Resource Cycling: Application of Anaerobic Utilization Methods
Abstract
:1. Introduction
2. Influence of Factors on Anaerobic Digestion of Organic Substrate
2.1. Substrate Pretreatment
2.2. Temperature
2.3. Acidity
2.4. C/N Ratio
2.5. Substrate Moisture
2.6. Particle Size
2.7. Mixing
2.8. Inoculum Application
3. Biogas Output
4. Process Performance Monitoring
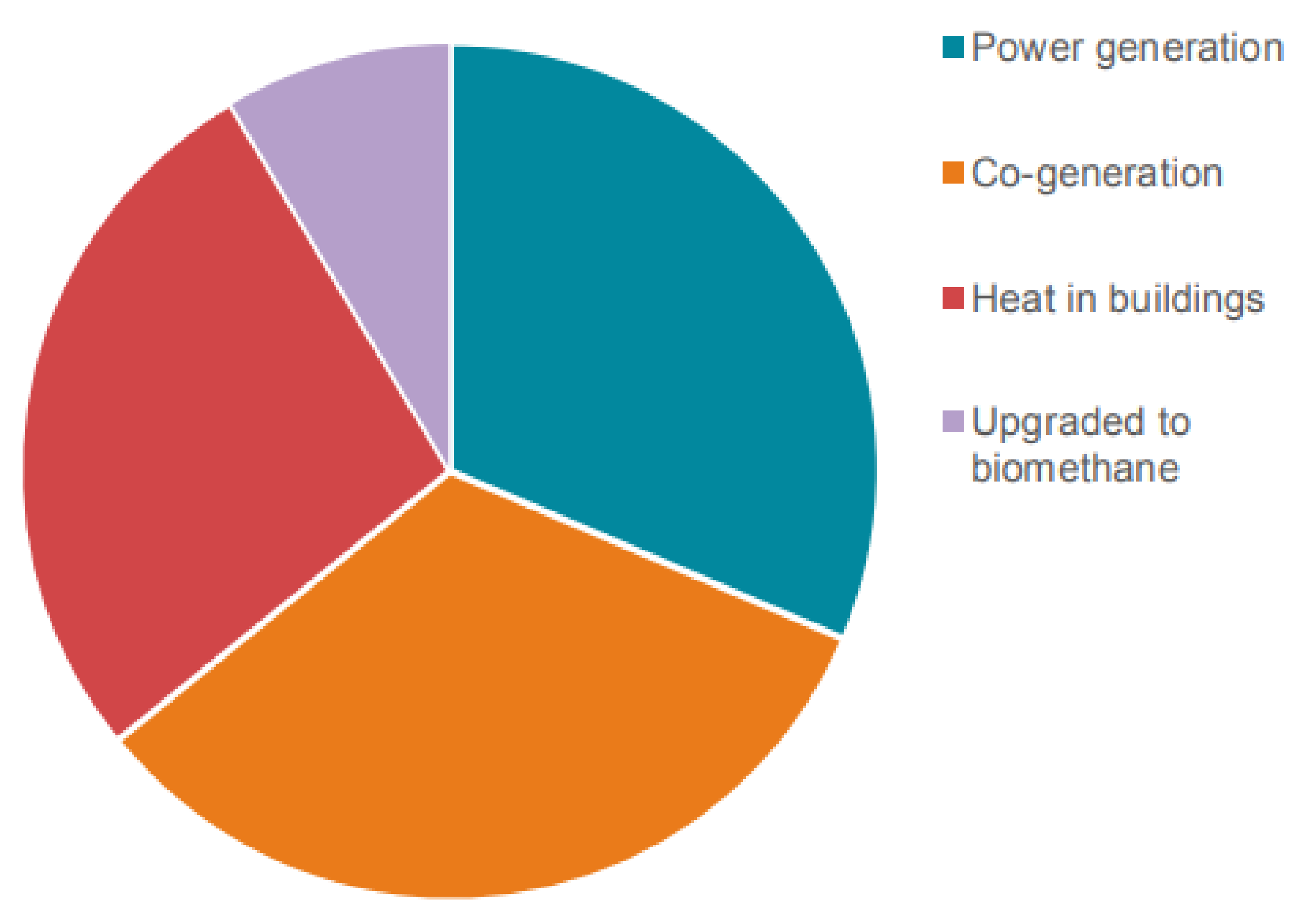
5. Biogas Application
6. Use of Digestate Formed in the Process of Anaerobic Digestion
7. Organic Waste: Challenges and Opportunities
8. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 9. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential–Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- De Baere, L. Will anaerobic digestion of solid waste survive in the future? Water Sci. Technol. 2006, 53, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Kurt, S. Creamer Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Wenjing, L.; Chenxi, J.; Junting, Z.; Junqing, X.; Dianhai, Y.; Guangming, L. Conversion of food waste into biofuel and biocarbon. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals; Woodhead Publishing: Sawston, UK, 2021; pp. 383–449. [Google Scholar]
- Liu, Z.-G.; Zhou, X.-F.; Zhang, Y.-L.; Zhu, H.-G. Enhanced anaerobic treatment of CSTR-digested effluent from chicken manure: The effect of ammonia inhibition. Waste Manag. 2012, 32, 137–143. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste–activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. A mathematical model for dynamic simulation of anaerobic digestion of complex substrates: Focusing on ammonia inhibition. Biotechnol. Bioeng. 1993, 42, 159–166. [Google Scholar] [CrossRef]
- Dekhtiar, I.; Dyvak, T.; Martsenyuk, Y. Features of biogas production process and methods of its modeling. In Proceedings of the 2013 12th International Conference on the Experience of Designing and Application of CAD Systems in Microelectronics (CADSM), Lviv, Ukraine, 19-23 February 2013. [Google Scholar]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Qasim, S.R. Wastewater Treatment Plants: Planning Design and Operation; CRC Press: Boca Raton, FL, USA, 1999; p. 1126. [Google Scholar]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Aquino, S.F.; Stuckey, D.C. Integrated model of the production of soluble microbial products (SMP) and extracellular polymeric substances (EPS) in anaerobic chemostats during transient conditions. Biochem. Eng. J. 2008, 38, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.K.; Tay, J.-H.; Tay, S.T.L.; Hung, Y.-T. Handbook of Environmental Engineering Volume 11 in Environmental Bioengineering; Humana Press: Totowa, NJ, USA, 2010; p. 867. [Google Scholar]
- Chynoweth, D.P. Anaerobic Digestion of Solid Waste; CPS Press: Kirazpınar, Turkey, 1996; p. 240. [Google Scholar]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Westerholm, M.; Hansson, M.; Schnurer, A. Improved biogas production from whole stillage by co–digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Foster, J.W. Microbiology: An Evolving Science, 2nd ed.; W.W. Norton & Company: New York, NY, USA, 2011; p. 1408. [Google Scholar]
- Nielfa, A.; Cano, R.; FdzPolanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Lerman, L.V.; Gerstlberger, W.; Lima, M.F.; Frank, A.G. How governments, universities, and companies contribute to renewable energy development? A municipal innovation policy perspective of the triple helix. Energy Res. Soc. Sci. 2021, 71, 101854. [Google Scholar] [CrossRef]
