Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 1: Characterization of Carbonaceous Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Carbonaceous Materials
2.2.1. Nonisothermal Thermogravimetric Analysis in Nitrogen Atmosphere (TGA-N2)
2.2.2. Pyrolysis in Nitrogen Atmosphere (py-N2)
2.2.3. Isothermal Reactivity in CO2 (TGA-CO2)
3. Results and Discussions
3.1. Characterization of Carbonaceous Materials
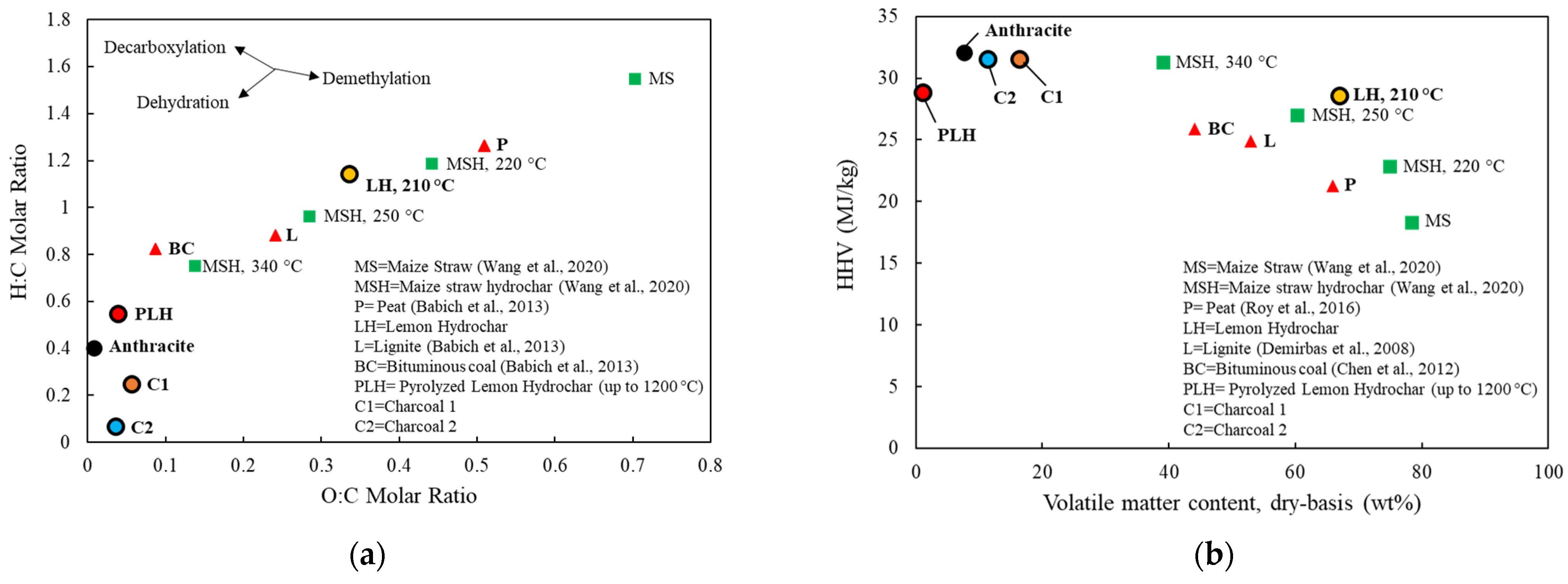
3.1.1. Compositions of Carbonaceous Materials
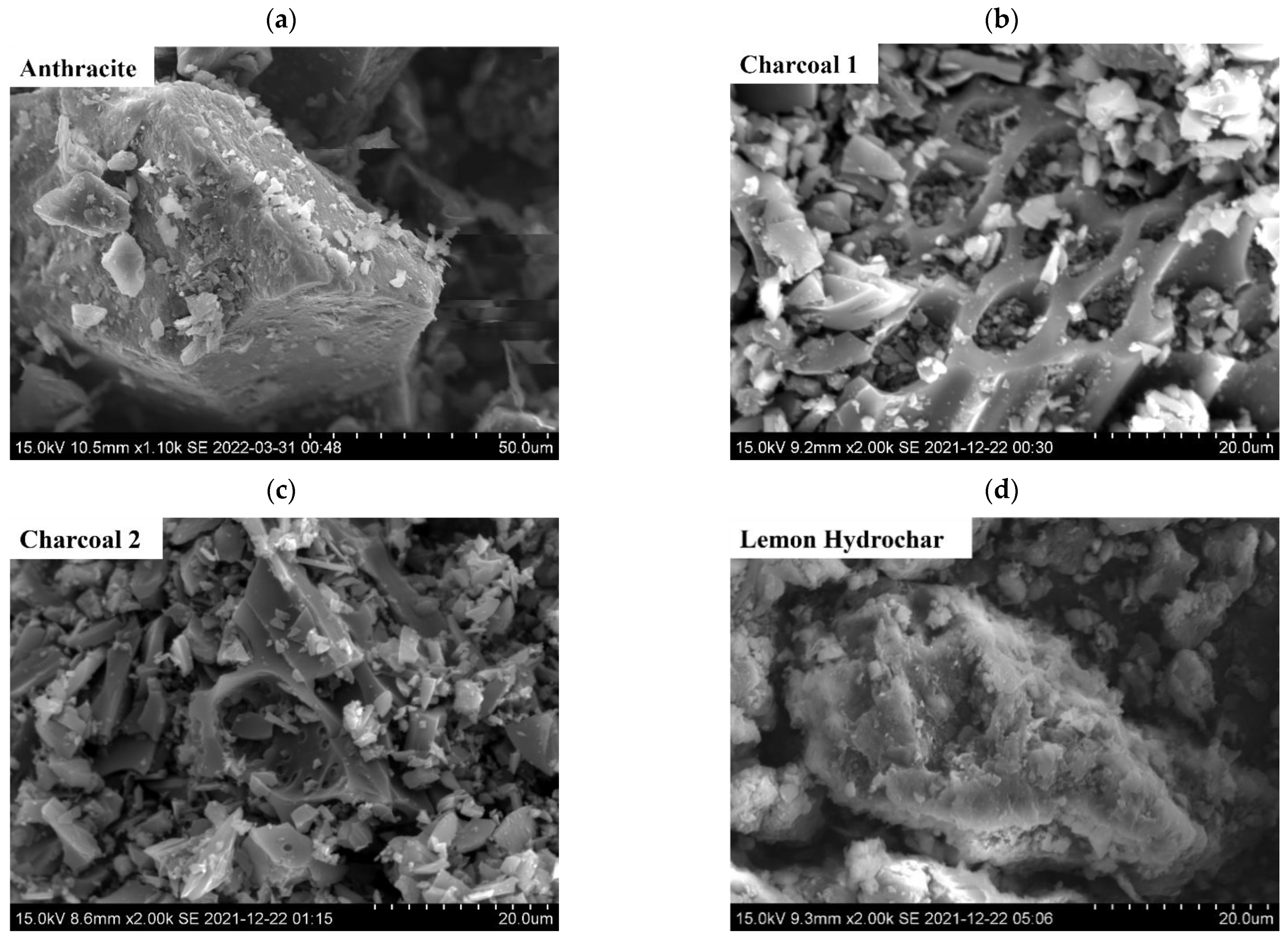
