Co-Combustion of Food Solid Wastes and Pulverized Coal for Blast Furnace Injection: Characteristics, Kinetics, and Superiority
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Experimental Setup
2.2.2. Determination of Combustion Characteristic Parameters
2.2.3. Kinetics Model
3. Results and Discussion
3.1. Analysis of Physical and Chemical Characteristics
3.2. Combustion Analysis
3.2.1. Effect of Heating Rate on PC and FSW Combustion
3.2.2. Effect of Different Proportions of FSW and PC on Combustion
3.2.3. Synergy of Different Proportions of Mixing
3.3. Kinetics Analysis
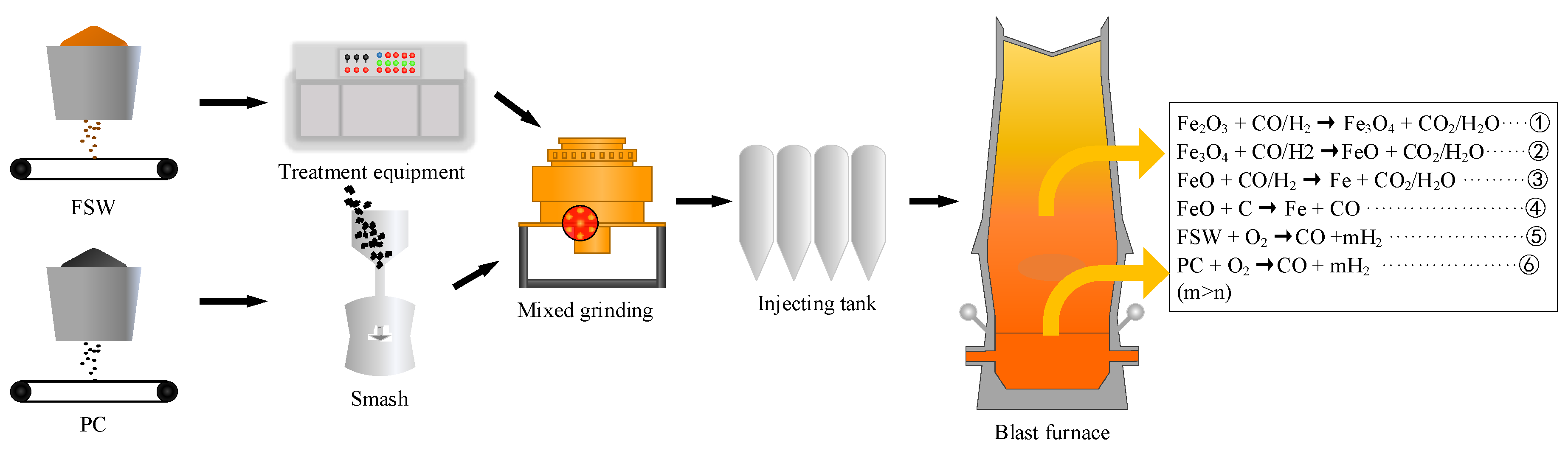
3.4. Superiority of FSW Injection into Blast Furnace
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Mishra, N.K.; Bhardwaj, N. Biofuels: A Renewable and Sustainable Way to Conserve the Fossil Fuels in India. Invertis J. Renew. Energy 2018, 8, 151–156. [Google Scholar]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Xue, M.; Xu, F.F.; Yuan, J.; Zong, P.J.; Wang, B.; Li, J.; Qiao, Y.Y.; Tian, Y.Y. Thermal degradation of food waste by TG-FTIR and Py-GC/MS: Pyrolysis behaviors, products, kinetic and thermodynamic analysis. J. Clean. Prod. 2020, 244, 118713. [Google Scholar]
- Cheng, Y.L.; Bi, G.C.; Wo, D.F.; Xie, J. Status Quo of Kitchen Waste and Its Treatment Measures at Home and Abroad. Adv. New Renew. Energy 2017, 5, 266–271. [Google Scholar]
- Zhang, X.X.; Jiao, P.B.; Yang, H.Y.; Wu, R.M.; Li, Y.M.; Ma, L.P. Recent advances in anaerobic co-digestion of excess sludge and food waste. China Environ. Sci. 2022, 42, 2179–2194. [Google Scholar] [CrossRef]