- Igoni, H.A.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of anaerobic digesters for producing biogas from municipal solid–waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Palela, M.; Socaciu, C. Biogas Technology in Germany and Romania: Comparative Aspects and Achievements. Bull. UASVM Agric. 2012, 69, 143–151. [Google Scholar] [CrossRef]
- Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques—A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar]
- Weinrich, S.; Nelles, M. Basics of Anaerobic Digestion: Biochemical Conversion and Process Modelling. DBFZ-Rep. 2021, 40, 9–76. [Google Scholar]
- Prabhu, A.V.; Raja, S.A.; Avinash, A.; Pugazhendhi, A. Parametric optimization of biogas potential in anaerobic co-digestion of biomass wastes. Fuel 2021, 288, 119574. [Google Scholar] [CrossRef]
- Sharma, D.K. Studies on Availability and Utilization of Onion Storage Waste in a Rural Habitat. Ph.D. Thesis, Centre for Rural Development and Technology, Indian Institute of Technology, Delhi, India, 2002. [Google Scholar]
- Lim, J.W.; Wang, J.-Y. Enhanced hydrolysis and methane yield by applying microaeration pretreatment to the anaerobic co-digestion of brown water and food waste. Waste Manag. 2013, 33, 813–819. [Google Scholar] [CrossRef]
- Benabdallah El-Hadj, T.; Dosta, J.; Marquez-Serrano, R.; Mata-Alvarez, J. Effect of ultrasound pretreatment in mesophilic and thermophilic anaerobic digestion with emphasis on naphthalene and pyrene removal. Water Res. 2007, 41, 87–94. [Google Scholar] [CrossRef]
- Dhar, B.R.; Nakhla, G.; Ray, M.B. Techno–economic evaluation of ultrasound and thermal pretreatments for enhanced anaerobic digestion of municipal waste activated sludge. Waste Manag. 2012, 32, 542–549. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Angelis, E.M.; Kornaros, M. Optimization of thermo–chemical hydrolysis of kitchen wastes. Waste Manag. 2013, 33, 740–745. [Google Scholar] [CrossRef]
- López Torres, M.; Lloréns, M.d.C.E. Effect of alkaline pretreatment on anaerobic digestion of solid wastes. Waste Manag. 2008, 28, 2229–2234. [Google Scholar] [CrossRef]
- Flore-Juarez, C.R.; Rodríguez-García, A.; Cárdenas-Mijangos, J.; Montoya-Herrera, L.; Mora-Tovar, L.A.G.; Bustos-Bustos, E.; Rodríguez-Valadez, F.; Manríquez-Rocha, J. Chemically pretreating slaughterhouse solid waste to increase the efficiency of anaerobic digestion. J. Biosci. Bioeng. 2014, 118, 415–419. [Google Scholar] [CrossRef]
- Bordeleau, E.L.; Droste, R.L. Comprehensive review and compilation of pretreatments for mesophilic and thermophilic anaerobic digestion. Water Sci. Technol. 2011, 63, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis–enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaeni, A.; Rasmin, N.; Patiung, G.W.; Susilowati, P.E. Evaluation of biogas potential from organic waste: Wastewater and solid wastes. J. Phys. Conf. Ser. 2021, 1899, 012005. [Google Scholar] [CrossRef]
- Garba, B. Effect of temperature and retention period on biogas production from ligrocellulosic material. Renew. Energy 1996, 9, 938–941. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Cascallana, J.G.; González, R.; Gómez, X. High-solid anaerobic digestion: Reviewing strategies for increasing reactor performance. Environments 2021, 8, 80. [Google Scholar] [CrossRef]