3.1.2. SEM Analyses of Surface Morphologies of Carbonaceous Materials
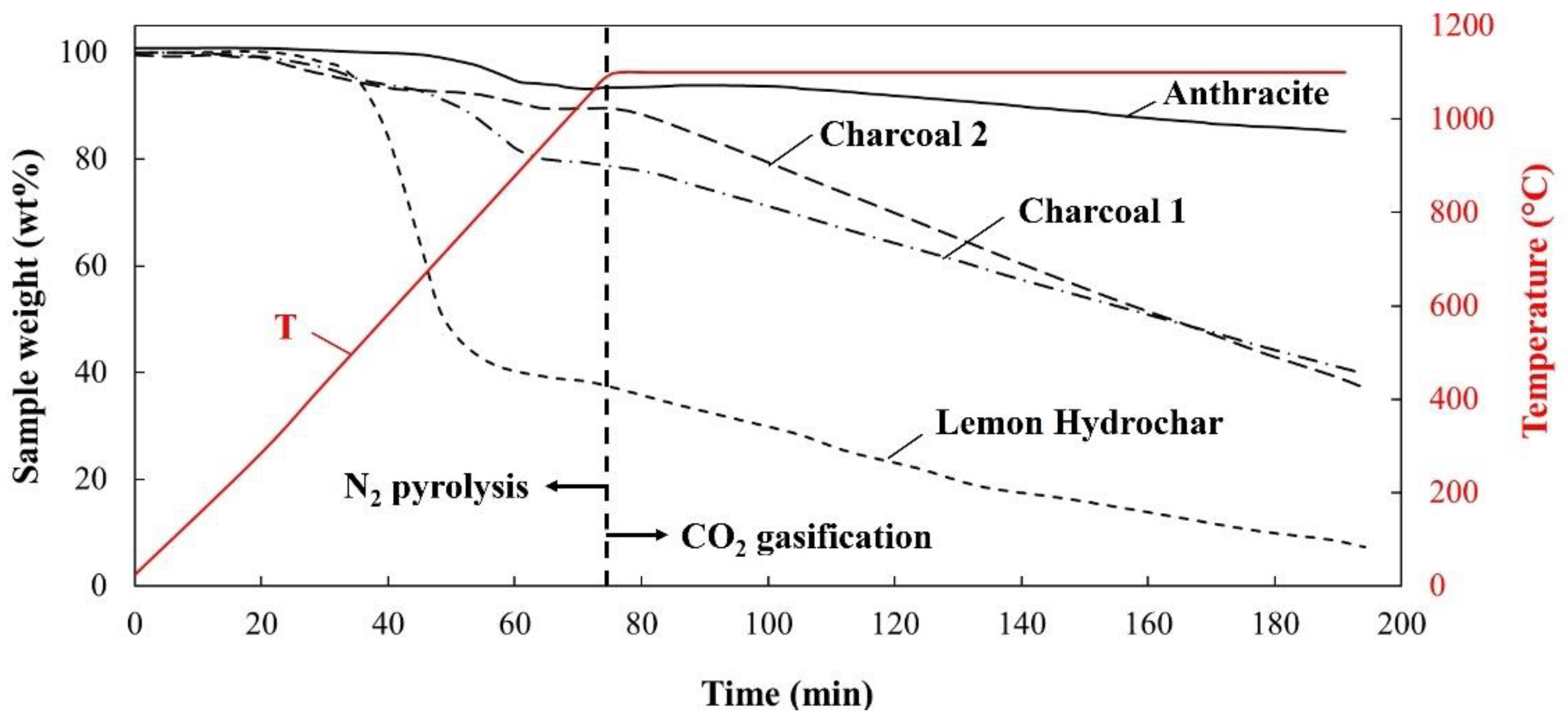
3.1.3. TGA-N2 Analyses
3.1.4. py-N2 Analyses
3.1.5. TGA-CO2 Analyses
3.1.6. Ash and Impurity Characteristics
3.2. Future Prospects
4. Conclusions
- Lemon Hydrochar is a medium carbon-containingmaterial (59.8 wt%) with a medium density (1.23 kg/m3), a medium lower heating value (23 MJ/kg), a high volatile matter content (67 wt%), a low moisture (2.6 wt%), a low ash, low impurity (S, P) concentrations, and a medium CO2 reactivity (0.25%/min) relative to charcoals and Anthracite.
