10 | - | 4.92 ± 0.55 |
| PS/PCL/E-GMA (70/25/5%) | 0.52 ± 0.03 | 18.6 ± 0.95 | - | 4.97 ± 0.5 |
| PS/PCL/EMA-GMA (70/25/5%) | 0.51 ± 0.03 | 19.21 ± 1.9 | - | 5.8 ± 0.77 |
| PS/PCL/SEBS-g-MA (70/25/5%) | 0.58 ± 0.04 | 24.76 ± 0.88 | 24.76 ± 0.88 | 10.3 ± 1.3 |
| PCL | 0.23 ± 0.02 | 18.79 ± 1.72 | 13.64 ± 0.77 | >418.0 * |
| Compositions | HDT (°C) |
|---|---|
| PS | 74.1 ± 0.9 |
| PS/PCL (75/25%) | 65.6 ± 1.8 |
| PS/PCL/E-GMA (70/25/5%) | 54.8 ± 0.9 |
| PS/PCL/EMA-GMA (70/25/5%) | 55.9 ± 0.3 |
| PS/PCL/SEBS-g-MA (70/25/5%) | 56.1 ± 0.2 |
| PCL | 41.5 ± 1.9 |
| Compositions | T10% (°C) | Tdmax (°C) | Residue at 500 °C (mg) |
|---|---|---|---|
| PS | 360.3 | 406.3 | 0.0 |
| PS/PCL | 354.3 | 409.6 | 0.05 |
| PCL/PCL/E-GMA | 331.8 | 411.8 | 0.0 |
| PCL/PCL/EMA-GMA | 338.5 | 418.4 | 0.04 |
| PCL/PCL/SEBS-g-MA | 371.6 | 414.6 | 0.0 |
| PCL | 377.4 | 408.6 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Morais, D.D.; Luna, C.B.B.; Bezerra, E.B.; de França, D.C.; Araújo, E.M.; do Nascimento, E.P.; de Oliveira, A.D.; de Mélo, T.J.A. Performance of Poly(caprolactone) (PCL) as an Impact Modifier for Polystyrene (PS): Effect of Functionalized Compatibilizers with Maleic Anhydride and Glycidyl Methacrylate. Sustainability 2022, 14, 9254. https://doi.org/10.3390/su14159254
de Souza Morais DD, Luna CBB, Bezerra EB, de França DC, Araújo EM, do Nascimento EP, de Oliveira AD, de Mélo TJA. Performance of Poly(caprolactone) (PCL) as an Impact Modifier for Polystyrene (PS): Effect of Functionalized Compatibilizers with Maleic Anhydride and Glycidyl Methacrylate. Sustainability. 2022; 14(15):9254. https://doi.org/10.3390/su14159254
Chicago/Turabian Stylede Souza Morais, Dayanne Diniz, Carlos Bruno Barreto Luna, Elieber Barros Bezerra, Danyelle Campos de França, Edcleide Maria Araújo, Emanuel Pereira do Nascimento, Amanda Dantas de Oliveira, and Tomás Jefferson Alves de Mélo. 2022. "Performance of Poly(caprolactone) (PCL) as an Impact Modifier for Polystyrene (PS): Effect of Functionalized Compatibilizers with Maleic Anhydride and Glycidyl Methacrylate" Sustainability 14, no. 15: 9254. https://doi.org/10.3390/su14159254