- Ameen, F.; Ranjitha, J.; Ahsan, N.; Shankar, V. Co-digestion of microbial biomass with animal manure in three-stage anaerobic digestion. Fuel 2021, 306, 121746. [Google Scholar] [CrossRef]
- Jeppu, G.P.; Janardhan, J.; Kaup, S.; Janardhanan, A.; Mohammed, S.; Acharya, S. Effect of feed slurry dilution and total solids on specific biogas production by anaerobic digestion in batch and semi-batch reactors. J. Mater. Cycles Waste Manag. 2022, 24, 97–110. [Google Scholar] [CrossRef]
- Barik, D. Energy extraction from toxic waste originating from food processing industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Woodhead Publishing: Sawston, UK, 2019; pp. 17–42. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1994, 38, 560–564. [Google Scholar] [CrossRef]
- Martín-González, L.; Castro, R.; Alcina Pereira, M.; Madalena Alves, M.; Font, X.; Vicent, T. Thermophilic co-digestion of organic fraction of municipal solid wastes with FOG wastes from a sewage treatment plant: Reactor performance and microbial community monitoring. Bioresour. Technol. 2011, 102, 4734–4741. [Google Scholar] [CrossRef] [Green Version]
- Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch anaerobic co-digestion and biochemical methane potential analysis of goat manure and food waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Fdez-Güelfo, L.A.; Álvarez-Gallego, C.; Sales Márquez, D.; Romero García, L.I. Biological pretreatment applied to industrial organic fraction of municipal solid wastes (OFMSW): Effect on anaerobic digestion. Chem. Eng. J. 2011, 172, 321–325. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef]
- Agdag, O.N.; Sponza, D.T. Co–digestion of mixed industrial sludge with municipal solid wastes in anaerobic simulated landfilling bioreactors. J. Hazard. Mater. 2007, 140, 75–85. [Google Scholar] [CrossRef]
- Elango, D.; Pulikesi, M.; Baskaralingam, P.; Ramamurthi, V.; Sivanesan, S. Production of biogas from municipal solid waste with domestic sewage. J. Hazard. Mater. 2007, 141, 301–304. [Google Scholar] [CrossRef]
- Kaya, D.; Imamoglu, I.; Dilek Sanin, F. Impact of PCB–118 and transformer oil toxicity on anaerobic digestion of sludge: Anaerobic toxicity assay results. Chemosphere 2013, 92, 821–827. [Google Scholar] [CrossRef]
- Braeutigam, P.; Franke, M.; Ondruschka, B. Effect of ultrasound amplitude and reaction time on the anaerobic fermentation of chicken manure for biogas production. Biomass Bioenergy 2014, 63, 109–113. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.-Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co–digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Niu, Q.; Hojo, T.; Qiao, W.; Qiang, H.; Li, Y.-Y. Characterization of methanogenesis, acidogenesis and hydrolysis in thermophilic methane fermentation of chicken manure. Chem. Eng. J. 2014, 244, 587–596. [Google Scholar] [CrossRef]
- Abouelenien, F.; Fujiwara, W.; Namba, Y.; Kosseva, M.; Nishio, N.; Nakashimada, Y. Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Bioresour. Technol. 2010, 101, 6368–6373. [Google Scholar] [CrossRef]
- Charles, W.; Walker, L.; Cord-Ruwisch, R. Effect of pre–aeration and inoculum on the start–up of batch thermophilic anaerobic digestion of municipal solid waste. Bioresour. Technol. 2009, 100, 2329–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanimeh, S.; Fadel, M.E.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source–sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012, 117, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, A.B.; Oyekola, O.O.; Welz, P.J. Anaerobic treatment of tannery wastewater in the context of a circular bioeconomy for developing countries. J. Clean. Prod. 2021, 296, 126490. [Google Scholar] [CrossRef]