- Charcoals are high carbon-containing materials (>86 wt%) with high heating energies and low volatile matter contents, and are more similar to Anthracite. Charcoals’ weaknesses include their lower densities (0.76–1.07 kg/m3), higher moisture contents, higher alkalis and P concentrations, and higher CO2 reactivities (0.33–0.44%/min). Densification resulted in an increased density, a reduced amount of moisture pickup, and a lowered CO2 reactivity of charcoal. The advantages of Lemon Hydrochar and charcoals compared to Anthracite are their lower ash and S concentrations and renewability.
- Pyrolysis of Anthracite and both charcoals up to 1200 °C indicated that their volatile matter mostly consisted of gases such as CO, CO2, H2, and CH4. Due to the high fixed carbon content and the low carbon concentrations of their volatile matter, more than 90% of carbon in the initial carbon material was retained in the residual char after a devolatilization up to 1200 °C. On the contrary, Lemon Hydrochar loses a significant portion of its total carbon to the volatile matter which includes liquid (17%) and pyrolysis gases (29%). The pyrolysis gas consisted of 17.6 wt% CO, 12.8 wt% CO2, 3.3 wt% CH4, 1.8 wt% H2, and other hydrocarbon gases which sums up to 4.3 wt%. Only 54% of carbon in Lemon Hydrochar remained in the solid up to 1200 °C. As a result, the amount of carbon available for high-temperature reactions such as combustion, generation of reducing gases, carburization, and slag foaming is significantly reduced. This makes Lemon Hydrochar a less-efficient material compared to charcoal and Anthracite. The extent to which tar could be utilized as a reducing agent requires further investigation.
- Based on the experimental findings from this work, it is speculated that charcoal could be used interchangeably with Anthracite in applications where its low density and CO2 reactivity do not create a problem, such as for carbon composite agglomerates, BF tuyere injection, and in EAFs. For hydrochar, it is recommended that two strategies should be investigated in the future to overcome its shortcomings in high-temperature metallurgical applications: (1) blending with either charcoal or Anthracite, or (2) removal of of its volatile matter by slow pyrolysis such that it becomes a comparable solid fuel to charcoal. Alternatively, hydrochar may be used for production of green syngas by gasification technology, which could be used for the production of direct-reduced iron. All three valorization routes of hydrochar should be investigated and assessed in future studies to identify the most efficient way to utilize hydrochar in the iron and steelmaking processes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | Blast furnace |
| BOF | Basic oxygen furnace |
| DRI | Direct-reduced iron |
| EAF | Electric arc furnace |
| SR | Smelting reduction |
| ISI | Iron and steelmaking industry |
| EU | European Union |
| ULCOS | Ultra-Low CO2 Steelmaking |
| CCS | Carbon capture storage |
| PC | Pulverized coal |
| HTC | Hydrothermal carbonization |
| LHV | Lower heating value |
| HHV | Higher heating value |
| SEM | Scanning electron microscope |
| TGA | Thermogravimetric analysis |
| Micro-GC | Micro-gas chromatography |
| CRI | Coke reactivity index |
| RHF | Rotary hearth furnace |
| CCA | Carbon composite agglomerate |
| TRZ | Thermal reserve zone |
| CSR | Coke strength after reaction |
| HBI | Hot-briquetted iron |
| AOD | Argon oxygen decarburization |
References
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass applications in iron and steel industry: An overview of challenges and opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive review of the opportunities to use biomass-based fuels in iron and steelmaking processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Abdul Quader, M.; Ahmed, S.; Dawal, S.Z.; Nukman, Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- European Commission. Towards Competitive and Clean European Steel Accompanying the Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Updating the 2020 New Industrial Strategy: Building a Stronger Single Market for Europe’s Recovery; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as blast furnace injectant—Considering availability, pretreatment and deployment in the Swedish steel industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Mathieson, J.G.; Somerville, M.A.; Deev, A.; Jahanshahi, S. 19-Utilization of biomass as an alternative fuel in ironmaking. In Iron Ore; Lu, L., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 581–613. [Google Scholar]
- Nwachukwu, C.M.; Wang, C.; Wetterlund, E. Exploring the role of forest biomass in abating fossil CO2 emissions in the iron and steel industry—The case of Sweden. Appl. Energy 2021, 288, 116558. [Google Scholar] [CrossRef]
