Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurements and Growth Parameters
2.3. Solution Compositions
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scordia, D.; Papazoglou, E.G.; Kotoula, D.; Sanz, M.; Ciria, C.S.; Pérez, J.; Maliarenko, O.; Prysiazhniuk, O.; von Cossel, M.; Greiner, B.E.; et al. Towards identifying industrial crop types and associated agronomies to improve biomass production from marginal lands in Europe. GCB Bioenergy 2022, 14, 710–734. [Google Scholar] [CrossRef]
- Zięty, J.J.; Olba-Zięty, E.; Stolarski, M.J.; Krzykowski, M.; Krzyżaniak, M. Legal Framework for the Sustainable Production of Short Rotation Coppice Biomass for Bioeconomy and Bioenergy. Energies 2022, 15, 1370. [Google Scholar] [CrossRef]
- Trava, I.D.; Borlea, G.F.; Hollerbach, W. Identification of the Most Productive Species from the Salix Genus and Its Use in Energetic Cultures. J. Hortic. For. Biotechnol. 2014, 18, 209–213. [Google Scholar]
- Johnston, C.R.; Walsh, L.R.; McCracken, A.R. Effect of two vs. three year harvest intervals on yields of Short Rotation Coppice (SRC) willow. Biomass Bioenergy 2022, 156, 106303. [Google Scholar] [CrossRef]
- Simon, L.; Szabó, B.; Szabó, M.; Vincze, G.; Varga, C.; Uri, Z.S.; Koncz, J. Effect of Various Soil Amendments on the Mineral Nutrition of Salix viminalis and Arundo donax Energy Crops. Eur. Chem. Bull 2013, 2, 18–21. [Google Scholar]
- Kulig, B.; Gacek, E.; Wojciechowski, R.; Oleksy, A.; Kołodziejczyk, M.; Szewczyk, W.; Klimek-Kopyra, A. Biomass Yield and Energy Efficiency of Willow Depending on Cultivar, Harvesting Frequency and Planting Density. Plant Soil Environ. 2019, 65, 377–386. [Google Scholar] [CrossRef]
- Demo, M.; Hauptvogl, M.; Prčík, M.; Húska, D. Comparison of production parameters of willow (Salix spp.) and poplar (Populus spp.) varieties in the last year of the first four-year harvest cycle. Wood Res. 2014, 59, 705–716. [Google Scholar]
- Macalpine, W.J. Identifying Drought Tolerant Short Rotation Coppice Willows. Master’s Thesis, Harper Adams University, Newport, UK, 2019. [Google Scholar]
- Liberacki, D.; Kocięcka, J.; Stachowski, P.; Rolbiecki, R.; Rolbiecki, S.; Sadan, H.A.; Figas, A.; Jagosz, B.; Wichrowska, D.; Ptach, W.; et al. Water Needs of Willow (Salix L.) in Western Poland. Energies 2022, 15, 484. [Google Scholar] [CrossRef]
- Digruber, T.; Sass, L.; Cseri, A.; Paul, K.; Nagy, A.V.; Remenyik, J.; Dudits, D. Stimulation of Energy Willow Biomass with Triacontanol and Seaweed Extract. Ind. Crops Prod. 2018, 120, 104–112. [Google Scholar] [CrossRef]
- Olba-Zięty, E.; Stolarski, M.J.; Krzyżaniak, M. Economic Evaluation of the Production of Perennial Crops for Energy Purposes—A Review. Energies 2021, 14, 7147. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Long, A.; Bose, A.; O’Shea, R.; Monaghan, R.; Murphy, J.D. Implications of European Union recast Renewable Energy Directive sustainability criteria for renewable heat and transport: Case study of willow biomethane in Ireland. Renew. Sustain. Energy Rev. 2021, 150, 111461. [Google Scholar] [CrossRef]
- Djomo, S.N.; Kasmioui, O.E.; Ceulemans, R. Energy and Greenhouse Gas Balance of Bioenergy Production from Poplar and Willow: A Review. GCB Bioenergy 2011, 3, 181–197. [Google Scholar] [CrossRef]
- Ivan, V.; Hoefnagels, R.; Junginger, M.; van der Hilst, F. Supply potential of lignocellulosic energy crops grown on marginal land and greenhouse gas footprint of advanced biofuels—A spatially explicit assessment under the sustainability criteria of the Renewable Energy Directive Recast. GCB Bioenergy 2021, 13, 1425–1447. [Google Scholar]
- Câmpeanu, V.; Pencea, S. Renewable energy sources in Romania: From a “paradise” of investors to a possible abandon or to another boom? The impact of a new paradigm in Romanian renewable sources policy. Procedia Econ. Financ. 2014, 8, 129–137. [Google Scholar] [CrossRef][Green Version]
- Oncioiu, I.; Petrescu, A.G.; Grecu, E.; Petrescu, M. Optimizing the renewable energy potential: Myth or future trend in Romania. Energies 2017, 10, 759. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Volk, T.A.; White, E.H.; Abrahamson, L.P.; Briggs, R.D.; Bickelhaupt, D.H. Biomass and Nutrient Removal by Willow Clones in Experimental Bioenergy Plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Influence of Plantation Site and Wastewater Sludge Fertilization on the Performance and Foliar Nutrient Status of Two Willow Species Grown under SRIC in Southern Quebec (Canada). For. Ecol. Manag. 2001, 150, 223–239. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field Performance and Biomass Production of 12 Willow and Poplar Clones in Short-Rotation Coppice in Southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Isebrands, J.G.; Aronsson, P.; Carlson, M.; Ceulemans, R.; Coleman, M.; Dickinson, N.; Dimitriou, J.; Doty, S.; Gardiner, E.; Heinsoo, K.; et al. Environmental Applications of Poplars and Willows. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; FAO: Roma, Italy, 2014; pp. 258–336. [Google Scholar]
- Cerrillo, T.; Álvarez, J.; Battistella, A.; Braccini, C.; Casaubón, E.; Ceballos, D.; Cortizo, S.; Fernandez Tschieder, E.; Fernández, P.C.; Faustino, L.; et al. Salicáceas afforestation as a contribution to the sustainable development of the Paraná Delta. Disertación. XXIX Jornadas Forestales de Entre Ríos. Concordia 2015, 14, 1–14. [Google Scholar]