- Silvestre, G.; Rodríguez-Abalde, A.; Fernández, B.; Flotats, X.; Bonmatí, A. Biomass adaptation over anaerobic co–digestion of sewage sludge and trapped grease waste. Bioresour. Technol. 2011, 102, 6830–6836. [Google Scholar] [CrossRef]
- Sosnowski, P.; Klepacz–Smolka, A.; Kaczorek, K.; Ledakowicz, S. Kinetic investigations of methane co–fermentation of sewage sludge and organic fraction of municipal solid wastes. Bioresour. Technol. 2007, 99, 5731–5737. [Google Scholar] [CrossRef]
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for olive wastes management and efficient bioenergy production. Energy Convers. Manag. 2021, 244, 114467. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Saady, N.M.C.; Zendehboudi, S.; Rajagopal, R.; Hung, Y.T. Biohydrogen production through mixed culture dark anaerobic fermentation of industrial waste. In Integrated Natural Resources Management; Springer: Cham, Switzerland, 2021; pp. 323–369. [Google Scholar]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. Biogas production—A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sustain. Energy Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Ke, L.; Liu, X.; Du, B.; Wang, Y.; Zheng, Y.; Li, Q. Component analysis and risk assessment of biogas slurry from biogas plants. Chin. J. Chem. Eng. 2022, 44, 182–191. [Google Scholar] [CrossRef]
- Noor, R.S.; Ahmed, A.; Abbas, I.; Hussain, F.; Umair, M.; Noor, R.; Sun, Y. Enhanced biomethane production by 2-stage anaerobic co-digestion of animal manure with pretreated organic waste. Biomass Convers. Biorefinery 2021, 1–15. [Google Scholar] [CrossRef]
- Astals, M.; Ariso, M.; Galí, A.; Mata-Alvarez, J. Co–digestion of pig manure and glycerine: Experimental and modelling study. J. Environ. Manag. 2011, 92, 1091–1096. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic co–digestion of pig manure and crude glycerol: Process performance and digestate stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef]
- Ali, S.; Zahra, N.; Nasreen, Z.; Usman, S. Impact of biogas technology in the development of rural population. Pak. J. Anal. Environ. Chem. 2013, 14, 10. [Google Scholar]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Nguyen, D.D. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Sawyerr, N.; Trois, C.; Workneh, T.; Okudoh, V.I. An overview of biogas production: Fundamentals, applications and future research. Int. J. Energy Econ. Policy 2019, 9, 105–116. [Google Scholar]
- Verma, M.L.; Saini, R.; Sharma, S.; Rani, V.; Jana, A.K. Suitability of the Lantana Weed as a substrate for biogas production. In Substrate Analysis for Effective Biofuels Production; Springer: Singapore, 2020; pp. 51–78. [Google Scholar]
- Lisboa, M.S.; Lansing, S. Characterizing food waste substrates for co-digestion through biochemical methane potential (BMP) experiments. Waste Manag. 2013, 33, 2664–2669. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bio. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Triolo, J.M.; Pedersen, L.; Qu, H.; Sommer, S.G. Biochemical methane potential and anaerobic biodegradability of non–herbaceous and herbaceous phytomass in biogas production. Bioresour. Technol. 2012, 125, 226–232. [Google Scholar] [CrossRef]