- Melikoglu, M. Reutilisation of food wastes for generating fuels and value added products: A global review. Environ. Technol. Innov. 2020, 19, 101040. [Google Scholar] [CrossRef]
- Pham, T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food wasteto-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Jin, C.X.; Sun, S.Q.; Yang, D.H.; Sheng, W.J.; Ma, Y.D.; He, W.Z.; Li, G.M. Anaerobic Digestion: An Alternative Resource Treatment Option for Food Waste in China. Sci. Total Environ. 2021, 779, 146397. [Google Scholar] [CrossRef]
- Salemdeeb, R.; Zu Ermgassen, E.K.H.J.; Kim, M.H.; Balmford, A.; Al-Tabbaa, A. Environmental and health impacts of using food waste as animal feed: A comparative analysis of food waste management options. J. Clean. Prod. 2017, 140, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Ilbery, B. Geographical aspects of the 2001 outbreak of foot and mouth disease in the UK. Geography 2002, 87, 142–147. [Google Scholar]
- zu Ermgassen, E.K.H.J.; Phalan, B.; Green, R.E.; Balmford, A. Reducing the land use of EU pork production: Where there’s swill, there’s a way. Food Policy 2016, 58, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Qi, H.; Wei, Z.; Xia, X. High-speed treatment of feed waste by continuous high-temperature composting enhanced by thermophilic microbial consortium. Environ. Eng. 2021, 39, 111–117. [Google Scholar]
- Yuan, T.G.; Bian, S.W.; Ko, J.H.; Liu, J.G.; Shi, X.Y.; Xu, Q.Y. Exploring the roles of zero-valent iron in two-stage food waste anaerobic digestion. Waste Manag. 2020, 107, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Tian, H.L.; Chen, Y.Y.; Ni, K.W.; Zhang, J.X.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef]