- Holm, B.; Heinsoo, K. Municipal wastewater application to Short Rotation Coppice of willows—Municipal wastewater application to Short Rotation Coppice of willows—Treatment efficiency and clone response in Estonian case study. Biomass Bioenergy 2013, 57, 126–135. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutowski, P.; Rissman, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazynska, K.; Stachowiak, A. Biomass Productivity and Phytoremediation Potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Corneanu, M.; Hernea, C.; Butnariu, M.; Corneanu, G.; Sărac, I.; Hollerbach, W.; Petcov, A.A. Preliminary Tests for Salix Sp. Tolerance to Heavy Metals (Cd, Ni, Pb). In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 27 April–2 May 2014; p. 10620. [Google Scholar]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small-and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Stanton, B.J.; Bourque, A.; Coleman, M.; Eisenbies, M.; Emerson, R.M.; Espinoza, J.; Gantz, C.; Himes, A.; Rodstrom, A.; Shuren, R.; et al. The practice and economics of hybrid poplar biomass production for biofuels and bioproducts in the Pacific Northwest. BioEnergy Res. 2021, 14, 543–560. [Google Scholar] [CrossRef]
- Dillen, M.; Vanhellemont, M.; Verdonckt, P.; Maes, W.H.; Steppe, K.; Verheyen, K. Productivity, stand dynamics and the selection effect in a mixed willow clone short rotation coppice plantation. Biomass Bioenergy 2016, 87, 46–54. [Google Scholar] [CrossRef]
- Njakou Djomo, S.; Ac, A.; Zenone, T.; De Groote, T.; Bergante, S.; Facciotto, G.; Sixto, H.; Ciria Ciria, P.; Weger, J.; Ceulemans, R. Energy performances of intensive and extensive short rotation cropping systems for woody biomass production in the EU. Renew. Sustain. Energy Rev. 2015, 41, 845–854. [Google Scholar] [CrossRef]
- Weih, M.; Glynn, C.; Baum, C. Willow short-rotation coppice as model system for exploring ecological theory on biodiversity–ecosystem function. Diversity 2019, 11, 125. [Google Scholar] [CrossRef]
- Matyka, M.; Radzikowski, P. Productivity and biometric characteristics of 11 varieties of willow cultivated on marginal soil. Agriculture 2020, 10, 616. [Google Scholar] [CrossRef]
- Hernea, C.; Corneanu, M.; Sarac, I.; Petrescu, I. The behavior of willow commercial clones in the first growing season. A case study for three different sites from Banat area. Ann. Univ. Craiova-Agric. Montanology Cadastre Ser. 2017, 46, 622–627. [Google Scholar]
- Soare, M.; Panita, O.; Salceanu, C. Partial results concerning the behavior of energy willow genotypes in cultivated improper areas. Ann. Univ. Craiova-Agric. Montanology Cadastre Ser. 2015, 45, 300–305. [Google Scholar]
- Buzatu-Goanta, C.; Corneanu, M.; Babeanu, C. Salix Accessions with Potential For New Hybrids. A Case Study from Banat Area. In Scientific Papers. Series E. Land Reclamation, Earth Observation & Surveying, Environmental Engineering; University of Agronomic Sciences and Veterinary Medicine of Bucharest: Bucharest, Romania, 2021; Volume X, ISSN 2285-6064. pp. 214–220. [Google Scholar]
- Hernea, C.; Trava, I.D.; Borlea, G.F. Biomass Production of Some Swedish Willow Hybrids on the West of Romania. A Case Study. J. Hortic. For. Biotechnol. 2015, 19, 103–106. [Google Scholar]
- Soare, M.; Iancu, P.; Panita, O.; Soare, R.; Bonciu, E. Researches Concerning the Possibility of Cultivating Energetic Willow on Deposit of Ash from Thermal Power Stations. SGEM2017 Conf. Proc. 2017, 17, 519–528. [Google Scholar]
- Scriba, C.; Lunguleasa, A.; Salca, E.A.; Ciobanu, V.D. Properties of Biomass Obtained from Short-Rotation Inger Willow Clone Grown on a Contaminated and Non-Contaminated Land. Maderas. Cienc. Tecnol. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Scriba, C.; Lunguleasa, A.; Spirchez, C.; Ciobanu, V. Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value. Forests 2021, 12, 826. [Google Scholar] [CrossRef]
- Orlovic, S.; Klasnja, B.; Pilipovic, A.; Radosavljevic, N. Physiological and growth characteristics of white willow (Salix alba L.). Clones 2003, 1, 223–226. [Google Scholar]
- Rewald, B.; Kunze, M.; Godbold, D.L. NH4: NO3 Nutrition Influence on Biomass Productivity and Root Respiration of Poplar and Willow Clones. GCB Bioenergy 2016, 8, 51–58. [Google Scholar] [CrossRef]
- Keller, C. Efficiency and Limitations of Phytoextraction by High Biomass Plants: The Example of Willows. In Trace Elements in the Environment; CRC Press: Boca Raton, FL, USA, 2005; pp. 629–650. [Google Scholar]
- Pulford, I.D.; Dickinson, N.M. Phytoremediation technologies using trees. In Trace Elements in the Environment. Biogeochemistry, Biotechnology, and Bioremediation; Prasad, M.N.V., Sajwan, K.S., Naidu, R., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2006; pp. 383–403. [Google Scholar]
- Cornelia, H.; Hollerbach, W.; Trava, D.; Mihaela, C. The Behaviour for SRC Willow Inger in Experimental Trial Ghilad, Romania. Bull. UASVM Hortic. 2015, 72, 376–380. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA, USA. 2021. Available online: http://www.rstudio.com/ (accessed on 20 April 2022).
