Sustainable Valorization of Four Types of Fruit Peel Waste for Biogas Recovery and Use of Digestate for Radish (Raphanus sativus L. cv. Pusa Himani) Cultivation
Abstract
:1. Introduction
2. Materials and Methods
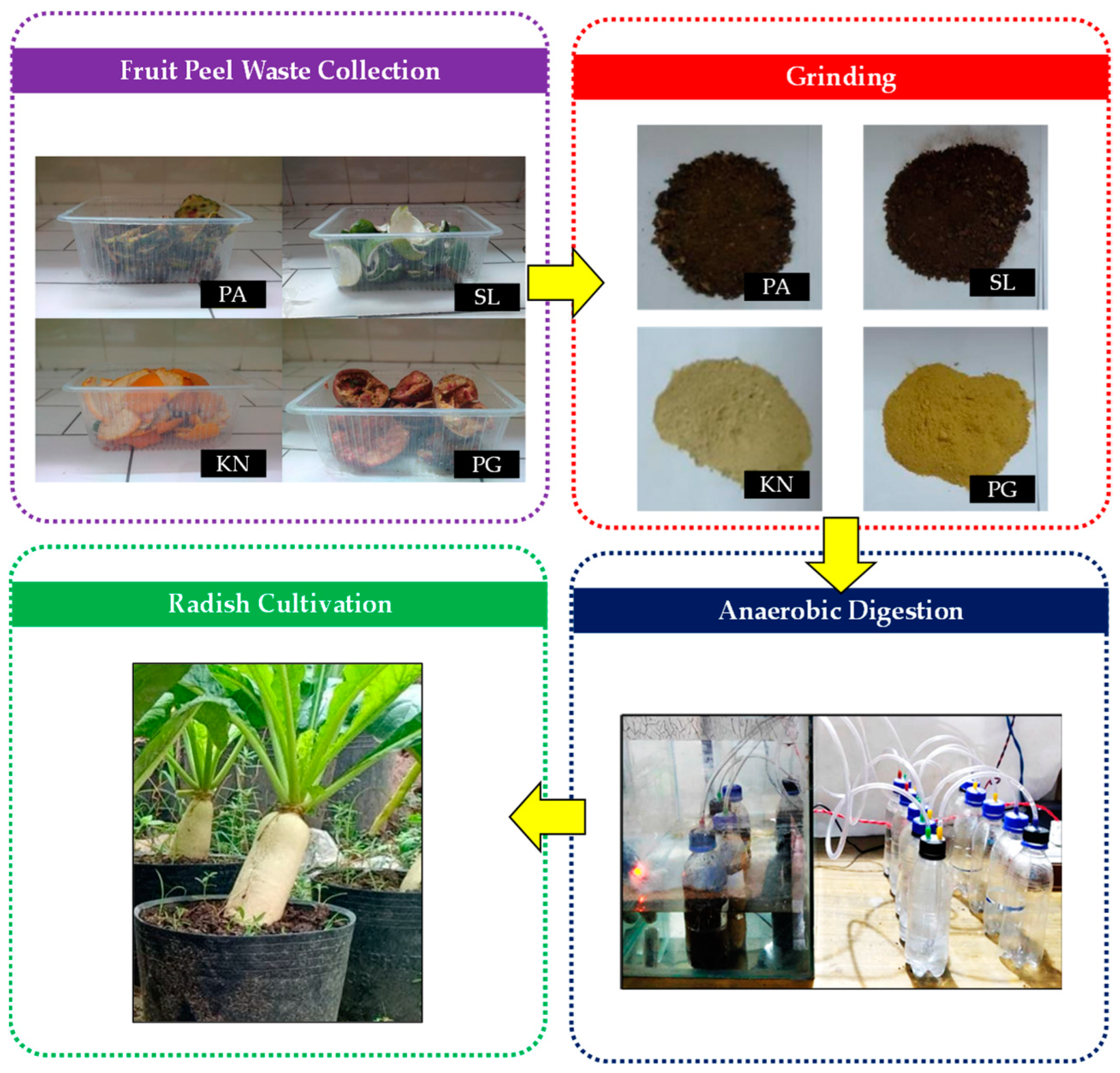
2.1. Collection of Experimental Materials
2.2. Experimental Design for Biogas Production
2.3. Experimental Design for Radish Cultivation
2.4. Analytical and Instrumental Procedures
2.5. Data Analysis
3. Results and Discussion
3.1. Properties of Fruit Peel Waste Used in This Study
3.2. Changes in FPW-Based Slurry before and after Biogas Production
3.3. Biogas Production Potential of Selected FPW and Kinetic Modeling Results
3.4. Effect of FPW Digestate on Soil Properties and Radish Crop
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adelodun, B.; Kareem, K.Y.; Kumar, P.; Kumar, V.; Choi, K.S.; Yadav, K.K.; Yadav, A.; El-Denglawey, A.; Cabral-Pinto, M.; Son, C.T.; et al. Understanding the impacts of the COVID-19 pandemic on sustainable agri-food system and agroecosystem decarbonization nexus: A review. J. Clean. Prod. 2021, 318, 128451. [Google Scholar] [CrossRef]
- Shahbandeh, M. Global Production of Fruit by Variety Selected 2019. 2021. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 15 July 2022).