- Sundqvist Ökvist, L.; Lundgren, M. Experiences of Bio-Coal Applications in the Blast Furnace Process—Opportunities and Limitations. Minerals 2021, 11, 863. [Google Scholar] [CrossRef]
- Mousa, E.; Babich, A.; Senk, D. Iron Ore Sintering Process with Biomass Utilization. In Proceedings of the METEC & 2nd ESTAD, Düsseldorf, Germany, 15–19 June 2015. [Google Scholar]
- Mathieson, J.G.; Rogers, H.; Somerville, M.A.; Jahanshahi, S. Reducing Net CO2 Emissions Using Charcoal as a Blast Furnace Tuyere Injectant. ISIJ Int. 2012, 52, 1489–1496. [Google Scholar] [CrossRef] [Green Version]
- Kowitwarangkul, P.; Babich, A.; Senk, D. Reduction Behavior of Self-Reducing Pellet (SRP) for Low Height Blast Furnace. Steel Res. Int. 2014, 85, 1501–1509. [Google Scholar] [CrossRef]
- Ueda, S.; Watanabe, K.; Yanagiya, K.; Ariyama, T. Improvement of Reactivity of Carbon Iron Ore Composite with Biomass Char for Blast Furnace. ISIJ Int. 2009, 49, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Konishi, H.; Ichikawa, K.; Usui, T. Effect of Residual Volatile Matter on Reduction of Iron Oxide in Semi-charcoal Composite Pellets. ISIJ Int. 2010, 50, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Ueki, Y.; Yoshiie, R.; Naruse, I.; Ohno, K.-I.; Maeda, T.; Nishioka, K.; Shimizu, M. Reaction behavior during heating biomass materials and iron oxide composites. Fuel 2013, 104, 58–61. [Google Scholar] [CrossRef]
- Marcos, M.; Bianco, L.; Cirilli, F.; Reichel, T.; Baracchini, G.; Echterhof, T.; Rekersdrees, T.; Mirabile, D.; Griessacher, T.; Sommerauer, H. Biochar for a Sustainable EAF Steel Production (GREENEAF2); Final Report; Publications Office: Luxembourg, 2019. [Google Scholar]
- Somerville, M.; Jahanshahi, S.; Ridgeway, P.; Davies, M.; Mathieson, J. Sustainable carbon in steelmaking—Plant trials at the Sydney Steel Mill, Sustainable. In Proceedings of the Sustainable Mining 2010—The Business Case, Kalgoorlie, WA, Australia, 17–19 August 2010; AusIMM: Melbourne, Australia, 2010; pp. 38–52. [Google Scholar]
- Norgate, T.; Haque, N.; Somerville, M.; Jahanshahi, S. Biomass as a Source of Renewable Carbon for Iron and Steelmaking. ISIJ Int. 2012, 52, 1472–1481. [Google Scholar] [CrossRef] [Green Version]
- Suopajärvi, H.; Fabritius, T. Towards More Sustainable Ironmaking—An Analysis of Energy Wood Availability in Finland and the Economics of Charcoal Production. Sustainability 2013, 5, 1188–1207. [Google Scholar] [CrossRef] [Green Version]
- Zahnen, J.; Haag, V.; Lewandrowski, T.; Hirschberger, P. 2020 Analysis of the EU Charcoal Market; WWF Germany: Berlin, Germany, 2020. [Google Scholar]
- Ferraz Filho, A.; Scolforo, J.; Mola-Yudego, B. The coppice-with-standards silvicultural system as applied to Eucalyptus plantations—A review. J. For. Res. 2014, 25, 237–248. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhan, H.; Song, Y.; Yin, X.; Wu, C. Structure-reactivity relationships of biowaste-derived hydrochar on subsequent pyrolysis and gasification performance. Energy Convers. Manag. 2019, 199, 112014. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zhou, S.; Shang, H.; Luo, J.; Tsang, D.C.W. Chapter 15—Hydrothermal Carbonization for Hydrochar Production and Its Application. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Cambridge, UK, 2019; pp. 275–294. [Google Scholar]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.-Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal carbonization of maize straw for hydrochar production and its injection for blast furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- Langone, M.; Basso, D. Process Waters from Hydrothermal Carbonization of Sludge: Characteristics and Possible Valorization Pathways. Int. J. Environ. Res. Public Health 2020, 17, 6618. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Final Report Summary—NEWAPP (New Technological Applications for Wet Biomass Waste Stream Products). Available online: https://cordis.europa.eu/project/id/605178/reporting (accessed on 18 April 2022).
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- European Compost Network. Treatment of Bio-Waste in Europe. Available online: https://www.compostnetwork.info/policy/biowaste-in-europe/treatment-bio-waste-europe/ (accessed on 28 May 2022).
- European Environment Agency. Bio-Waste in Europe—Turning Challenges into Opportunities. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 28 May 2022).
- European Commission. Waste Framework Directive. Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/waste-framework-directive_en (accessed on 28 May 2022).
- Ingelia. Ingelia Model. Available online: https://ingelia.com/index.php/negocio-sostenible/modelo-ingelia/?lang=en (accessed on 15 March 2022).