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/ (accessed on 20 April 2022).
- Revelle, W. Psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA. 2021. Available online: https://CRAN.R-project.org/package=psych (accessed on 20 April 2022).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 20 April 2022).
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles. R Package Version 0.7.8. 2021. Available online: https://CRAN.R-project.org/package=broom (accessed on 20 April 2022).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Biomass Yield, Nutrient Concentration and Nutrient Uptake by SRC Willow Cultivars Grown on Different Sites in Denmark. Biomass Bioenergy 2018, 116, 161–170. [Google Scholar] [CrossRef]
- Kałuża-Haładyn, A.; Jamroz, E.; Bekier, J. Humic substances of differently matured composts produced from municipal solid wastes and biomass of energetic plants. Soil Sci. Annu. 2019, 70, 292–297. [Google Scholar] [CrossRef]
- Rajan, R.P.; Singh, G. A review on the use of organic rooting substances for propagation of horticulture crops. Plant Arch. 2021, 21, 685–692. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Makan, A. (Ed.) Humic Substances; IntechOpen: London, UK, 2021. [Google Scholar]
- Toetz, D.; Payton, M. The role of dissolved organic matter in accrual of periphytic biomass in a subalpine stream, Colorado Front Range. J. Freshw. Ecol. 2006, 21, 613–620. [Google Scholar] [CrossRef][Green Version]
- Jia, W.; Hu, C.; Xu, J.; Ming, J.; Zhao, Y.; Cai, M.; Zhao, X. Dissolved organic matter derived from rape straw pretreated with selenium in soil improves the inhibition of Sclerotinia sclerotiorum growth. J. Hazard. Mater. 2019, 369, 601–610. [Google Scholar] [CrossRef]
- Lange, M.; Roth, V.N.; Eisenhauer, N.; Roscher, C.; Dittmar, T.; Fischer-Bedtke, C.; Gleixner, G. Plant diversity enhances production and downward transport of biodegradable dissolved organic matter. J. Ecol. 2021, 109, 1284–1297. [Google Scholar] [CrossRef]
- Kaschl, A.; Chen, Y. Interactions of humic substances with trace metals and their stimulatory effects on plant growth. In Use of Humic Substances to Remediate Polluted Environments: From Theory to Practice; Springer: Dordrecht, The Netherlands, 2005; pp. 83–113. [Google Scholar]