- MoAFW Horticultural Statistics at a Glance 2018; Department of Agriculture, Cooperation & Farmers’ Welfare, Horticulture Sta-tistics Division, Ministry of Agriculture & Farmers’ Welfare, Government of India: New Delhi, India, 2018.
- Hussain, H.; Mamadalieva, N.Z.; Hussain, A.; Hassan, U.; Rabnawaz, A.; Ahmed, I.; Green, I.R. Fruit Peels: Food Waste as a Valuable Source of Bioactive Natural Products for Drug Discovery. Curr. Issues Mol. Biol. 2022, 44, 1960–1994. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Soh, T.; Chan, J.J.; Meng, S.; Meyer, D.; Srinivasan, M.; Tay, C.Y. Repurposing of Fruit Peel Waste as a Green Reductant for Recycling of Spent Lithium-Ion Batteries. Environ. Sci. Technol. 2020, 54, 9681–9692. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; López-Hernández, L.H. Food Vegetable and Fruit Waste Used in Meat Products. Food Rev. Int. 2020, 38, 628–654. [Google Scholar] [CrossRef]
- Abatal, M.; Lima, E.C.; Giannakoudakis, D.A.; Vargas, J.; Anastopoulos, I.; Olguin, M.T.; Alfonso, I. Pitahaya Fruit (Hylocereus spp.) Peels Evaluation for Removal of Pb(II), Cd(II), Co(II), and Ni(II) from the Waters. Sustainability 2022, 14, 1685. [Google Scholar] [CrossRef]
- Cavalheiro, T.R.T.; Alcoforado, R.D.O.; Silva, V.S.D.A.; Coimbra, P.P.S.; Mendes, N.D.S.; Cavalcanti, E.D.C.; Jurelevicius, D.D.A.; Gonçalves, C.B.D.A. The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity. Sustainability 2021, 13, 128. [Google Scholar] [CrossRef]
- O’Hara, I.M. The sugarcane industry, biofuel, and bioproduct perspectives. Sugarcane Based Biofuels Bioprod. 2013, 14, 1–22. [Google Scholar] [CrossRef]
- Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.E.; Osman, H.E.M.; Al-Bakre, D.A.; Adelodun, B.; Fayssal, S.A.; Goala, M.; Mioč, B.; et al. Biotransforming the Spent Substrate of Shiitake Mushroom (Lentinula edodes Berk.): A Synergistic Approach to Biogas Production and Tomato (Solanum lycopersicum L.) Fertilization. Horticulturae 2022, 8, 479. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Weichgrebe, D.; Srinivasan, S.V. Enhancement of Methane Production from Vegetable, Fruit and Flower Market Wastes Using Extrusion as Pretreatment and Kinetic Modeling. Water Air Soil Pollut. 2020, 231, 126. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; Van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed With Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Valença, R.B.; da Silva, L.C.S.; Holanda, S.H.D.B.; da Silva, A.F.V.; Jucá, J.F.T.; Santos, A.F.M.S. Methane generation potential through anaerobic digestion of fruit waste. J. Clean. Prod. 2020, 256, 120389. [Google Scholar] [CrossRef]
- Rani, D.; Nand, K. Ensilage of pineapple processing waste for methane generation. Waste Manag. 2004, 24, 523–528. [Google Scholar] [CrossRef]
- Ofomatah, A.C.; Ugwu, E.K.; Ani, J.U. Biogas Production and Storage from Pig Dung Co-Digested With Pineapple Peel. IOP Conf. Ser. Earth Environ. Sci. 2021, 730, 012004. [Google Scholar] [CrossRef]
- Jain, K.; Suryawanshi, P.; Chaudhari, A. Recovery of Acerbic Anaerobic Digester for Biogas Production from Pomegranate Shells Using Organic Loading Approach. Indian J. Biochem. Biophys. 2020, 57, 86–94. [Google Scholar]
- Liu, S.Y.; Charles, W.; Ho, G.; Cord-Ruwisch, R.; Cheng, K.Y. Bioelectrochemical enhancement of anaerobic digestion: Comparing single- and two-chamber reactor configurations at thermophilic conditions. Bioresour. Technol. 2017, 245, 1168–1175. [Google Scholar] [CrossRef]
- Prajapati, K.B.; Singh, R. Enhancement of biogas production in bio-electrochemical digester from agricultural waste mixed with wastewater. Renew. Energy 2020, 146, 460–468. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, V.; Singh, J.; Kumar, P. Electrokinetic assisted anaerobic digestion of spent mushroom substrate supplemented with sugar mill wastewater for enhanced biogas production. Renew. Energy 2021, 179, 418–426. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International, 21st ed. 2019. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 4 July 2022).