- Bevan, E.; Fu, J.; Zheng, Y. Retracted Article: Challenges and opportunities of hydrothermal carbonisation in the UK; case study in Chirnside. RSC Adv. 2020, 10, 31586–31610. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Salimbeni, A.; Hitzl, M.; Zhang, J.; Wang, G.-W.; Wang, K.; Wang, C. Evaluation of Utilising Ingelia Hydrochar Produced from Organic Residues for Blast Furnaces Injection Comparison with Anthracite and Bituminous Coal. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–17 May 2018. [Google Scholar]
- Li, J.; Xu, R.; Wang, G.; Zhang, J.; Song, B.; Liang, W.; Wang, C. Study on the feasibility and co-combustion mechanism of mixed injection of biomass hydrochar and anthracite in blast furnace. Fuel 2021, 304, 121465. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.; Zhou, H.; Ning, X.; Zhang, C. Numerical Simulation Study on the Effects of Co-Injection of Pulverized Coal and Hydrochar into the Blast Furnace. Sustainability 2022, 14, 4407. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, C.; Wang, G.; Ning, X.; Zhang, J.; Guo, X.; Xu, R.; Wang, P.; Ye, L.; Li, J.; et al. Research on the co-combustion characteristics and kinetics of agricultural waste hydrochar and anthracite. Renew. Energy 2022, 194, 1119–1130. [Google Scholar] [CrossRef]
- Liang, W.; Nanou, P.; Wray, H.; Zhang, J.; Lundstrom, I.; Lundqvist, S.; Wang, C. Feasibility Study of Bio-Sludge Hydrochar as Blast Furnace Injectant. Sustainability 2022, 14, 5510. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, J.; Li, K.; Barati, M.; Liu, Z. Co-gasification characteristics of coke blended with hydro-char and pyro-char from bamboo. Energy 2022, 241, 122890. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, E.; Chionoso, O.P. Numerical simulation of the synergistic effect of combustion for the hydrochar/coal blends in a blast furnace. Energy 2022, 238, 121722. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Xu, R.; Ning, X.; Zhang, N.; Wang, C.; Mao, X.; Li, J.; Wang, G.; Wang, C. Co-combustion kinetic analysis of biomass hydrochar and anthracite in blast furnace injection. Fuel 2022, 316, 123299. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Ning, X.; Zhang, J.; Wang, C. Numerical simulation of combustion behaviors of hydrochar derived from low-rank coal in the raceway of blast furnace. Fuel 2020, 278, 118267. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, G.; Zhang, J.; Ning, X.; Li, Y.; Liang, W.; Wang, C. Study on co-combustion characteristics of hydrochar and anthracite coal. J. Energy Inst. 2020, 93, 1125–1137. [Google Scholar] [CrossRef]
- Jarnerud, T.; Karasev, A.V.; Wang, C.; Bäck, F.; Jönsson, P.G. Utilization of Organic Mixed Biosludge from Pulp and Paper Industries and Green Waste as Carbon Sources in Blast Furnace Hot Metal Production. Sustainability 2021, 13, 7706. [Google Scholar] [CrossRef]
- Basu, P. Chapter 3—Biomass Characteristics. In Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Basu, P., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 47–86. [Google Scholar]
- Lu, Y.-C.; Brabie, L.; Karasev, A.V.; Wang, C. Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 2: Carburization of Liquid Iron by Addition of Iron-Carbon Briquettes. Sustainability 2022, 14, 5383. [Google Scholar] [CrossRef]
- Basu, P. Chapter 7—Gasification Theory. In Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Basu, P., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 199–248. [Google Scholar]
- Speight, J.G. Chapter 13—Upgrading by Gasification. In Heavy Oil Recovery and Upgrading; Speight, J.G., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 559–614. [Google Scholar]
- Murakami, T.; Nagata, K. New Ironmaking Process From The Viewpoint Of Carburization and Iron Melting at Low Temperature. Miner. Process. Extr. Metall. Rev. 2003, 24, 253–267. [Google Scholar] [CrossRef]
- Blažič, A.; Škrjanc, I.; Logar, V. Soft sensor of bath temperature in an electric arc furnace based on a data-driven Takagi–Sugeno fuzzy model. Appl. Soft Comput. 2021, 113, 107949. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Z.; Zhang, Q.; Yu, Z.; Li, C.; Huang, J.; Fang, Y. Evaluation of CO2 Gasification Reactivity of Different Coal Rank Chars by Physicochemical Properties. Energy Fuels 2013, 27, 7287–7293. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D. Biomass use in the steel industry: Back to the future? Stahl Und Eisen 2013, 133, 57–67. [Google Scholar]
