Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Processing and Characterization of Raw Red Mud and Spent Fluorescent Lamps
2.3. Screening of Solvents for Leaching Using Pure Rare Earth Oxides
2.4. Experimental Design for Identifying Optimal Leaching Parameters
2.5. Quantification of REEs and Morphological and Compositional Analysis of the Solids
3. Results and Discussion
3.1. Screening of Leaching Solvents Using Pure Rare Earth Oxides
3.2. Quantification of REEs in Original Red Mud and Fluorescent Phosphors by ICP–OES
3.3. Identification of Optimal Conditions for Maximal Leaching Efficiency by LevA
3.4. Validation of Models under Optimal and Randomly Selected Conditions
3.5. Comparison of Leaching Efficiency between This Study and the Literature
3.6. Morphological and Compositional Comparisons between Solids before and after Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, A.B.; Tarik, M.; Struis, R.P.; Ludwig, C. Exploiting end-of-life lamps fluorescent powder e-waste as a secondary resource for critical rare earth metals. Resour. Conserv. Recycl. 2021, 164, 105153. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Opare, E.O.; Struhs, E.; Mirkouei, A. A comparative state-of-technology review and future directions for rare earth element separation. Renew. Sustain. Energy Rev. 2021, 143, 110917. [Google Scholar] [CrossRef]
- Riba, J.-R.; López-Torres, C.; Romeral, L.; Garcia, A. Rare-earth-free propulsion motors for electric vehicles: A technology review. Renew. Sustain. Energy Rev. 2016, 57, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Chang, M.-H.; Pecht, M. Rare-earth elements in lighting and optical applications and their recycling. Jom-Us 2013, 65, 1276–1282. [Google Scholar] [CrossRef]
- Mancheri, N.A. World trade in rare earths, Chinese export restrictions, and implications. Resour. Policy 2015, 46, 262–271. [Google Scholar] [CrossRef]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative life cycle analysis for value recovery of precious metals and rare earth elements from electronic waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Evans, K. Successes and challenges in the management and use of bauxite residue. In Proceedings of the Bauxite Residue Valorisation and Best Practices Conference, Leuven, Belgium, 5–7 October 2015; pp. 113–128. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Ujaczki, É.; Zimmermann, Y.S.; Gasser, C.A.; Molnár, M.; Feigl, V.; Lenz, M. Red mud as secondary source for critical raw materials–extraction study. J. Chem. Technol. Biotechnol. 2017, 92, 2835–2844. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Wagner, T.P. Compact fluorescent lights and the impact of convenience and knowledge on household recycling rates. Waste Manag. 2011, 31, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Cheong, R. Application of Green Solvents for Rare Earth Element Recovery from Aluminate Phosphors. Minerals 2021, 11, 287. [Google Scholar] [CrossRef]
- Alaoui, Y.T.H.; Aouragh, N.S.; Kitane, S. Recovery and Characterization of Phosphor Powders From Waste Linear Tube Fluorescent Lamps. Int. J. Mech. Eng. Technol. 2018, 9, 1357–1366. [Google Scholar]
- Ecolamp. 2021. Available online: https://ecolamp.co.uk/lamps/ (accessed on 10 March 2022).
- Hopfe, S.; Flemming, K.; Lehmann, F.; Möckel, R.; Kutschke, S.; Pollmann, K. Leaching of rare earth elements from fluorescent powder using the tea fungus Kombucha. Waste Manag. 2017, 62, 211–221. [Google Scholar] [CrossRef]
- Gambogi, J. Rare Earths; U.S. Geological Survey (USGS) National Minerals Information Center: Reston, VA, USA, 2021.
- Statista. Available online: https://www.statista.com (accessed on 10 April 2022).
- Stormcrow. 2021. Available online: https://www.stormcrow.ca/wp-content/uploads/2021/03/20210308-Stormcrow-UCore-Initiation-Final.pdf (accessed on 10 April 2022).