- Ghosh, S.; Halder, S. Effect of different kinds of gibberellin on temperate fruit crops: A review. Pharma Innov. J. 2018, 7, 315–319. [Google Scholar]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J. Sci. Food Agric. 2015, 95, 1777–1785. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, A.; Khare, S.; Yadav, V.; Bano, C.; Yadav, R.K. Mitigating strategies of gibberellins in various environmental cues and their crosstalk with other hormonal pathways in plants: A review. Plant Mol. Biol. Report. 2021, 39, 34–49. [Google Scholar]
- Pal, S.L. Role of plant growth regulators in floriculture: An overview. J. Pharmacogn. Phytochem. 2019, 8, 789–796. [Google Scholar]
- Rademacher, W. Chemical regulators of gibberellin status and their application in plant production. Annu. Plant Rev. Online 2018, 49, 359–403. [Google Scholar]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. Annu. Plant Rev. 2016, 49, 253–284. [Google Scholar]
- Gollagi, S.G.; Lokesha, R.; Dharmpal, S.; Sathish, B.R. Effects of growth regulators on growth, yield and quality of fruits crops: A review. J Pharm. Phytochem. 2019, 8, 979–981. [Google Scholar]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2021, 49, 617–628. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New insights into gibberellin signaling in regulating plant growth–metabolic coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Rizza, A.; Jones, A.M. The makings of a gradient: Spatiotemporal distribution of gibberellins in plant development. Curr. Opin. Plant Biol. 2019, 47, 9–15. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar]
- Richards, D.E.; King, K.E.; Ait-Ali, T.; Harberd, N.P. How gibberellin regulates plant growth and development: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biol. 2015, 15, 290. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 2017, 254, 839–848. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, J.; Chen, R.; Zhang, Y.; Ouyang, B.; Ye, Z. Dissection of GA 20-oxidase members affecting tomato morphology by RNAi-mediated silencing. Plant Growth Regul. 2006, 50, 179–189. [Google Scholar] [CrossRef]