- Yuan, T.G.; Shi, X.Y.; Sun, R.; Ko, J.H.; Xu, Q.Y. Simultaneous addition of biochar and zero-valent iron to improve food waste anaerobic digestion. J. Clean. Prod. 2021, 278, 123627. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, H.P.; Fan, Q.M.; Long, S.L.; Hua, D.L. Research progress on anaerobic digestion treatment technology for kitchen waste. Mod. Chem. Ind. 2022, 42, 10–14. [Google Scholar]
- Zhang, F.M. Research and design on low-carbon and high-efficiency blast furnace. China Metall. 2021, 31, 1–8. [Google Scholar]
- Raygan, S.; Abdizadeh, H.; Rizi, A.E. Evaluation of four coals for blast furnace pulverized coal injection. J. Iron Steel Res. Int. 2010, 17, 8–20. [Google Scholar] [CrossRef]
- Long, H.M.; Wei, R.F.; Li, N.; Zhou, D.; Meng, Q.M.; Li, J.X. Disposal of city combustible solid waste by blast furnace. Iron Steel. 2018, 53, 1–9. [Google Scholar]
- Zhou, D.; Wei, R.F.; Long, H.M.; Li, J.X.; Qi, L.Y.; Xu, C.B. Combustion characteristics and kinetics of different food solid wastes treatment by blast furnace. Renew. Energy 2020, 145, 530–541. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, G.W.; Ning, X.J.; Zhang, J.L.; Wang, C. Numerical simulation of combustion behaviors of hydrochar derived from low-rank coal in the raceway of blast furnace. Fuel 2020, 278, 118267. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, R.S.; Wang, G.W.; Zhang, J.L.; Song, B.; Liang, W.; Wang, C. Study on the feasibility and co-combustion mechanism of mixed injection of biomass hydrochar and anthracite in blast furnace. Fuel 2021, 304, 121465. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G.W.; Zhang, J.L.; Lee, J.-Y.; Li, Y.J.; Wang, C. Co-combustion characteristics and kinetic study of anthracite coal and palm kernel shell char. Appl. Therm. Eng. 2018, 143, 736–745. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China Coal Industry Press: Beijing, China, 2012.