- Demirbas, A. Relationships Proximate Analysis Results and Higher Heating Values of Lignites. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1876–1883. [Google Scholar] [CrossRef]
- Chen, W.-H.; Du, S.-W.; Tsai, C.-H.; Wang, Z.-Y. Torrefied biomasses in a drop tube furnace to evaluate their utility in blast furnaces. Bioresour. Technol. 2012, 111, 433–438. [Google Scholar] [CrossRef]
- Roy, P.; Dutta, A.; Gallant, J. Hydrothermal Carbonization of Peat Moss and Herbaceous Biomass (Miscanthus): A Potential Route for Bioenergy. Energies 2018, 11, 2794. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal Carbonization of Fruit Wastes: A Promising Technique for Generating Hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Terada, A.; Sasaki, M.; Goto, M.; Hoshino, M. Separation of Pectin from Citrus junos Flavedo using a Hot- Compressed Water-Flow Reactor Separation of Pectin from Citrus junos Flavedo using a Hot-Compressed Water-Flow Reactor. In Proceedings of the SUPERGREEN, Seoul, Korea, 28–30 November 2007. [Google Scholar]
- Lagazzo, A.; Finocchio, E.; Petrini, P.; Ruggiero, C.; Pastorino, L. Hydrothermal synthesis of pectin derived nanoporous carbon material. Mater. Lett. 2016, 171, 212–215. [Google Scholar] [CrossRef]
- Zong, P.; Jiang, Y.; Tian, Y.; Li, J.; Yuan, M.; Ji, Y.; Chen, M.; Li, D.; Qiao, Y. Pyrolysis behavior and product distributions of biomass six group components: Starch, cellulose, hemicellulose, lignin, protein and oil. Energy Convers. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]
- Czajka, K.M. The impact of the thermal lag on the interpretation of cellulose pyrolysis. Energy 2021, 236, 121497. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin Pyrolysis Components and Upgrading—Technology Review. BioEnergy Res. 2013, 6, 1183–1204. [Google Scholar] [CrossRef]
- Valderrama Rios, M.L.; González, A.M.; Lora, E.E.S.; Almazán del Olmo, O.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- York, A.; Xiao, T.; Green, M. Brief Overview of the Partial Oxidation of Methane to Synthesis Gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Sohn, I.; Fruehan, R. The reduction of iron oxides by volatiles in a rotary hearth furnace process: Part III. The simulation of volatile reduction in a multi-layer rotary hearth furnace process. Metall. Mater. Trans. B 2006, 37, 231–238. [Google Scholar] [CrossRef]
- Davydenko, A.; Karasev, A.; Lindstrand, G.; Jönsson, P. Investigation of Slag Foaming by Additions of Briquettes in the EAF during Stainless Steel Production. Steel Res. Int. 2015, 86, 146–153. [Google Scholar] [CrossRef]
- Hayes, P.C. The kinetics of formation of H2O and CO2 during iron oxide reduction. Metall. Trans. B 1979, 10, 211–217. [Google Scholar] [CrossRef]
- Kongkarat, S.; Khanna, R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Recycling Waste Polymers in EAF Steelmaking: Influence of Polymer Composition on Carbon/Slag Interactions. ISIJ Int. 2012, 52, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Kaffash, H.; Surup, G.R.; Tangstad, M. Densification of Biocarbon and Its Effect on CO2 Reactivity. Processes 2021, 9, 193. [Google Scholar] [CrossRef]
- Semberg, P. Hearth Coke Bed Buoyancy in the Blast Furnace: Experimental Study with a 3-Dimensional Cold Model. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, March 2006. [Google Scholar]
- Yang, Y.; Raipala, K.; Holappa, L. Chapter 1.1—Ironmaking. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 2–88. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Yasipourtehrani, S.; Strezov, V.; Bliznyukov, S.; Evans, T. Investigation of thermal properties of blast furnace slag to improve process energy efficiency. J. Clean. Prod. 2017, 149, 137–145. [Google Scholar] [CrossRef]
- Cham, S.T.; Sahajwalla, V.; Sakurovs, R.; Sun, H.; Dubikova, M. Factors Influencing Carbon Dissolution from Cokes into Liquid Iron. ISIJ Int. 2004, 44, 1835–1841. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Kasai, A.; Matsui, Y. Lowering of Thermal Reserve Zone Temperature in Blast Furnace by Adjoining Carbonaceous Material and Iron Ore. ISIJ Int. 2004, 44, 2073–2078. [Google Scholar] [CrossRef]