- Tagliavini, M.; Looney, N.E. Response of peach seedlings to root-zone temperature and root-applied growth regulators. HortScience 1991, 26, 870–872. [Google Scholar] [CrossRef]
- Rytter, R.M. The effect of limited availability of N or water on C allocation to fine roots and annual fine root turnover in Alnus incana and Salix viminalis. Tree Physiol. 2013, 33, 924–939. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, W.; Wang, Z.; Chen, L.; Ma, Z.; Wang, Q. Water use of Salix in the variably unsaturated zone of a semiarid desert region based on in-situ observation. J. Hydrol. 2020, 591, 125579. [Google Scholar] [CrossRef]
- Vigl, F.; Rewald, B. Size Matters?–The Diverging Influence of Cutting Length on Growth and Allometry of Two Salicaceae Clones. Biomass Bioenergy 2014, 60, 130–136. [Google Scholar] [CrossRef]
- Styszko, L.; Dabrowski, J. Impact of the Nitrogen Fertilization on Yield, Dry Matter, Ash and Total Nitrogen Content in the Second 4-Year Rotation of Basket Willow Cultivation. Rocz. Ochr. Srodowiska 2018, 20, 1234–1251. [Google Scholar]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Willow yield is highly dependent on clone and site. BioEnergy Res. 2014, 7, 1280–1292. [Google Scholar] [CrossRef]
- Liu, N.; Jørgensen, U.; Lærke, P.E. Concentrations of chemical elements in willow biomass depend on clone, site and management in the field. BioEnergy Res. 2016, 9, 1216–1230. [Google Scholar] [CrossRef]
- Borzymowska, A.; Styszko, L. Influence of Planting Density on Lenght, Thikness and Number of Shoots in Willow Carp During Four-year Cultivation Cycle. Rocz. Ochr. Srodowiska 2012, 14, 481–490. [Google Scholar]
- Graß, R.; Malec, S.; Wachendorf, M. Biomass performance and competition effects in an established temperate agroforestry system of willow and grassland—Results of the 2nd rotation. Agronomy 2020, 10, 1819. [Google Scholar] [CrossRef]
- Finnan, J.M.; Donnelly, I.; Burke, B. The effect of cutting back willow after one year of growth on biomass production over two harvest cycles. Biomass Bioenergy 2016, 92, 76–80. [Google Scholar] [CrossRef]