- GB/T 212-2008; Proximate Analysis of Coal. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China Coal Industry Press: Beijing, China, 2008.
- GB/T 31391-2015; Ultimate Analysis of Coal. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, China Coal Industry Press: Beijing, China, 2008.
- Yuzbasi, N.S.; Selçuk, N. Air and oxy-fuel combustion characteristics of biomass/lignite blends in TGA-FTIR. Fuel Process. Technol. 2011, 92, 1101–1108. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Fraga, L.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Influence of operating conditions on the thermal behavior and kinetics of pine wood particles using thermogravimetric analysis. Energies 2020, 13, 2756. [Google Scholar] [CrossRef]
- Toptas, A.; Yildirim, Y.; Duman, G.; Yanik, J. Combustion behavior of different kinds of torrefied biomass and their blends with lignite. Bioresour. Technol. 2015, 177, 328–336. [Google Scholar] [CrossRef]
- Xing, D.D.; Wang, S.; Zhang, Q.L.; Mo, C.; Chen, Y.F. Mixed combustion and kinetics of waste plastics injected into blast furnace and anthracite. J. Iron Steel Res. 2019, 31, 258–264. [Google Scholar]
- Ding, J.; Liu, Q.C.; Jiang, L.J.; Liu, G.Q.; Ren, S.; Yang, J.; Meng, F. Thermal behavior and kinetics of raw/pyrolytic wood and coal blends during co-combustion process. J. Iron Steel Res. Int. 2016, 23, 917–923. [Google Scholar] [CrossRef]