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3: Eu 3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Alkan, G.; Yagmurlu, B.; Gronen, L.; Dittrich, C.; Ma, Y.; Stopic, S.; Friedrich, B. Selective silica gel free scandium extraction from Iron-depleted red mud slags by dry digestion. Hydrometallurgy 2019, 185, 266–272. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Selective process steps for the recovery of scandium from Jamaican bauxite residue (red mud). ACS Sustain. Chem. Eng. 2018, 6, 1478–1488. [Google Scholar] [CrossRef]
- Strauss, M.L. The Recovery of Rare Earth Oxides Fromwaste Fluorescent Lamps; Colorado School of Mines: Golden, CO, USA, 2016. [Google Scholar]
- Wang, Q.; Ma, L.; He, X.; Chen, Y.; Tan, C.; Su, T. CRT (Cathode Ray Tube) Fluorescent Powder Processing Method. Chinese Patent CN102312095B; filed 24 May 2011, and issued 17 April 2013,
- Mudali, U.K.; Patil, M.; Saravanabhavan, R.; Saraswat, V. Review on E-waste Recycling: Part II—Technologies for Recovery of Rare Earth Metals. Trans. Indian Natl. Acad. Eng. 2021, 6, 613–631. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of Valuable Elements from Red Mud with a Focus on Using Liquid Media—A Review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Bonomi, C.; Alexandri, A.; Vind, J.; Panagiotopoulou, A.; Tsakiridis, P.; Panias, D. Scandium and titanium recovery from bauxite residue by direct leaching with a Brønsted acidic ionic liquid. Metals 2018, 8, 834. [Google Scholar] [CrossRef] [Green Version]