- Sommer, J.; Hartmann, L.; Dippold, M.A.; Lamersdorf, N.P. Specific Nmin uptake patterns of two widely applied poplar and willow clones for short rotation coppices–Implications for management practices. Biomass Bioenergy 2017, 98, 236–242. [Google Scholar] [CrossRef]
- Heinsoo, K.; Dimitriou, I. Growth performance of willow clones in short rotation coppice after sewage sludge application. Balt. For. 2014, 20, 70–77. [Google Scholar]
- Castaño-Díaz, M.; Barrio-Anta, M.; Afif-Khouri, E.; Cámara-Obregón, A. Willow short rotation coppice trial in a former mining area in northern Spain: Effects of clone, fertilization and planting density on yield after five years. Forests 2018, 9, 154. [Google Scholar] [CrossRef]
- Mikó, P.; Kovács, G.P.; Alexa, L.; Balla, I.; Póti, P.; Gyuricza, C.S. Biomass production of energy willow under unfavourable field conditions. Appl. Ecol. Environ. Res. 2014, 12, 1–11. [Google Scholar] [CrossRef]
- Van Slycken, S.; Witters, N.; Meiresonne, L.; Meers, E.; Ruttens, A.; Van Peteghem, P.; Weyens, N.; Tack, F.M.; Vangronsveld, J. Field evaluation of willow under short rotation coppice for phytomanagement of metal-polluted agricultural soils. Int. J. Phytoremediation 2013, 15, 677–689. [Google Scholar] [CrossRef]


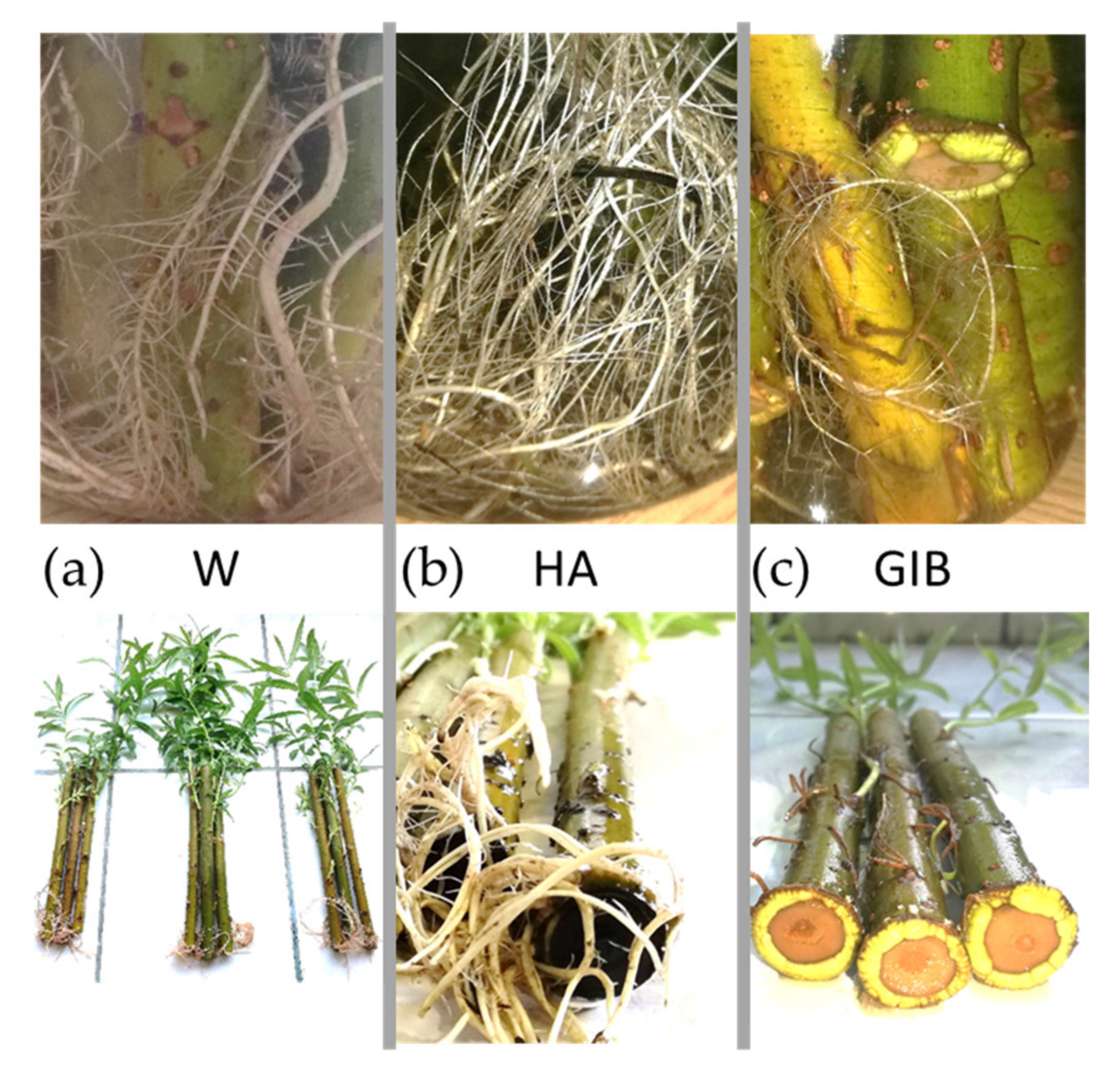
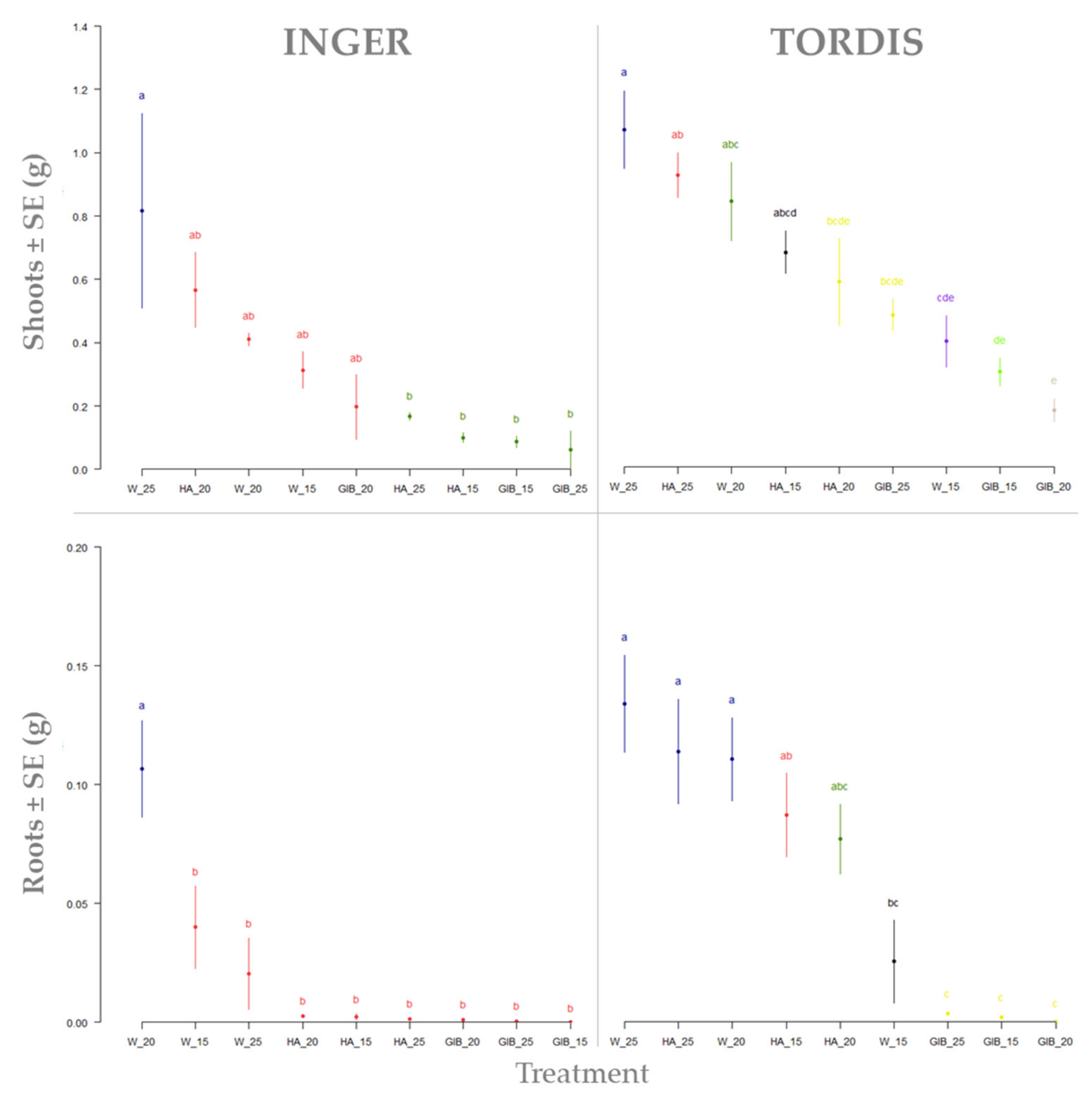
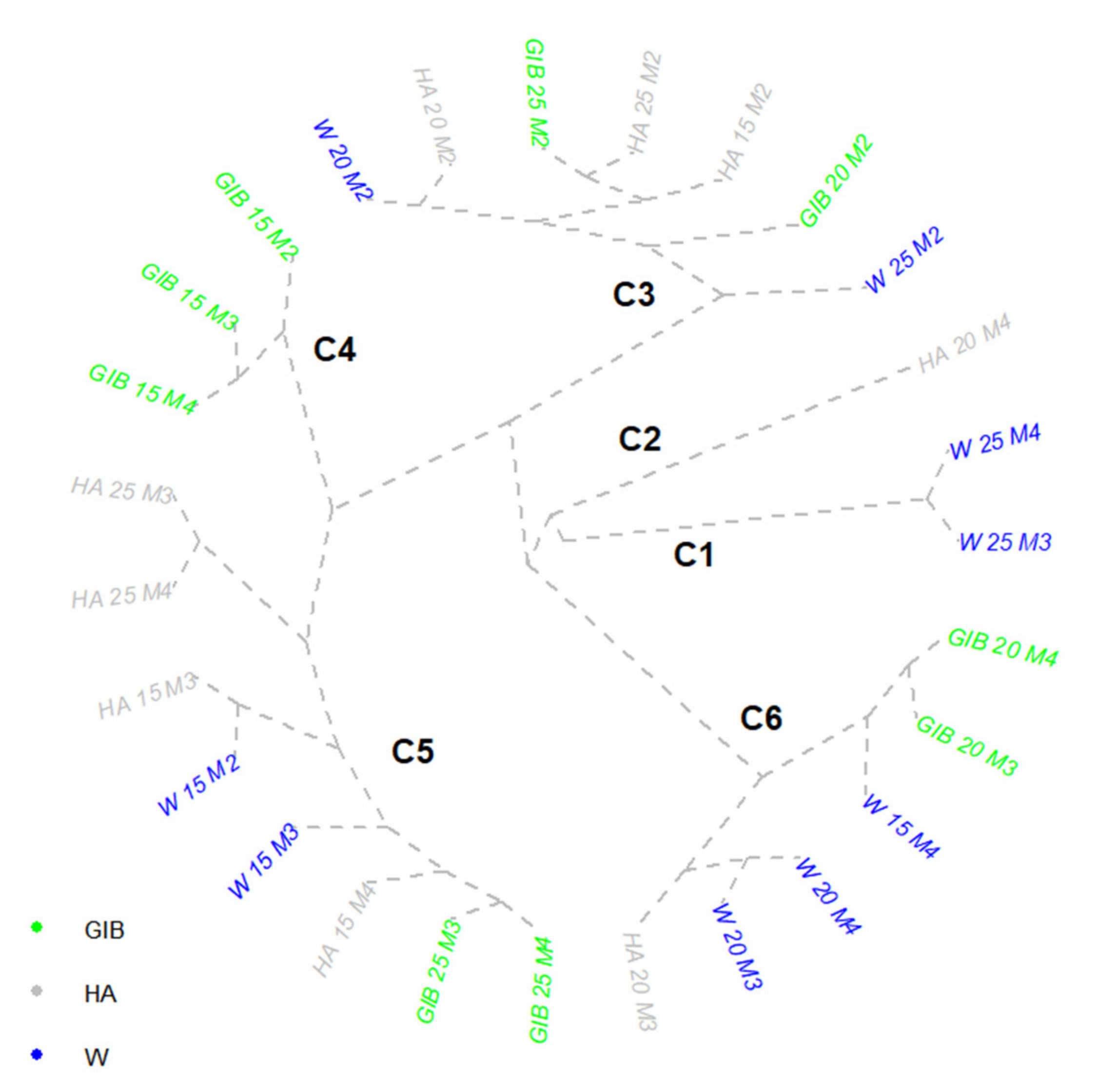
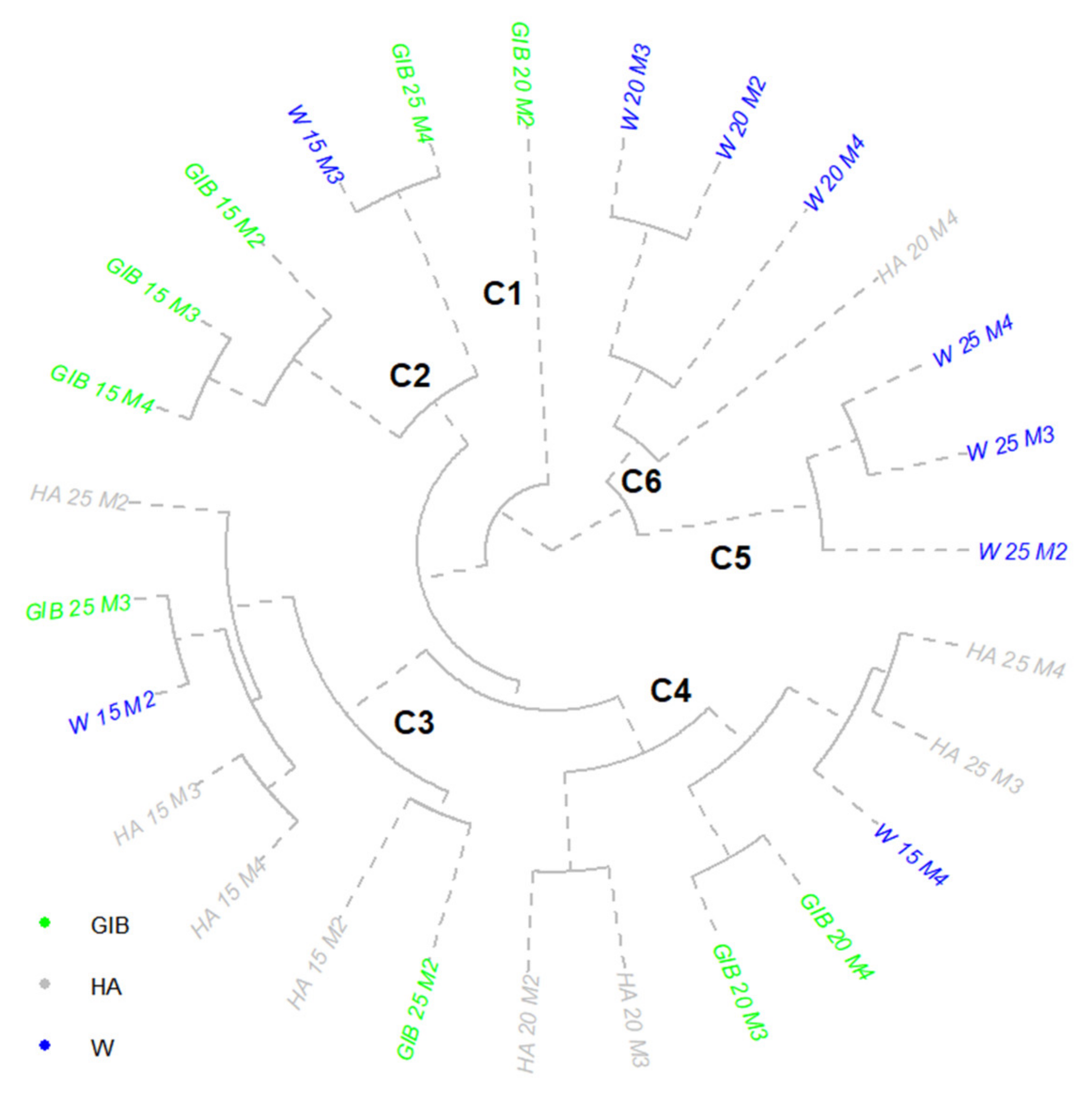

| Var | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| IN_15_GIB | 0.67 ± 0.17 b | 1.11 ± 0.26 c | 1.56 ± 0.34 c | 1.56 ± 0.34 c |
| IN_15_HA | 0.67 ± 0.24 b | 1.56 ± 0.29 c | 2.00 ± 0.29 bc | 2.00 ± 0.29 bc |
| IN_15_W | 1.44 ± 0.44 ab | 1.89 ± 0.35 bc | 2.00 ± 0.37 bc | 2.11 ± 0.42 bc |
| IN_20_GIB | 0.89 ± 0.45 b | 1.78 ± 0.49 bc | 2.11 ± 0.68 bc | 2.22 ± 0.64 bc |
| IN_20_HA | 2.00 ± 0.62 ab | 2.22 ± 0.57 bc | 2.67 ± 0.47 abc | 2.89 ± 0.59 bc |
| IN_20_W | 2.78 ± 0.66 ab | 3.67 ± 0.53 ab | 4.00 ± 0.53 ab | 4.00 ± 0.53 ab |
| IN_25_GIB | 0.78 ± 0.36 b | 1.33 ± 0.29 c | 1.33 ± 0.29 c | 1.33 ± 0.29 c |
| IN_25_HA | 0.89 ± 0.20 b | 1.78 ± 0.22 bc | 2.22 ± 0.32 bc | 2.22 ± 0.32 bc |
| IN_25_W | 3.33 ± 0.80 a | 4.33 ± 0.69 a | 4.78 ± 0.60 a | 5.11 ± 0.63 a |
| Length (F test) | 3.41 | 7.21 | 6.25 | 6.65 |
| p value | 0.069 | 0.009 | 0.015 | 0.012 |