- Wu, H.X.; Li, H.B.; Zhao, Z.L. Thermogravimetric analysis and pyrolytic kinetic study on coal/biomass blends. J. Fuel Chem. Technol. 2009, 37, 538–545. [Google Scholar]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Zuo, H.B. Thermogravimetric analysis of coal char combustion kinetics. J. Iron Steel Res. Int. 2014, 21, 897–904. [Google Scholar] [CrossRef]
- Huang, Y.L.; Guo, Q.J.; Tian, H.J.; Hou, L.Y. Study on pyrolysis of kitchen waste (KW) by Using thermogravimetric analyzer (TGA) and tube furnace. J. Chem. Eng. Chin. Univ. 2012, 26, 721–728. [Google Scholar]
- Chen, D.Y.; Gao, A.J.; Cen, K.H.; Zhang, J.; Cao, X.B.; Ma, Z.Q. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energ. Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Z.Z.; Zhu, M.M.; Cheng, F.Q.; Zhang, D.K. Interactions of coal gangue and pine sawdust during combustion of their blends studied using differential thermogravimetric analysis. Bioresour. Technol. 2016, 214, 396–403. [Google Scholar] [CrossRef]
- Zhou, L.M.; Zhang, G.J.; Reinmöller, M.; Meyer, B. Effect of inherent mineral matter on the co-pyrolysis of highly reactive brown coal and wheat straw. Fuel 2019, 239, 1194–1203. [Google Scholar] [CrossRef]
- Jiang, G.D.; Wei, L.P.; Teng, H.P.; Hao, H.D. A kinetic model based on TGA data for pyrolysis of Zhundong coal. CIESC J. 2017, 68, 1415–1422. [Google Scholar]
- Magalhaes, D.; Kazanc, F.; Riaza, J.; Erensoy, S.; Kabaklı, O.; Chalmers, H. Combustion of Turkish lignites and olive residue: Experiments and kinetic modelling. Fuel 2017, 203, 868–876. [Google Scholar] [CrossRef]
- Abdel Halim, K.S. Effective utilization of using natural gas injection in the production of pig iron. Mater. Lett. 2007, 61, 3281–3286. [Google Scholar] [CrossRef]