- Pateli, I.M.; Abbott, A.P.; Binnemans, K.; Rodriguez, N.R. Recovery of yttrium and europium from spent fluorescent lamps using pure levulinic acid and the deep eutectic solvent levulinic acid–choline chloride. RSC Adv. 2020, 10, 28879–28890. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Grymonprez, B.; Binnemans, K. Integrated Process for Recovery of Rare-Earth Elements from Lamp Phosphor Waste Using Methanesulfonic Acid. Ind. Eng. Chem. Res. 2021, 60, 10319–10326. [Google Scholar] [CrossRef]
- Box, G.E.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Hopfe, S.; Konsulke, S.; Barthen, R.; Lehmann, F.; Kutschke, S.; Pollmann, K. Screening and selection of technologically applicable microorganisms for recovery of rare earth elements from fluorescent powder. Waste Manag. 2018, 79, 554–563. [Google Scholar] [CrossRef]
- U.S. EPA. Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; Revision 4.4 ed.; Method 200.7; U.S. EPA: Cincinnati, OH, USA, 1994.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Li, Z.; Din, J.; Xu, J.; Liao, C.; Yin, F.; Lǚ, T.; Cheng, L.; Li, J. Discovery of the REE minerals in the Wulong–Nanchuan bauxite deposits, Chongqing, China: Insights on conditions of formation and processes. J. Geochem. Explor. 2013, 133, 88–102. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric mining of rare earth elements from bauxite residue (red mud): Process optimization, kinetic investigation, and microwave pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [Green Version]