- Elkader, M.; Fathy, A.; Megahed, G.; Eissa, M.; Shama, S.; El-Sharkawy, A. Influence of Direct-Reduced Iron in Charge Mix on EAF Operation Performance. In Proceedings of the 12th International Conference on Petroleum Engineering, Suez, Egypt, 20–22 October 2014. [Google Scholar]
- Anameric, B.; Kawatra, S.K. Properties and Features of Direct Reduced Iron. Miner. Process. Extr. Metall. Rev. 2007, 28, 59–116. [Google Scholar] [CrossRef]
- Surup, G.R.; Nielsen, H.K.; Heidelmann, M.; Anna, T. Characterization and reactivity of charcoal from high temperature pyrolysis (800–1600 °C). Fuel 2019, 235, 1544–1554. [Google Scholar] [CrossRef]
- Fan, X.; Ji, Z.; Gan, M.; Chen, X.; Jiang, T. Integrated assessment on the characteristics of straw-based fuels and their effects on iron ore sintering performance. Fuel Process. Technol. 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Lovel, R.R.; Vining, K.R.; Dell’amico, M. The Influence of Fuel Reactivity on Iron Ore Sintering. ISIJ Int. 2009, 49, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Ooi, T.C.; Aries, E.; Ewan, B.C.R.; Thompson, D.; Anderson, D.R.; Fisher, R.; Fray, T.; Tognarelli, D. The study of sunflower seed husks as a fuel in the iron ore sintering process. Miner. Eng. 2008, 21, 167–177. [Google Scholar] [CrossRef]
- Diez, M.A.; Alvarez, R.; Fernández, M. Biomass derived products as modifiers of the rheological properties of coking coals. Fuel 2012, 96, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]





| Material | Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar |
|---|---|---|---|---|
| Moisture content (wb (1)) | 1.2 | 7.1 | 66.8 | 2.6 |
| Proximate analysis (db (2)) | ||||
| Volatile matter | 7.6 | 16.4 | 11.4 | 67.0 |
| Fixed carbon | 81.1 | 79.1 | 81.2 | 26.9 |
| Ash content | 11.3 | 4.5 | 7.4 | 6.1 |
| Alkalis (db) | 0.33 | 0.58 | 1.95 | 0.44 |
| Ultimate analysis (db) | ||||
| C | 83.2 | 86.6 | 89.0 | 59.8 |
| H | 2.8 | 1.8 | 0.5 | 5.7 |
| N | 1.16 | 0.57 | 0.61 | 1.45 |
| O | 0.9 | 6.5 | 4.2 | 26.8 |
| S | 0.587 | 0.029 | 0.070 | 0.074 |
| P | 0.03 | 0.07 | 0.20 | 0.16 |
| Others (by difference) | 11.323 | 4.450 | 5.420 | 6.016 |
| HHV (MJ/kg) | 32.05 | 31.58 | 31.07 | 24.68 |
| LHV (MJ/kg) | 31.41 | 31.01 | 30.78 | 23.36 |
| Density (g/cm3) [46] | 1.81 ± 0.003 | 1.07 ± 0.08 | 0.76 ± 0.06 | 1.23 ± 0.02 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Na2O | 0.70 | 1.85 | 1.62 | 1.33 |
| K2O | 2.19 | 10.98 | 24.77 | 5.93 |
| MgO | 1.77 | 5.34 | 4.31 | 2.60 |
| MnO2 | <0.01 | 2.48 | 0.92 | 0.14 |
| Al2O3 | 24.9 | 5.34 | 1.83 | 2.46 |
| TiO2 | <0.01 | 0.26 | 0.12 | 0.27 |
| Fe2O3 | 20.12 | 2.82 | 1.08 | 17.37 |
| SiO2 | 44.31 | 20.40 | 21.54 | 15.92 |
| P2O5 | 0.65 | 3.45 | 6.14 | 6.22 |
| CaO | 4.48 | 47.08 | 37.69 | 47.75 |
| Others | 0.88 | 0.00 | 0.00 | 0.00 |
| Experiment | Aim | Sample Weight (g) | T (°C) | Heating Rate (K/min) |
|---|---|---|---|---|
| Nonisothermal TGA in N2 (TGA-N2) | To understand the devolatilization behavior. | 0.01–0.03 | 100–1000 | 10, 50 |
| Pyrolysis in N2 (py-N2) |
| 10–30 | 20–1200 | 50 |
| Isothermal reactivity in CO2 (TGA-CO2) | To evaluate the carbon reactivity in CO2 according to a standard for CRI (2) measurements (ISO-18894). | 100 | 20–1100 | 15 (1) |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Heating rate 10 K/min | ||||
| VM<300 °C (%) | 1.19 | 2.49 | 2.31 | 9.87 |
| VM300–400 °C (%) | 0.07 | 1.55 | 0.63 | 28.50 |
| VM400–700 °C (%) | 0.65 | 6.70 | 5.00 | 20.36 |
| VM700–1000 °C (%) | 2.3 | 7.69 | 10.59 | 4.71 |
| CY1000 °C (1) (%) | 95.79 | 81.57 | 81.47 | 36.56 |
| Tmax (2) (°C) | 823 | 694 | 697 | 360 |
| d(wt%)/dTmax (3) (%/K) | 0.006 | 0.055 | 0.082 | 0.570 |
| Heating rate 50 K/min | ||||
| VM<300 °C (%) | 4.02 | 1.20 | 2.19 | 6.03 |
| VM300–400 °C (%) | 0.20 | 1.91 | 0.93 | 26.48 |
| VM400–700 °C (%) | 0.60 | 4.91 | 2.87 | 25.03 |
| VM700–1000 °C (%) | 2.45 | 14.19 | 12.96 | 5.30 |
| CY1000 °C (%) | 92.73 | 77.79 | 81.05 | 37.16 |
| Tmax (°C) | 875 | 747 | 732 | 392 |
| d(wt%)/dTmax (%/K) | 0.006 | 0.065 | 0.037 | 0.500 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Solid | 91.8 | 82.6 | 87.4 | 38.3 |
| Liquid | 1.7 | 2.6 | 3.5 | 22.1 |
| Aqueous phase | 1.7 | 2.6 | 3.5 | 5.0 |
| Tarry phase | 0.0 | 0.0 | 0.0 | 17.1 |
| Gas | 6.5 | 14.8 | 9.1 | 39.6 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| C | 84.51 | 94.20 | 92.00 | 85.00 |
| H | 0.85 | 0.06 | 0.09 | 0.08 |
| N | 0.66 | 0.48 | 0.55 | 0.51 |
| S | 2.67 | 0.10 | 0.13 | 0.23 |