- Wei, R.F.; Xiang, D.W.; Long, H.M.; Xu, C.B.; Li, J.X. Reduction of iron oxide by lignin: Characteristics, kinetics and superiority. Energy 2020, 197, 117203. [Google Scholar] [CrossRef]
- Verma, M.; Loha, C.; Chatterjee, P.K. Drying of biomass for utilising in co-firing with coal and its impact on environment–A review. Renew. Sustain. Energy Rev. 2016, 71, 732–741. [Google Scholar] [CrossRef]
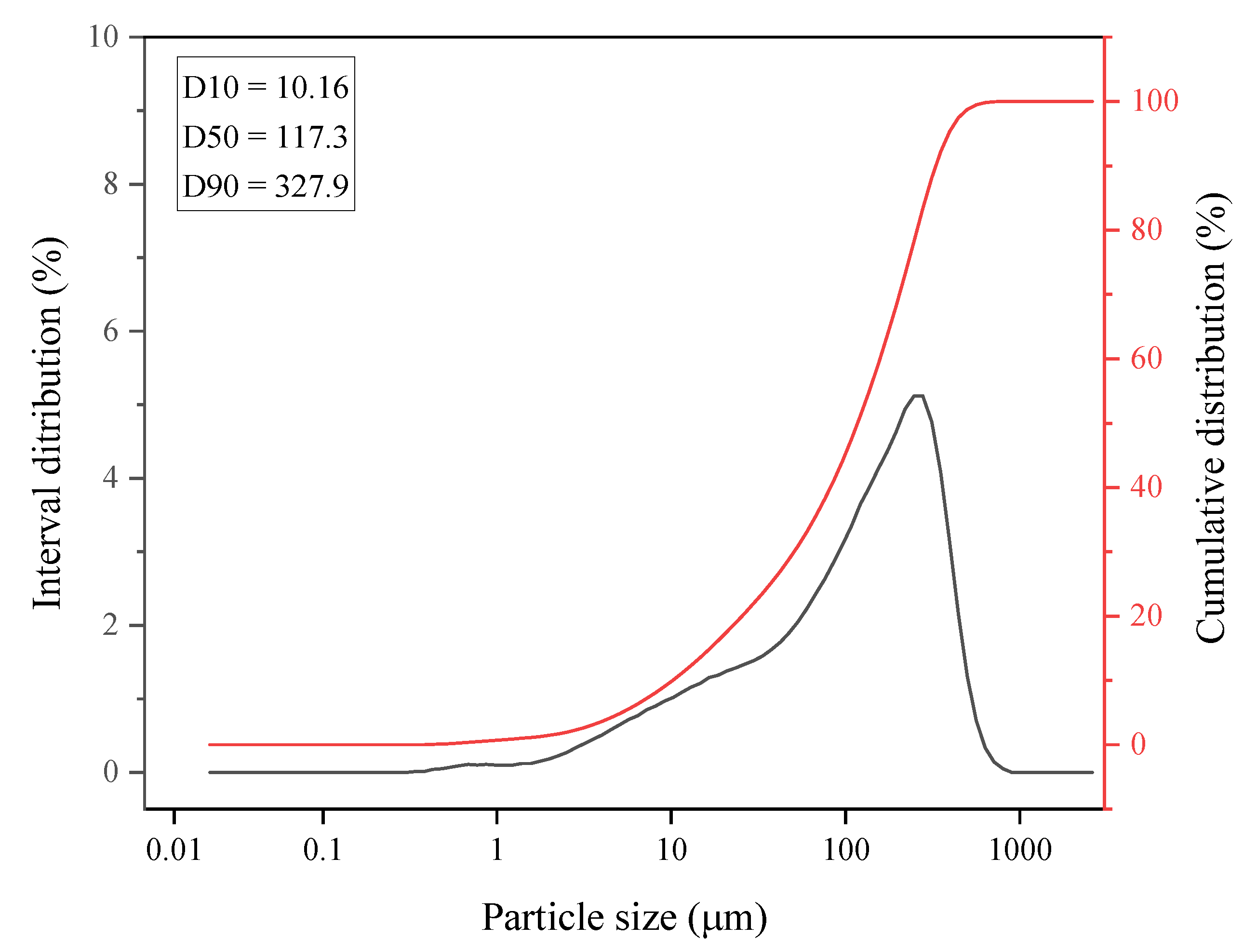

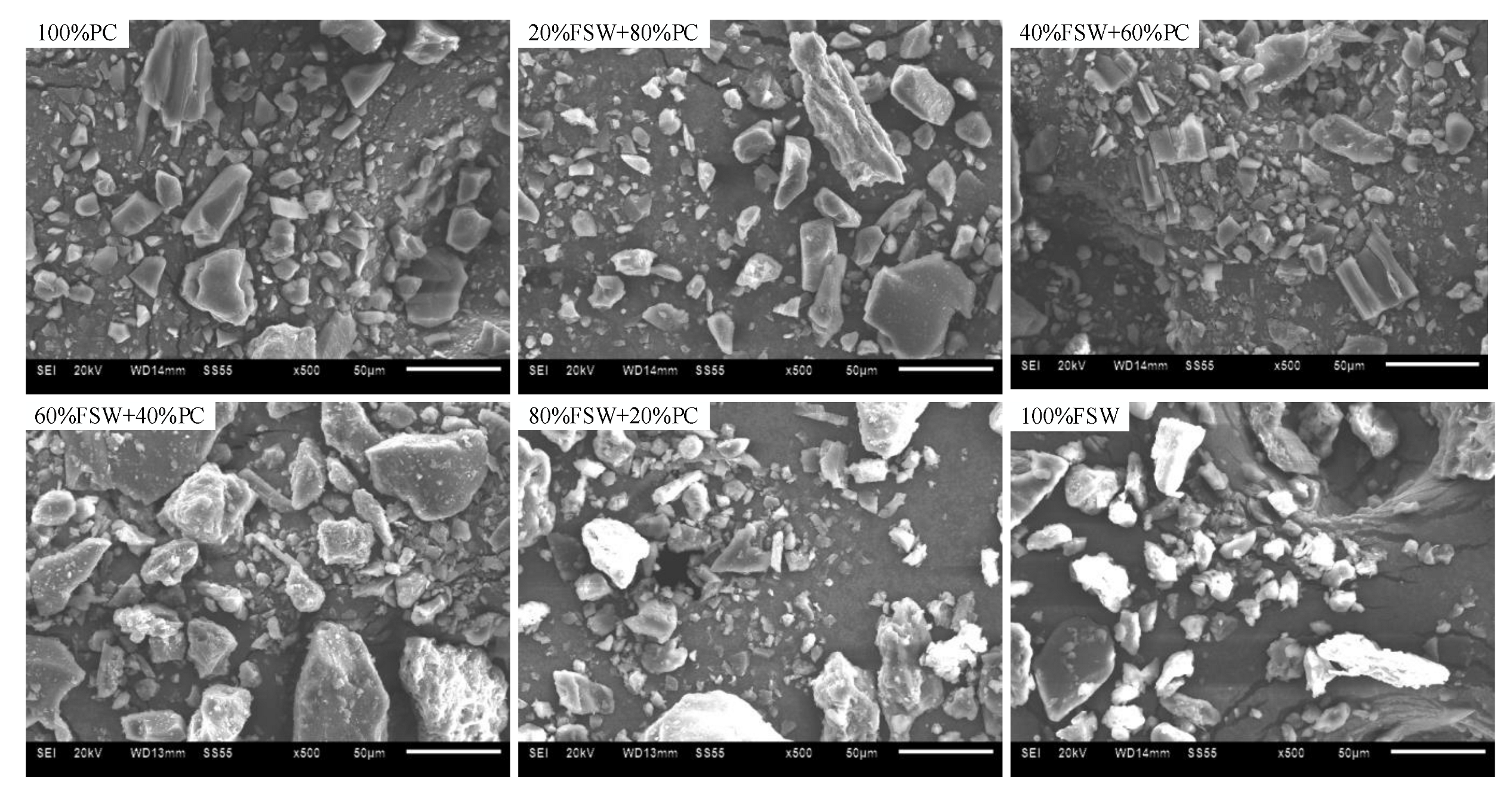



| Sample | WV | Mixture | |||||
|---|---|---|---|---|---|---|---|
| WC | WCF | WBP | WM | WSB | WR | WV | |
| Content | 53.6 | 11.8 | 34.6 | 15 | 15 | 15 | 55 |
| Samples | Proximate Analysis | Ultimate Analysis | LHV(MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCd | Ad | Vd | Cd | Hd | Od | Nd | Sd | Qd | |
| FSW | 10.19 | 0.77 | 79.06 | 46.02 | 17.2 | 33.57 | 2.81 | 0.40 | 29.39 |
| PC | 61.55 | 7.43 | 31.02 | 75.33 | 3.98 | 13.82 | 1.02 | 0.42 | 28.06 |
| Wave Number (cm−1) | Functional Grops | Compounds |
|---|---|---|
| 3600–3000 | OH stretching | Acid, methanol |
| 2860–2970 | C-Hn stretching | Alkyl, aliphatic, aromatic |
| 1700–1730 | C=O stretching | Ketone and carbonyl |
| 1632 | C=C | Benzene stretching ring |
| 1613,1450 | C=C stretching | Aromatic skeletal mode |
| 1470–1430 | O-CH3 | Methoxyl-O-CH3 |
| 1440–1400 | OH bending | Acid |
| 1402 | CH bending | - |
| 1232 | C-O-C stretching | Aryl–alkyl ether linkage |
| 1215 | C-O stretching | Phenol |
| 1170,1082 | C-O-C stretching vibration | Pyranose ring skeletal |
| 1108 | OH association | C-OH |
| 1060 | C-O stretching and C-O deformation | C-OH (ethanol) |
| 700–900 | C-H | Aromatic hydrogen |