- Anawati, J.; Azimi, G. Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manag. 2019, 95, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F. Availability of bauxite reserves. Nat. Resour. Res. 2004, 13, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Brookins, D.G. Scandium. In Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 120–121. [Google Scholar]
- Ashokraju, M.; Mohan, V.; Murali, K.; Rao, M.V.; Raju, B.D.; Rao, K.S.R. Formic acid assisted hydrogenation of levulinic acid to γ-valerolactone over ordered mesoporous Cu/Fe2O3 catalyst prepared by hard template method. J. Chem. Sci. 2018, 130, 16. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Deng, F.; Kommalapati, R.R.; Amarasekara, A.S. Iron based catalysts in biomass processing. Renew. Sustain. Energy Rev. 2020, 134, 110292. [Google Scholar]
- Shoppert, A.; Loginova, I.; Napol’skikh, J.; Valeev, D. High-Selective Extraction of Scandium (Sc) from Bauxite Residue (Red Mud) by Acid Leaching with MgSO4. Materials 2022, 15, 1343. [Google Scholar] [CrossRef]
- Borra, C.R.; Mermans, J.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Selective recovery of rare earths from bauxite residue by combination of sulfation, roasting and leaching. Miner. Eng. 2016, 92, 151–159. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; Pietrelli, L.; Centofanti, M.; Piga, L.; Vegliò, F. Application of solvent extraction operation to recover rare earths from fluorescent lamps. J. Clean. Prod. 2018, 172, 2840–2852. [Google Scholar] [CrossRef]

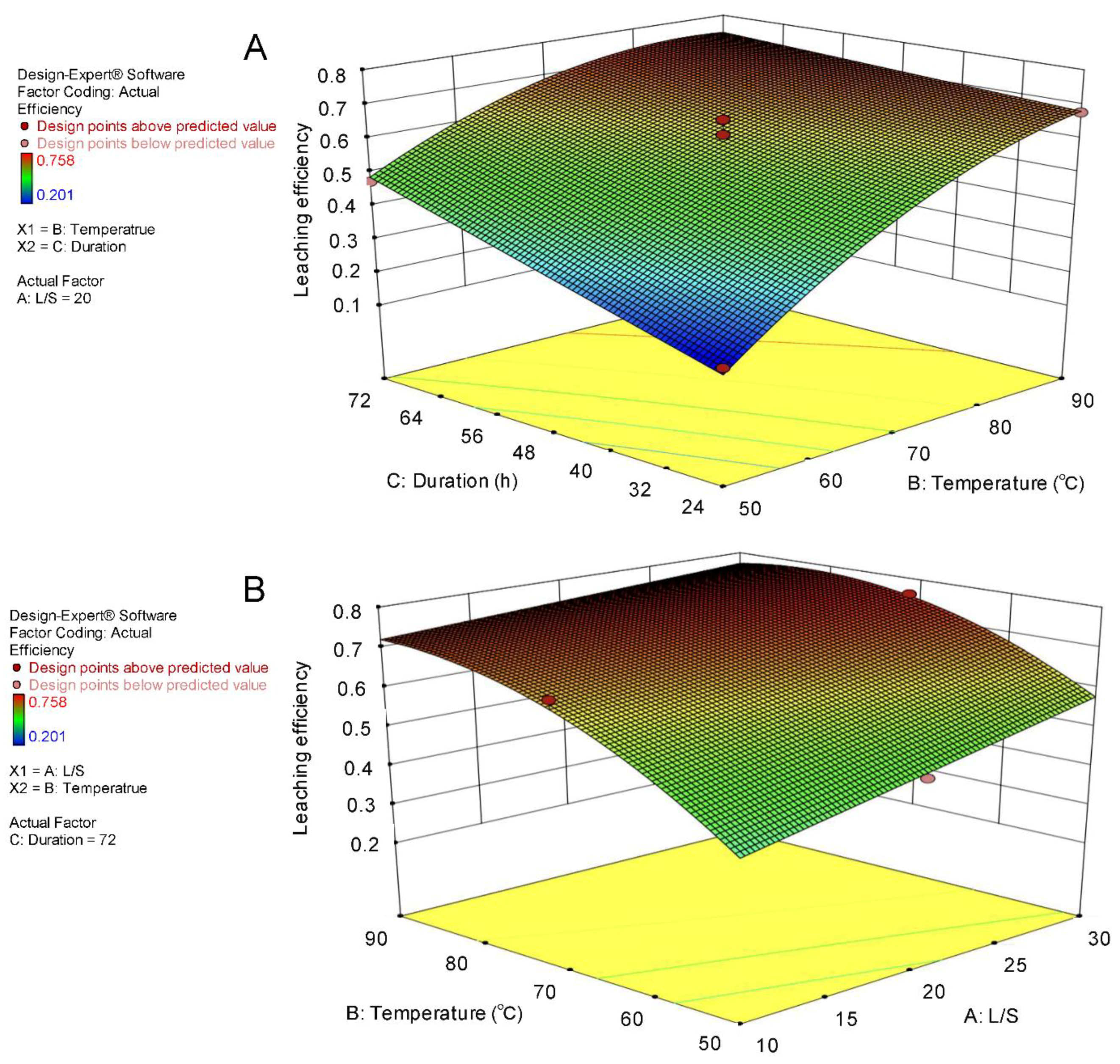
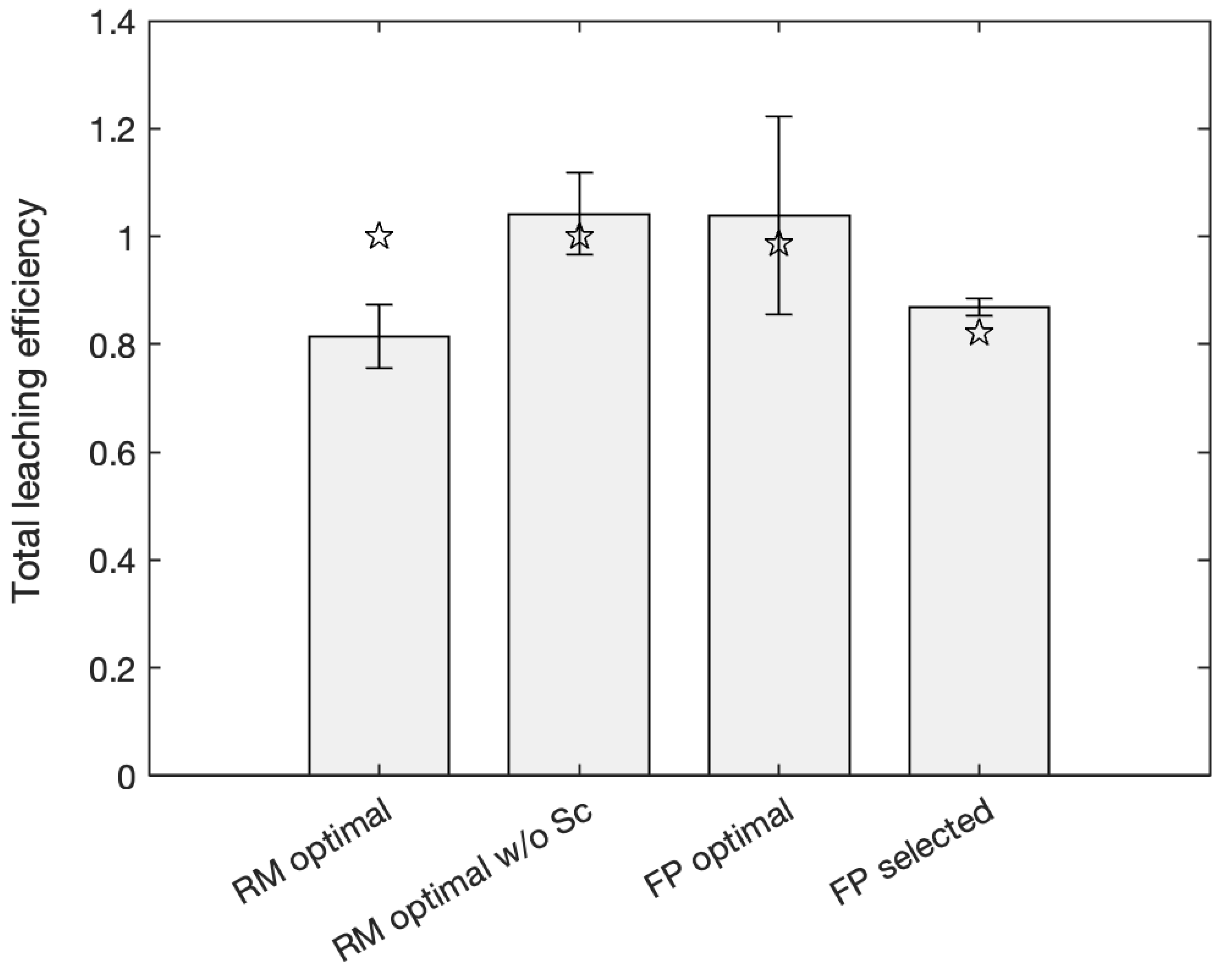
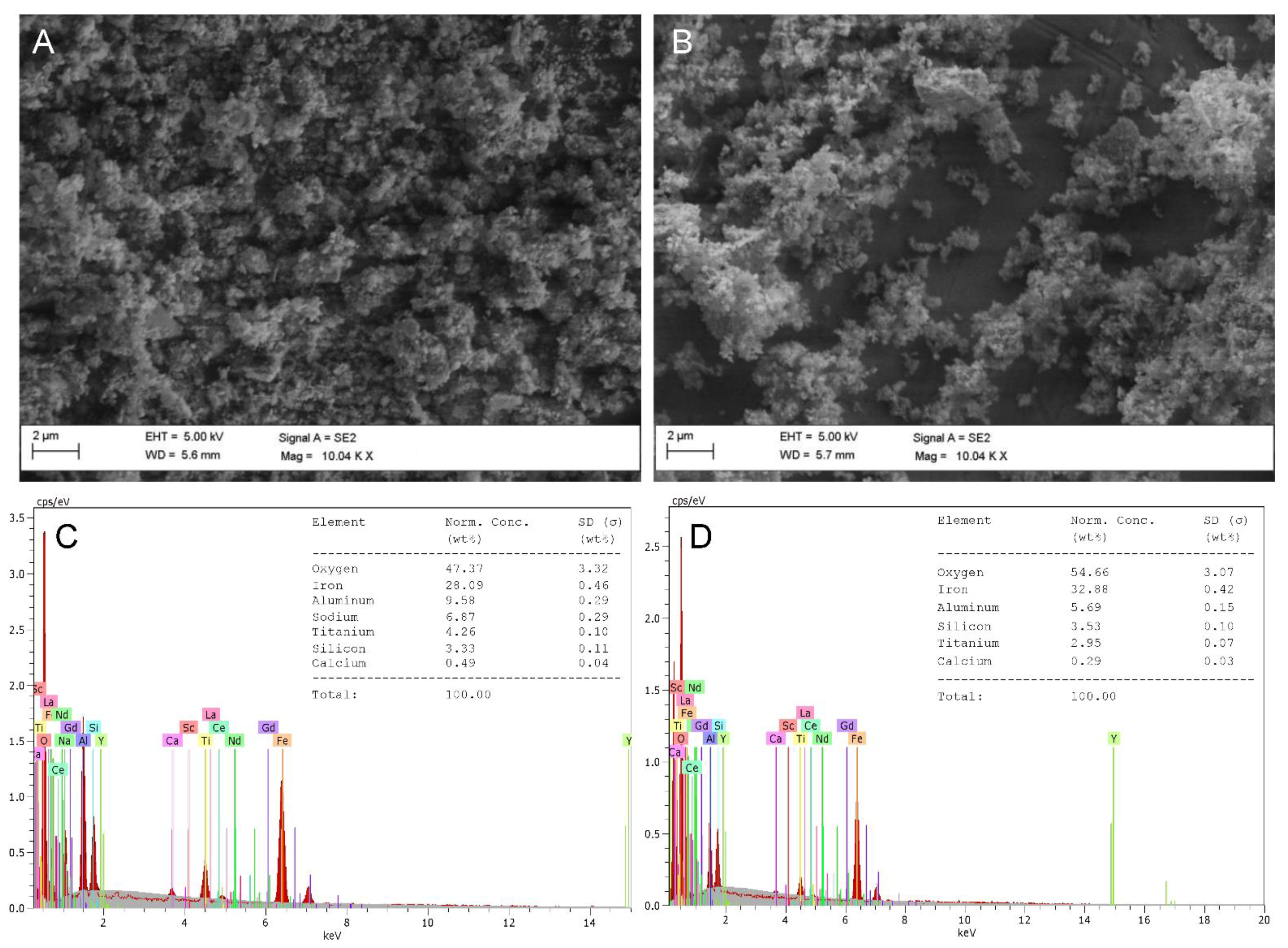
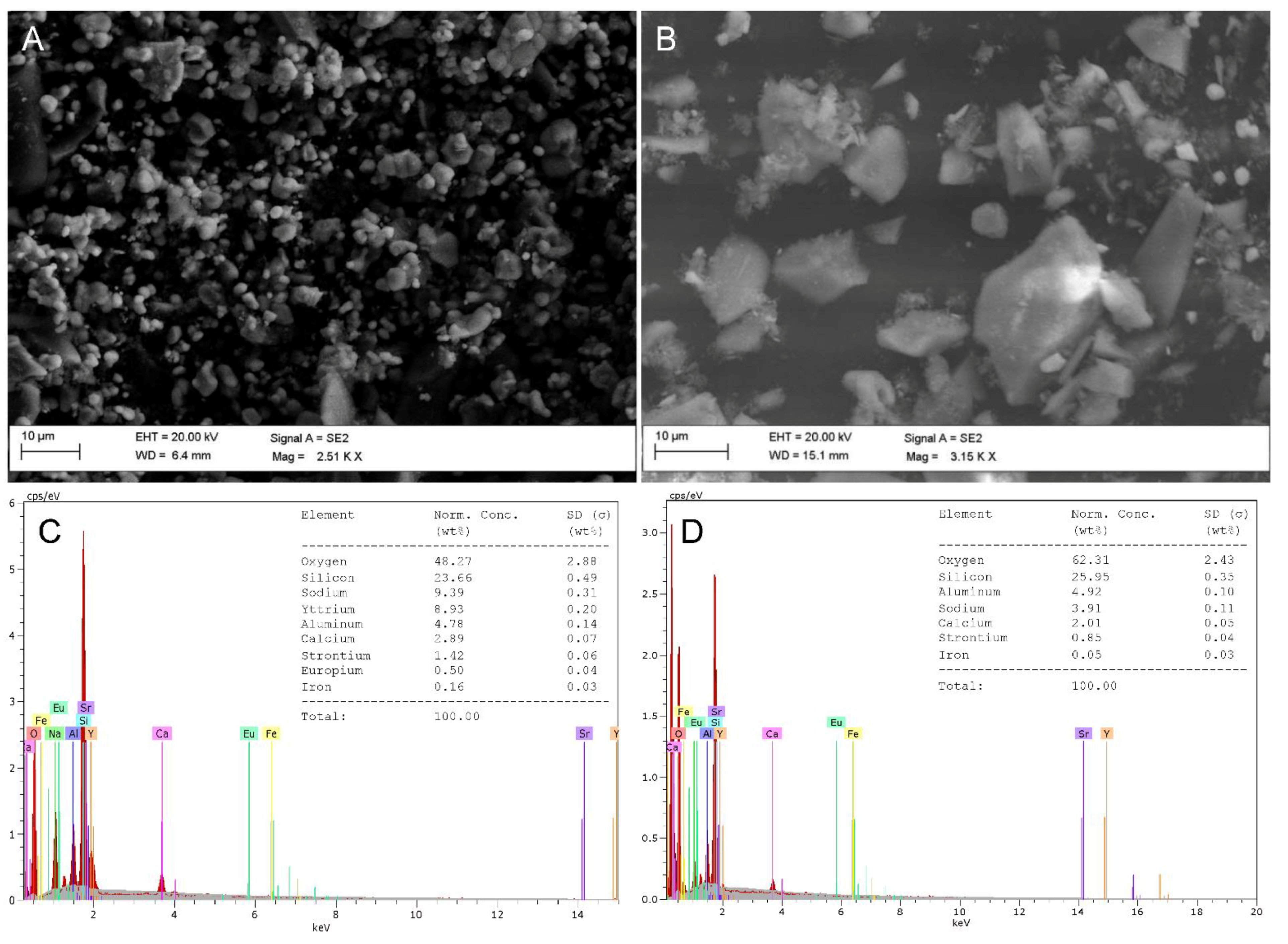
| REE | Red Mud (mg/kg) | Fluorescent Phosphors (g/kg) |
|---|---|---|
| Ce | 79.67 ± 1.53 | - |
| Sc | 46.33 ± 0.58 | - |
| Nd | 38.70 ± 0.58 | - |
| La | 38.67 ± 0.58 | - |
| Gd | 5.00 ± 0.01 | - |
| Y | 3.47 ± 0.12 | 87.80 ± 1.83 |
| Eu | - | 6.54 ± 0.12 |
| Run | L/S (L/kg) | Temperature (°C) | Duration (h) | Red Mud | Fluorescent Phosphors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ce | Sc | Nd | La | Gd | Y | Total | Y | Eu | Total | ||||
| 1 | 20 | 90 | 72 | 0.881 | 0.099 | 0.129 | 0.238 | 1.000 | 0.923 | 0.462 | 0.712 | 0.679 | 0.709 |
| 2 | 20 | 90 | 24 | 0.773 | 0.069 | 0.460 | 0.781 | 0.800 | 0.750 | 0.564 | 0.679 | 0.640 | 0.677 |
| 3 | 20 | 70 | 48 | 0.695 | 0.065 | 0.414 | 0.916 | 0.920 | 0.635 | 0.551 | 0.603 | 0.575 | 0.601 |
| 4 | 10 | 70 | 72 | 0.759 | 0.069 | 0.494 | 0.799 | 1.000 | 0.750 | 0.578 | 0.663 | 0.626 | 0.661 |
| 5 | 10 | 50 | 48 | 0.698 | 0.050 | 0.502 | 0.794 | 0.620 | 0.692 | 0.536 | 0.276 | 0.259 | 0.275 |