| Treat (F test) | 10.29 | 15.17 | 14.05 | 14.35 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Length × Treat (F test) | 2.05 | 4.27 | 6.37 | 6.76 |
| p value | 0.136 | 0.018 | 0.003 | 0.002 |
| Var | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| TO_15_GIB | 1.56 ± 0.24 d | 2.00 ± 0.00 d | 2.00 ± 0.00 d | 1.44 ± 0.18 e |
| TO_15_HA | 2.89 ± 0.31 abcd | 3.44 ± 0.18 cd | 4.11 ± 0.35 bcd | 4.22 ± 0.36 bcd |
| TO_15_W | 2.56 ± 0.44 bcd | 4.00 ± 0.71 bcd | 4.33 ± 0.53 bcd | 4.56 ± 0.58 bcd |
| TO_20_GIB | 1.89 ± 0.26 cd | 2.00 ± 0.24 d | 2.11 ± 0.31 d | 1.56 ± 0.18 e |
| TO_20_HA | 2.33 ± 0.69 bcd | 3.22 ± 0.52 cd | 3.44 ± 0.50 cd | 3.78 ± 0.55 cde |
| TO_20_W | 5.11 ± 0.59 a | 6.78 ± 0.64 a | 7.00 ± 0.55 a | 7.22 ± 0.55 a |
| TO_25_GIB | 1.78 ± 0.52 d | 2.56 ± 0.50 d | 2.67 ± 0.55 d | 2.56 ± 0.65 de |
| TO_25_HA | 4.33 ± 0.94 abc | 5.44 ± 0.63 abc | 5.78 ± 0.68 abc | 5.89 ± 0.72 abc |
| TO_25_W | 4.56 ± 0.41 ab | 5.89 ± 0.51 ab | 6.33 ± 0.62 ab | 6.33 ± 0.62 ab |
| Length (F test) | 7.22 | 11.93 | 11.12 | 11.31 |
| p value | 0.009 | 0.001 | 0.001 | 0.001 |
| Treat (F test) | 13.40 | 30.98 | 35.59 | 44.50 |
| p value | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Length × Treat (F test) | 1.33 | 1.17 | 0.86 | 0.21 |
| p value | 0.270 | 0.316 | 0.429 | 0.812 |
| Var | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| IN_15_GIB | ||||
| IN_15_HA | 1.56 ± 0.63 ab | 1.89 ± 0.68 bcd | ||
| IN_15_W | 0.67 ± 0.44 a | 2.33 ± 1.09 ab | 3.44 ± 1.19 abcd | |
| IN_20_GIB | 0.11 ± 0.11 a | 0.67 ± 0.44 a | 0.67 ± 0.44 ab | 1.00 ± 0.44 cd |
| IN_20_HA | 0.11 ± 0.11 a | 1.78 ± 0.78 a | 6.78 ± 3.47 a | 8.33 ± 3.21 a |
| IN_20_W | 0.33 ± 0.17 a | 1.56 ± 0.75 a | 4.11 ± 0.61 ab | 6.89 ± 1.20 abc |
| IN_25_GIB | 0.11 ± 0.11 a | 0.11 ± 0.11 b | 0.11 ± 0.11 d | |
| IN_25_HA | 0.11 ± 0.11 a | 0.67 ± 0.29 ab | 1.33 ± 0.41 cd | |
| IN_25_W | 0.33 ± 0.24 a | 1.67 ± 0.71 a | 4.56 ± 1.24 ab | 7.56 ± 1.03 ab |
| Length (F test) | 0.61 | 1.33 | 0.12 | |
| p value | 0.440 | 0.255 | 0.729 | |
| Treat (F test) | 0.61 | 1.72 | 3.04 | 8.10 |
| p value | 0.550 | 0.189 | 0.055 | p < 0.001 |
| Length × Treat (F test) | 2.580 | 0.62 | 1.489 | |
| p value | 0.085 | 0.543 | 0.232 |