- Jarwar, A.I.; Laghari, A.Q.; Maitlo, G.; Qureshi, K.; Bhutto, A.W.; Shah, A.K.; Ahmed, S. Biological assisted treatment of buffalo dung and poultry manure for biogas generation using laboratory-scale bioreactor. Biomass Convers. Biorefinery 2021, 1–8. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Ayyadurai, S.; Arunachalam, K.D.; Mishra, G.; Chen, W.H.; Juan, J.C.; Naqvi, S.R. Current technologies of biochemical conversion of food waste into biogas production: A review. Fuel 2022, 323, 124321. [Google Scholar] [CrossRef]
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrog. Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- German Technical and Scientific Association for Gas and Water (DVGW). Utilisation of Gases from Renewable Sources in the Public Gas Supply; Technische Regeln Arbeitsblatt G262; DVGW: Bonn, Germany, 2004. [Google Scholar]
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Ghekiere, G.; Accoe, F.; Tack, F.M.G. Closing the nutrient cycle by using bio–digestion waste derivatives as synthetic fertilizer substitutes: A field experiment. Biomass Bioenergy 2013, 55, 175–189. [Google Scholar] [CrossRef] [Green Version]
- IEA. Outlook for Biogas and Biomethane Prospects for Organic Growth—An Introduction to Biogas and Biomethane. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/an-introduction-to-biogas-and-biomethane (accessed on 20 April 2022).
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Buysse, J.; Tack, F.M.G. Ecological and economic benefits of the application of bio–based mineral fertilizers in modern agriculture. Biomass Bioenergy 2013, 49, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Jury, C.; Benetto, E.; Koster, D.; Schmitt, B.; Welfring, J. Life Cycle Assessment of biogas production by monofermentation of energy crops and injection into the natural gas grid. Biomass Bioenergy 2010, 34, 54–66. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilizers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–2954. [Google Scholar] [CrossRef]
- Mangwandi, C.; Tao, L.J.; Albadarin, A.B.; Allen, S.J.; Walker, G.M. Alternative method for producing organic fertiliser from anaerobic digestion liquor and limestone powder: High Shear wet granulation. Powder Technol. 2013, 233, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Blagodatskaya, E.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Kuzyakov, Y. Decomposition of biogas residues in soil and their effects on microbial growth kinetics and enzyme activities. Biomass Bioenergy 2012, 45, 221–229. [Google Scholar] [CrossRef]
- Bond, T.; Templeton, M.R. History and future of domestic biogas plants in the developing world. Energy Sustain. Dev. 2011, 15, 347–354. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Hchaichi, I.; Bandini, F.; Spini, G.; Banni, M.; Cocconcelli, P.S.; Puglisi, E. Enterococcus faecalis and Vibrio harveyi colonize low-density polyethylene and biodegradable plastics under marine conditions. FEMS Microbiol. Lett. 2020, 367, fnaa125. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, T.; Aravinthan, A.; Lakshmi, K.; Venkatesan, R.; Vedaprakash, L.; Doble, M. Fouling and stability of polymers and composites in marine environment. Int. Biodeterior. Biodegrad. 2011, 65, 276–284. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2020, 759, 143536. [Google Scholar] [CrossRef]