| 6 | 20 | 50 | 24 | 0.547 | 0.043 | 0.383 | 0.641 | 0.560 | 0.635 | 0.426 | 0.202 | 0.188 | 0.201 |
| 7 | 20 | 50 | 72 | 0.776 | 0.052 | 0.528 | 0.890 | 0.480 | 0.692 | 0.585 | 0.477 | 0.444 | 0.474 |
| 8 | 20 | 70 | 48 | 0.673 | 0.065 | 0.403 | 0.859 | 0.920 | 1.000 | 0.536 | 0.615 | 0.581 | 0.612 |
| 9 | 20 | 70 | 48 | 0.640 | 0.052 | 0.424 | 0.745 | 0.680 | 0.635 | 0.492 | 0.615 | 0.572 | 0.612 |
| 10 | 10 | 90 | 48 | 0.865 | 0.097 | 0.509 | 0.835 | 0.960 | 0.923 | 0.630 | 0.784 | 0.147 | 0.740 |
| 11 | 30 | 70 | 72 | 0.753 | 0.091 | 0.512 | 1.000 | 0.960 | 1.000 | 0.711 | 0.762 | 0.716 | 0.758 |
| 12 | 20 | 70 | 48 | 0.758 | 0.069 | 0.440 | 0.869 | 1.000 | 1.000 | 0.583 | 0.659 | 0.615 | 0.656 |
| 13 | 20 | 70 | 48 | 0.846 | 0.082 | 0.548 | 0.978 | 1.000 | 1.000 | 0.655 | 0.561 | 0.520 | 0.558 |
| 14 | 30 | 50 | 48 | 0.798 | 0.052 | 0.520 | 1.000 | 0.780 | 0.727 | 0.629 | 0.394 | 0.372 | 0.393 |
| 15 | 10 | 70 | 24 | 0.512 | 0.052 | 0.313 | 0.584 | 0.540 | 0.635 | 0.391 | 0.534 | 0.398 | 0.525 |
| 16 | 30 | 70 | 24 | 0.550 | 0.049 | 0.388 | 0.884 | 0.660 | 0.952 | 0.481 | 0.461 | 0.441 | 0.459 |
| 17 | 30 | 90 | 48 | 0.889 | 0.091 | 0.504 | 1.000 | 1.000 | 0.865 | 0.680 | 0.729 | 0.702 | 0.728 |
| Source | Red Mud | Fluorescent Phosphors | ||||
|---|---|---|---|---|---|---|
| Degree of Freedom | F-Value | p-Value | Degree of Freedom | F-Value | p-Value | |
| Model | 8 | 6.90 | 0.0065 | 7 | 55.29 | <0.0001 |
| Χ1 (L/S) | 1 | 7.46 | 0.0258 | 1 | 2.22 | 0.1703 |