| O | 10.72 | 0.66 | 1.61 | 8.00 |
| Other | 0.59 | 4.50 | 5.62 | 6.18 |
| Ash | 12.30 | 5.45 | 8.47 | 16.10 |
| LHV (MJ/kg) | 29.21 | 32.77 | 31.86 | 28.60 |
| Na2O | K2O | MgO | MnO2 | Al2O3 | TiO2 | Fe2O3 | SiO2 | P2O5 | CaO |
|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 6.49 | 2.53 | 0.09 | 5.37 | 0.35 | 14.19 | 29.4 | 5.27 | 35.48 |
| Aqueous Phase | Tarry Phase | |
|---|---|---|
| C | 3.77 | 60.31 |
| H | 11.52 | 6.83 |
| N | 0.78 | 1.78 |
| S | 0.03 | 0.13 |
| O | 83.90 | 30.95 |
| LHV (MJ/kg) | 3.67 | 24.41 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Gas composition (wt% of carbon material before pyrolysis) | ||||
| H2 | 2.12 | 1.44 | 0.37 | 1.78 |
| CH4 | 1.84 | 1.00 | 0.25 | 3.25 |
| CO | 1.15 | 7.24 | 5.97 | 17.56 |
| CO2 | 0.44 | 3.61 | 2.50 | 12.76 |
| C2H2 | 0.75 | 1.50 | 0.00 | 2.05 |
| C2H4 | 0.06 | 0.00 | 0.01 | 0.75 |
| C2H6 | 0.00 | 0.00 | 0.00 | 0.54 |
| C3H6 | 0.14 | 0.01 | 0.00 | 0.83 |
| C3H8 | 0.00 | 0.00 | 0.00 | 0.08 |
| Elemental composition of gas (wt% of gas) | ||||
| C | 43.96 | 42.06 | 37.79 | 43.40 |
| H | 41.01 | 12.24 | 4.78 | 7.83 |
| O | 15.03 | 45.70 | 57.44 | 48.77 |
| LHV (MJ/kg) | 53.10 | 21.69 | 11.83 | 17.61 |
| Total volume of gas (L/g) (1) | 0.31 | 0.34 | 0.22 | 0.53 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Normalized carbon distribution | ||||
| Solid | 93.0 | 90.0 | 90.0 | 54.0 |
| Liquid | 3.6 | 2.8 | 6.1 | 17.3 |
| Gas | 3.4 | 7.2 | 3.9 | 28.7 |
| Normalized LHV distribution | ||||
| Solid | 85 | 87 | 91 | 47 |
| Liquid | 4 | 2 | 6 | 23 |
| Gas | 11 | 10 | 3 | 30 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| Volatile matter | ||||
| H2 | 2.12 | 1.44 | 0.37 | 1.78 |
| CH4 | 1.84 | 1.00 | 0.25 | 3.25 |
| CO | 1.15 | 7.24 | 5.97 | 17.56 |
| Other hydrocarbons gases | 0.95 | 1.51 | 0.01 | 4.25 |
| Sum | 6.06 | 11.19 | 6.60 | 26.84 |
| Residual char | ||||
| C in solid () | 77.58 | 77.81 | 80.41 | 32.52 |
| CO gas produced by reaction of CO2 with residual char () | ||||
| (a) X = 1 | 181.02 | 181.55 | 187.62 | 75.88 |
| (b) X = CRI | 15.95 | 89.47 | 109.12 | 61.21 |
| Total amount of reducing gases | ||||
| Based on by (a) | 187.08 | 192.74 | 194.22 | 102.72 |
| Based on by (b) | 22.01 | 100.66 | 115.72 | 88.05 |
| Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | |
|---|---|---|---|---|
| CRI (%) | 8.81 | 49.28 | 58.16 | 80.67 |
| (1/min) | NA (1) | NA (1) | 0.006 | 0.013 |
| (wt%/min) | 0.07 | 0.33 | 0.44 | 0.25 |
| Material | Anthracite | Charcoal 1 | Charcoal 2 | Lemon Hydrochar | Pyrolyzed Hydrochar |
|---|---|---|---|---|---|
| CaO/SiO2 | 0.1 | 2.3 | 1.8 | 3 | 1.21 |
| %Ash-solid at 1600 °C | 0 | 7.9 | 4.5 | 0.3 | 0.1 |
| %Ash-liquid at 1600 °C | 100 | 92.1 | 95.5 | 99.7 | 99.9 |
| Alkalinity index | 5 | 12 | 22 | 25 | 28 |
| Ash | Acidic Ash (1) | Basic Ash (2) | Alkalis | S | P | |
|---|---|---|---|---|---|---|
| European BF [1] | <11.35 | - | - | <0.38 | <0.930 | <0.06 |
| EAF [84] | <5.2 | - | - | - | <0.051 | <0.07 |
| DRI [85] | - | <3 | <3 | - | <0.008 | <0.03 |
| Anthracite | 11.3 | 7.8 | 3.3 | 0.33 | 0.587 | 0.03 |
| Charcoal 1 | 4.5 | 1.1 | 3.1 | 0.58 | 0.029 | 0.07 |
| Charcoal 2 | 7.4 | 1.7 | 5.1 | 1.95 | 0.070 | 0.20 |
| Lemon Hydrochar | 6.1 | 1.1 | 4.6 | 0.44 | 0.074 | 0.16 |
| Pyrolyzed Lemon Hydrochar | 16.1 | 5.6 | 9.6 | 1.18 | 0.230 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Yang, H.; Karasev, A.V.; Wang, C.; Jönsson, P.G. Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 1: Characterization of Carbonaceous Materials. Sustainability 2022, 14, 9488. https://doi.org/10.3390/su14159488
Lu Y, Yang H, Karasev AV, Wang C, Jönsson PG. Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 1: Characterization of Carbonaceous Materials. Sustainability. 2022; 14(15):9488. https://doi.org/10.3390/su14159488
Chicago/Turabian StyleLu, Yuchiao, Hanmin Yang, Andrey V. Karasev, Chuan Wang, and Pär G. Jönsson. 2022. "Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 1: Characterization of Carbonaceous Materials" Sustainability 14, no. 15: 9488. https://doi.org/10.3390/su14159488
APA StyleLu, Y., Yang, H., Karasev, A. V., Wang, C., & Jönsson, P. G. (2022). Applications of Hydrochar and Charcoal in the Iron and Steelmaking Industry—Part 1: Characterization of Carbonaceous Materials. Sustainability, 14(15), 9488. https://doi.org/10.3390/su14159488