- Huang, X.; Cao, L.; Qin, Z.; Li, S.; Kong, W.; Liu, Y. Tat-independent secretion of polyethylene terephthalate hydrolase petase in Bacillus subtilis 168 mediated by its native signal peptide. J. Agric. Food Chem. 2018, 66, 13217–13227. [Google Scholar] [CrossRef]
- Danso, Z. Supplemental figures new insights into the function and global distribution of polyethylene terephthalate (PET)—Degrading bacteria and terrestrial metagenome. Appl. Environ. Microbiol. 2018, 53, 1689–1699. [Google Scholar]
- Barth, M.; Honak, A.; Oeser, T.; Wei, R.; Belisário-Ferrari, M.R.; Then, J.; Schmidt, J.; Zimmermann, W. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol. J. 2016, 11, 1082–1087. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2020, 14, 374–385. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, X.; Shi, X.; Zhang, Y. A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total Environ. 2020, 726, 138564. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Mirończuk, A.M.; García-Martín, A.; Saborido, A.; de la Mata, I.; Arroyo, M. Biochemical properties and biotechnological applications of microbial enzymes involved in the degradation of polyester-type plastics. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140315. [Google Scholar] [CrossRef]
- Espinosa, M.J.C.; Blanco, A.C.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Skariyachan, S.; Taskeen, N.; Kishore, A.P.; Krishna, B.V. Recent advances in plastic degradation—From microbial consortia-based methods to data sciences and computational biology driven approaches. J. Hazard. Mater. 2021, 426, 128086. [Google Scholar] [CrossRef]
- Moharir, R.V.; Kumar, S. Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J. Clean. Prod. 2019, 208, 65–76. [Google Scholar] [CrossRef]
- Shi, C.; Reilly, L.T.; Kumar VS, P.; Coile, M.W.; Nicholson, S.R.; Broadbelt, L.J.; Chen EY, X. Design principles for intrinsically circular polymers with tunable properties. Chem 2021, 7, 2896–2912. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Gomez, X.; Cuetos, M.J.; Cara, J.; Moran, A.; Garcia, A.I. Anaerobic co-digestion of primary sludge and the fruit and vegetable fraction of the municipal solid wastes: Conditions for mixing and evaluation of the organic loading rate. Renew. Energy 2006, 31, 2017–2024. [Google Scholar] [CrossRef]
- Cristancho, D.E.; Arellano, A.V. Study of the operational conditions for anaerobic digestion of urban solid wastes. Waste Manag. 2006, 26, 546–556. [Google Scholar]
- Zhang, Y.; Banks, C.J. Impact of different particle size distributions on anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2013, 33, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Okishio, Y.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikhareva, I.N.; Aminova, G.K.; Mazitova, A.K. Resource Cycling: Application of Anaerobic Utilization Methods. Sustainability 2022, 14, 9278. https://doi.org/10.3390/su14159278
Vikhareva IN, Aminova GK, Mazitova AK. Resource Cycling: Application of Anaerobic Utilization Methods. Sustainability. 2022; 14(15):9278. https://doi.org/10.3390/su14159278
Chicago/Turabian StyleVikhareva, Irina N., Guliya K. Aminova, and Aliya K. Mazitova. 2022. "Resource Cycling: Application of Anaerobic Utilization Methods" Sustainability 14, no. 15: 9278. https://doi.org/10.3390/su14159278
APA StyleVikhareva, I. N., Aminova, G. K., & Mazitova, A. K. (2022). Resource Cycling: Application of Anaerobic Utilization Methods. Sustainability, 14(15), 9278. https://doi.org/10.3390/su14159278