| 700–400 | C-C stretching | - |
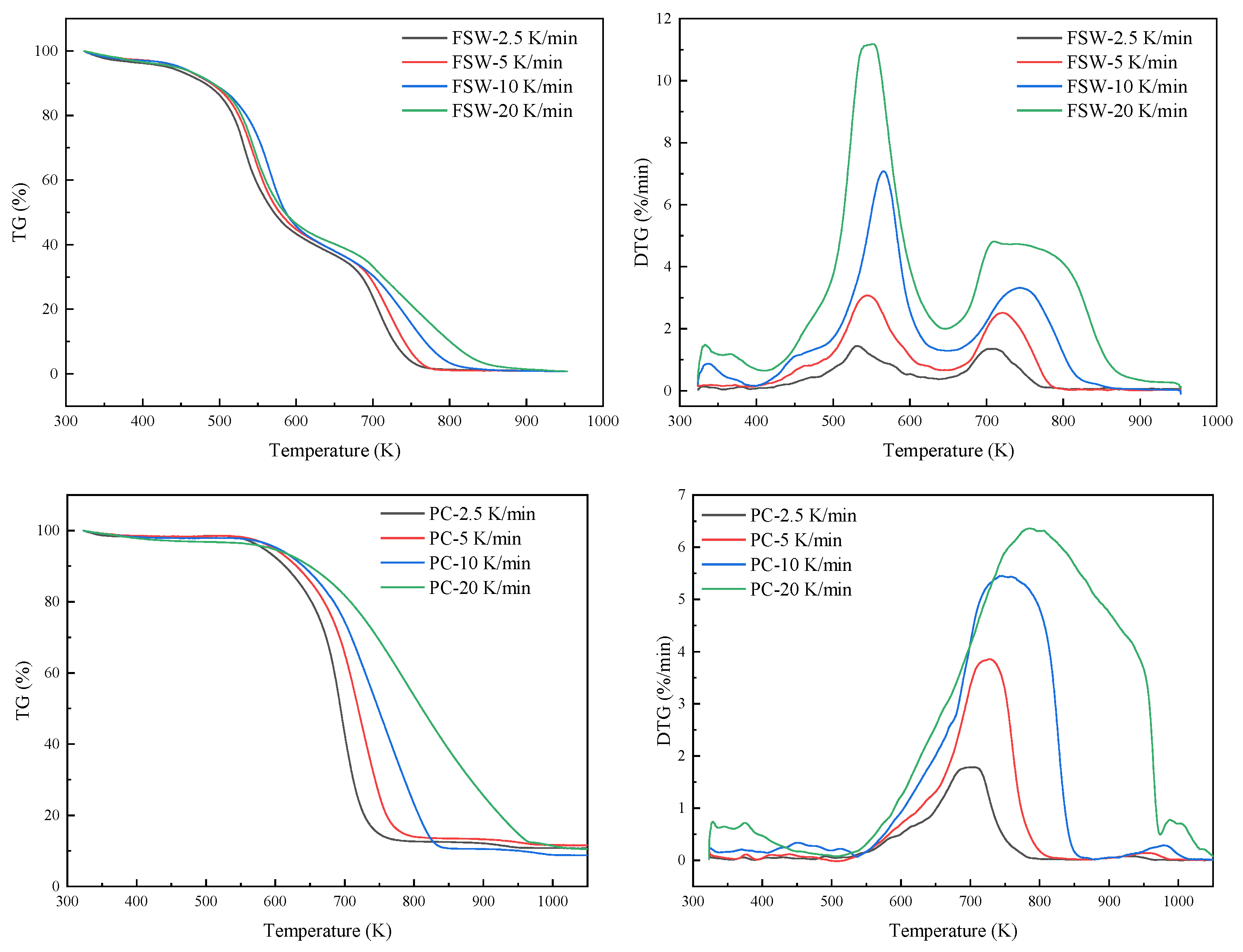
| Sample | β (K/min) | Tin (K) | Tp (K) | Ti (K) | T1max (K) | DTG1max (%/min) | T2max (K) | DTG2max (%/min) |
|---|---|---|---|---|---|---|---|---|
| FSW | 2.5 | 515.69 | 729.44 | 497.35 | 531.9 | 1.43 | 707.12 | 1.35 |
| 5 | 487.66 | 764.2 | 503.12 | 545.35 | 3.05 | 720.41 | 2.5 | |
| 10 | 444.76 | 803.58 | 513.87 | 566.44 | 7 | 743.05 | 3.2 | |
| 20 | 437.31 | 856.38 | 501.48 | 545.77 | 11 | 754.69 | 4.6 | |
| PC | 2.5 | 651.12 | 731.14 | 653.02 | - | - | 704.85 | 1.81 |
| 5 | 627.14 | 763.1 | 667.63 | - | - | 728.95 | 3.88 | |
| 10 | 604.14 | 836.16 | 641.66 | - | - | 738.62 | 5.5 | |
| 20 | 590.27 | 967.61 | 657.24 | - | - | 787.35 | 6.4 |
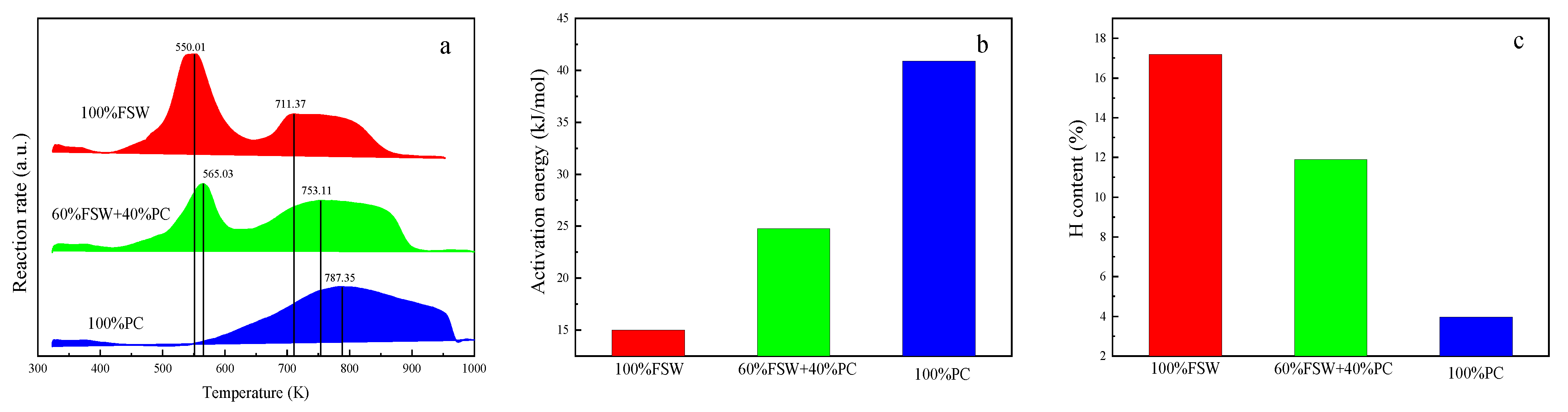
| FSW Content (%) | Tin (K) | Tp (K) | Ti (K) | Cb∗10−5 | Sn∗10−9 | First Stage | Second Stage | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TR (K) | Tpeak (K) | Rate (%/min) | TR (K) | Tpeak (K) | Rate (%/min) | ||||||
| 0 | 590.27 | 967.61 | 657.24 | 1.48 | 6.26 | - | - | - | 757–817 | 787.4 | 6.4 |
| 20 | 516.56 | 942.83 | 502.22 | 2.69 | 10.64 | 539–599 | 569.73 | 2.93 | 750–810 | 780.8 | 6.79 |
| 40 | 482.58 | 923.7 | 504.58 | 2.43 | 9.89 | 535–595 | 565.68 | 5.1 | 746–806 | 776.1 | 6.19 |
| 60 | 457.11 | 893.69 | 503.19 | 2.9 | 12.48 | 535–595 | 565.03 | 7.34 | 723–783 | 753.1 | 5.58 |
| 80 | 447.08 | 877.34 | 509.1 | 3.54 | 15.64 | 526–586 | 556.6 | 9.17 | 748–808 | 778.7 | 4.84 |
| 100 | 437.31 | 856.38 | 499.26 | 4.49 | 16.63 | 520–580 | 550.01 | 11.18 | 681–741 | 711.4 | 4.81 |
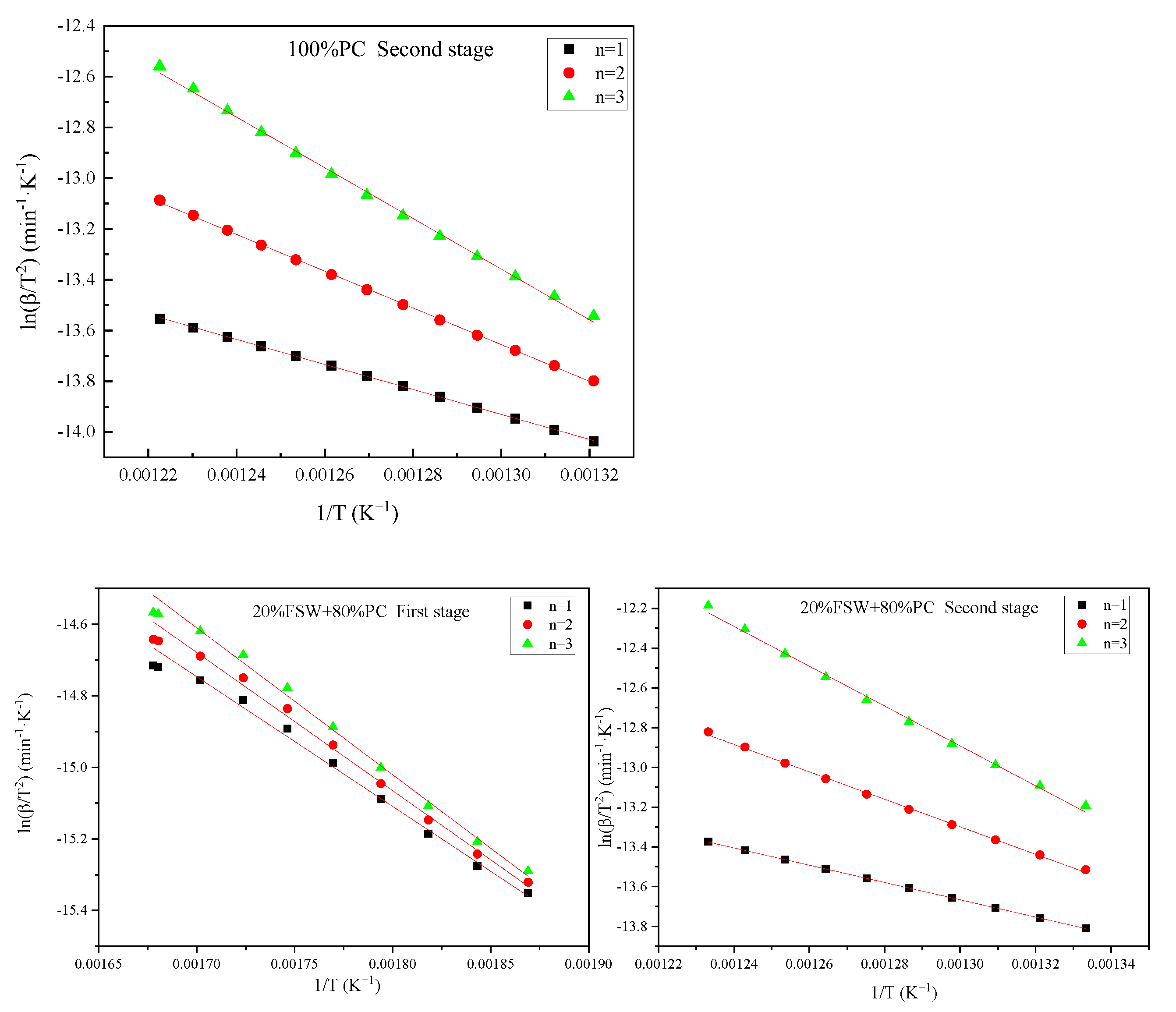
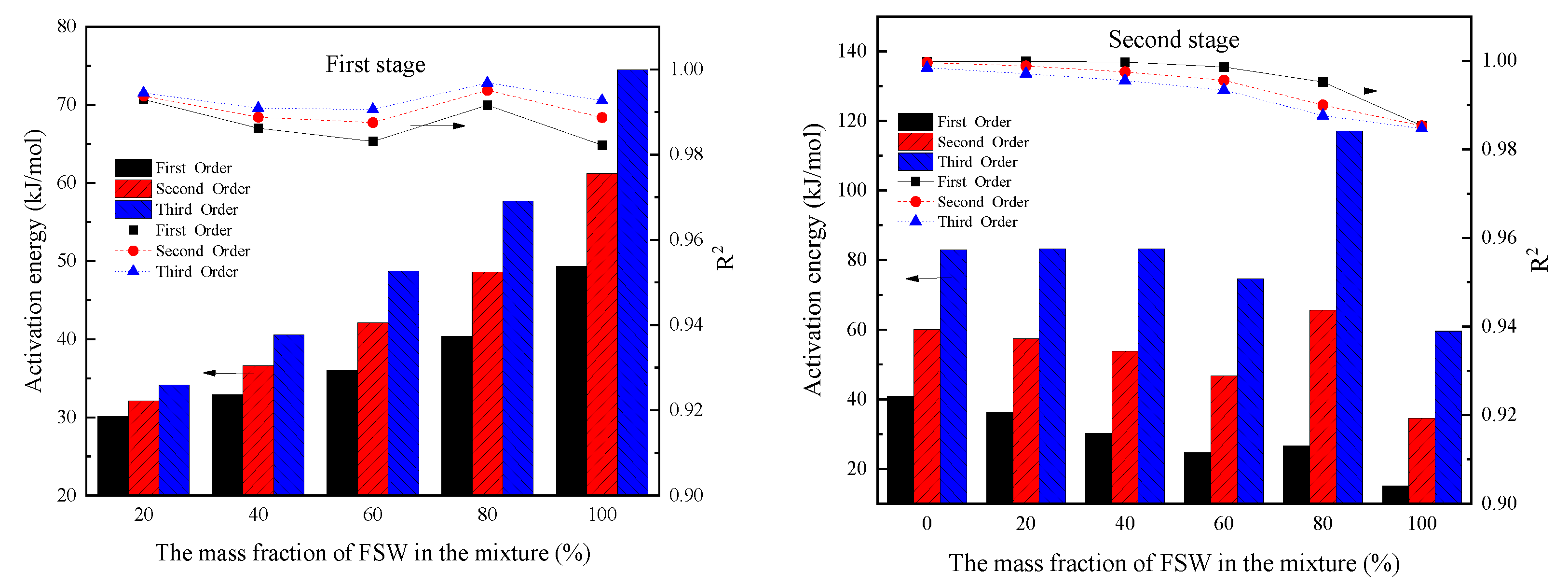