| Χ2 (temperature) | 1 | 1.40 | 0.2712 | 1 | 270.25 | <0.0001 |
| Χ3 (duration) | 1 | 0.56 | 0.4747 | 1 | 64.82 | <0.0001 |
| Χ1Χ2 | - | - | - | 1 | 4.00 | 0.0765 |
| Χ1Χ3 | 1 | 0.064 | 0.8061 | 1 | 6.29 | 0.0334 |
| Χ2Χ3 | 1 | 10.25 | 0.0126 | 1 | 13.75 | 0.0049 |
| Χ12 | 1 | 2.61 | 0.1447 | - | - | - |
| Χ22 | - | - | - | 1 | 25.69 | 0.0007 |
| Χ32 | 1 | 10.84 | 0.0110 | - | - | - |
| Χ12Χ3 | 1 | 7.99 | 0.0223 | - | - | - |
| Residual | 8 | - | - | 9 | - | - |
| Lack-of-fit | 4 | 0.31 | 0.8576 | 5 | 0.76 | 0.6237 |
| Pure Error | 4 | - | - | 4 | - | - |
| Cor Total | 16 | - | - | 16 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Singh, S.; Dunn, K.A.; Liang, Y. Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid. Sustainability 2022, 14, 9682. https://doi.org/10.3390/su14159682
Jiang T, Singh S, Dunn KA, Liang Y. Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid. Sustainability. 2022; 14(15):9682. https://doi.org/10.3390/su14159682
Chicago/Turabian StyleJiang, Tao, Sarabjot Singh, Kathleen A. Dunn, and Yanna Liang. 2022. "Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid" Sustainability 14, no. 15: 9682. https://doi.org/10.3390/su14159682
APA StyleJiang, T., Singh, S., Dunn, K. A., & Liang, Y. (2022). Optimizing Leaching of Rare Earth Elements from Red Mud and Spent Fluorescent Lamp Phosphors Using Levulinic Acid. Sustainability, 14(15), 9682. https://doi.org/10.3390/su14159682