| Var | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| TO_15_GIB | 0.56 ± 0.24 ab | 0.56 ± 0.24 b | 1.00 ± 0.37 c | 1.00 ± 0.37 d |
| TO_15_HA | 0.33 ± 0.24 b | 1.44 ± 0.44 b | 4.44 ± 0.73 bc | 9.78 ± 1.47 bc |
| TO_15_W | 0.33 ± 0.17 b | 2.78 ± 0.49 ab | 3.33 ± 0.47 bc | 4.56 ± 0.60 cd |
| TO_20_GIB | 0.11 ± 0.11 b | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| TO_20_HA | 0.11 ± 0.11 b | 0.56 ± 0.29 b | 4.78 ± 1.47 bc | 15.22 ± 3.21 ab |
| TO_20_W | 0.89 ± 0.35 ab | 4.56 ± 0.97 a | 10.11 ± 1.41 ab | 13.89 ± 1.43 b |
| TO_25_GIB | 0.67 ± 0.29 ab | 1.33 ± 0.47 b | 1.78 ± 0.64 c | 1.67 ± 0.60 cd |
| TO_25_HA | 0.22 ± 0.22 b | 2.11 ± 0.7 ab | 12.89 ± 4.03 a | 23.67 ± 3.02 a |
| TO_25_W | 1.44 ± 0.24 a | 4.89 ± 1.25 a | 9.67 ± 1.59 ab | 14.67 ± 2.23 b |
| Length (F test) | 3.82 | 4.88 | 14.17 | 30.34 |
| p value | 0.054 | 0.030 | p < 0.001 | p < 0.001 |
| Treat (F test) | 6.42 | 22.82 | 15.38 | 54.60 |
| p value | 0.003 | p < 0.001 | p < 0.001 | p < 0.001 |
| Length × Treat (F test) | 3.94 | 0.75 | 2.75 | 6.94 |
| p value | 0.024 | 0.477 | 0.070 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vâtcă, S.D.; Gâdea, Ș.; Vidican, R.; Șandor, M.; Stoian, V.; Vâtcă, A.; Horvath, A.; Stoian, V.A. Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments. Sustainability 2022, 14, 9272. https://doi.org/10.3390/su14159272
Vâtcă SD, Gâdea Ș, Vidican R, Șandor M, Stoian V, Vâtcă A, Horvath A, Stoian VA. Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments. Sustainability. 2022; 14(15):9272. https://doi.org/10.3390/su14159272
Chicago/Turabian StyleVâtcă, Sorin Daniel, Ștefania Gâdea, Roxana Vidican, Mignon Șandor, Vlad Stoian, Anamaria Vâtcă, Adrian Horvath, and Valentina Ancuța Stoian. 2022. "Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments" Sustainability 14, no. 15: 9272. https://doi.org/10.3390/su14159272
APA StyleVâtcă, S. D., Gâdea, Ș., Vidican, R., Șandor, M., Stoian, V., Vâtcă, A., Horvath, A., & Stoian, V. A. (2022). Primary Growth Effect of Salix viminalis L. CV. Inger and Tordis in Controlled Conditions by Exploring Optimum Cutting Lengths and Rhizogenesis Treatments. Sustainability, 14(15), 9272. https://doi.org/10.3390/su14159272