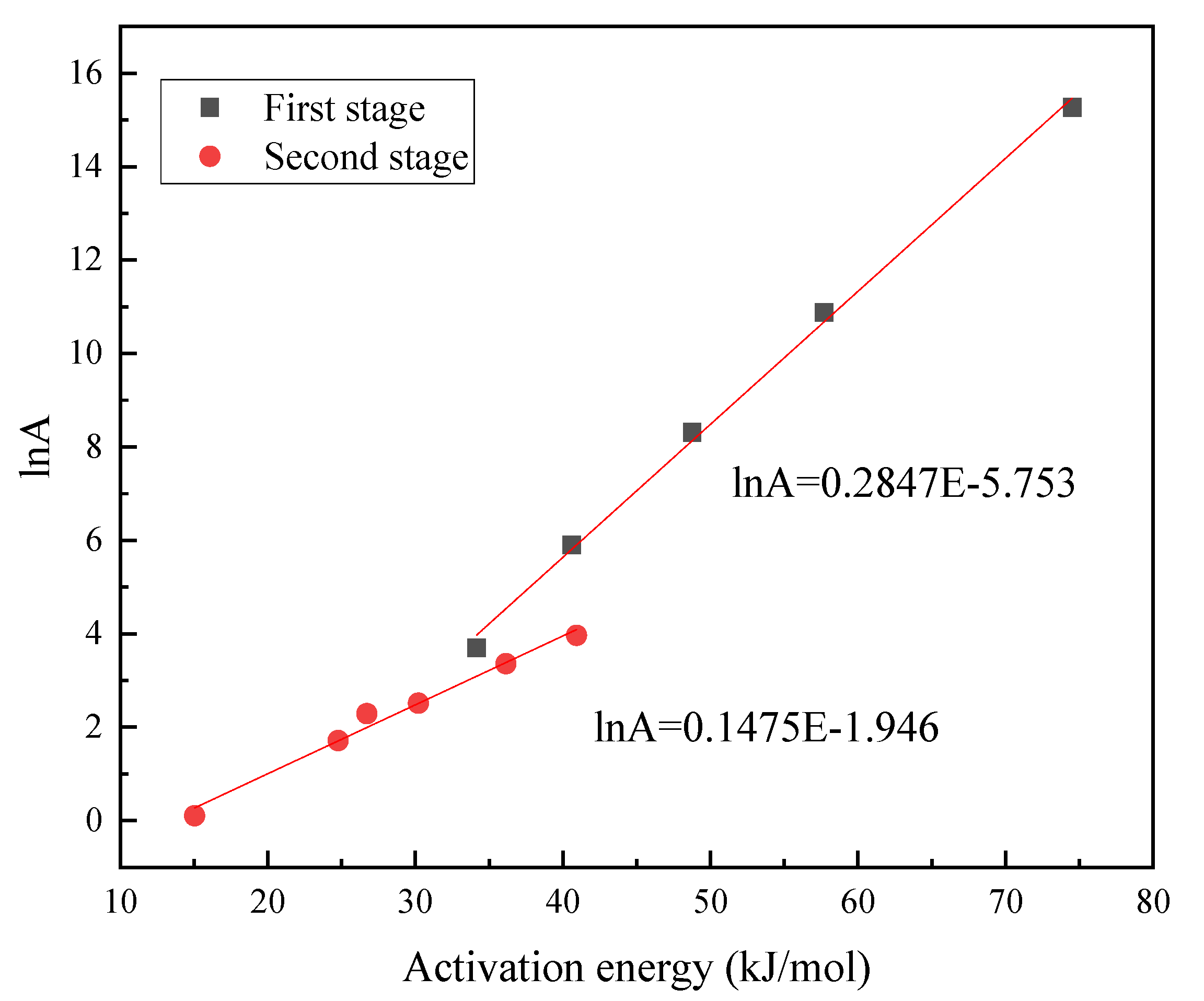
| FSW Content (%) | The First Stage | The Second Stage | ||||
|---|---|---|---|---|---|---|
| E (kJ/mol) | R2 | E (kJ/mol) | R2 | |||
| 0 | - | - | - | 40.92 | 52.73 | 0.9998 |
| 20 | 34.16 | 4.01 × 10 | 0.9944 | 36.15 | 28.76 | 0.9999 |
| 40 | 40.61 | 3.46 × 102 | 0.9909 | 30.21 | 11.69 | 0.9997 |
| 60 | 48.76 | 4.05 × 103 | 0.9906 | 24.76 | 5.52 | 0.9986 |
| 80 | 57.70 | 5.27 × 104 | 0.9968 | 26.71 | 9.79 | 0.9952 |
| 100 | 74.52 | 4.27 × 105 | 0.9927 | 15.04 | 1.11 | 0.9853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, Z.; Wei, R.; Zhou, D.; Long, H.; Li, J.; Xu, C. Co-Combustion of Food Solid Wastes and Pulverized Coal for Blast Furnace Injection: Characteristics, Kinetics, and Superiority. Sustainability 2022, 14, 7156. https://doi.org/10.3390/su14127156
Yang J, Li Z, Wei R, Zhou D, Long H, Li J, Xu C. Co-Combustion of Food Solid Wastes and Pulverized Coal for Blast Furnace Injection: Characteristics, Kinetics, and Superiority. Sustainability. 2022; 14(12):7156. https://doi.org/10.3390/su14127156
Chicago/Turabian StyleYang, Jian, Zhenying Li, Rufei Wei, Di Zhou, Hongming Long, Jiaxin Li, and Chunbao (Charles) Xu. 2022. "Co-Combustion of Food Solid Wastes and Pulverized Coal for Blast Furnace Injection: Characteristics, Kinetics, and Superiority" Sustainability 14, no. 12: 7156. https://doi.org/10.3390/su14127156
APA StyleYang, J., Li, Z., Wei, R., Zhou, D., Long, H., Li, J., & Xu, C. (2022). Co-Combustion of Food Solid Wastes and Pulverized Coal for Blast Furnace Injection: Characteristics, Kinetics, and Superiority. Sustainability, 14(12), 7156. https://doi.org/10.3390/su14127156





_Xu.png)