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Laferrriere, J.E. CRC Handbook of Proximate Analysis Tables of Higher Plants; CRC Press: Boca Raton, FL, USA, 1987; Volume 7. [Google Scholar]
- Kumar, V.; Kumar, P.; Kumar, P.; Singh, J. Anaerobic Digestion of Azolla pinnata Biomass Grown in Integrated Industrial Effluent for Enhanced Biogas Production and COD Reduction: Optimization and Kinetics Studies. Environ. Technol. Innov. 2020, 17, 100627. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Nadeem, M.; Kumar, P.; Pathak, V.V. Experimental and Kinetics Studies for Biogas Production Using Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Sugar Mill Effluent. Waste Biomass Valorization 2020, 11, 109–119. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Biogas from Wastes: Processes and Applications. In Sustainable Resource Recovery and Zero Waste Approaches; Elsevier B.V.: Amsterdam, The Netherlands, 2019; ISBN 9780444642004. [Google Scholar]
- Fayssal, S.A.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Böhme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV–vis spectroscopic data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 35. [Google Scholar]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A Review of Biogas Utilisation, Purification and Upgrading Technologies. Waste Biomass Valorization 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Bouallagui, H.; ben Cheikh, R.; Marouani, L.; Hamdi, M. Mesophilic biogas production from fruit and vegetable waste in a tubular digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef]
- Ariyanto, T.; Cahyono, R.B.; Vente, A.; Mattheij, S.; Millati, R.; Sarto, S.; Taherzadeh, M.; Syamsiah, S. Utilization of Fruit Waste as Biogas Plant Feed and its Superiority Compared to Landfill. Int. J. Technol. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Silva, A.; DOS Santos, L.A.; Valença, R.; Porto, T.; Sobrinho, M.D.M.; Gomes, G.; Jucá, J.F.T.; Santos, A.F.M.S. Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J. Environ. Chem. Eng. 2019, 7, 103510. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D.; Baksh, H. Performance of Different Genotypes of Radish (Raphanus sativus L.). J. Agric. Search 2021, 8, 338–341. [Google Scholar] [CrossRef]
- Lee, M.E.; Steiman, M.W.; Angelo, S.K.S. Biogas digestate as a renewable fertilizer: Effects of digestate application on crop growth and nutrient composition. Renew. Agric. Food Syst. 2021, 36, 173–181. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W. Effect of biofertilizer amendments on the concentration of elements in edible parts of radish and green bean grown in a cropping sequence. J. Elem. 2018, 23, 69–83. [Google Scholar] [CrossRef]


| Properties | Cow Dung (CD) | Fruit Peel Wastes | |||
|---|---|---|---|---|---|
| Pineapple (PA) | Sweet Lemon (SL) | Kinnow (KN) | Pomegranate (PG) | ||
| pH | 8.10 ± 0.03 a | 5.83 ± 0.02 d | 6.17 ± 0.05 b | 6.02 ± 0.02 c | 6.13 ± 0.04 b |
| Electrical Conductivity (dS/m) | 4.82 ± 0.10 a | 5.08 ± 0.31 a | 3.70 ± 0.17 d | 4.10 ± 0.09 cd | 4.46 ± 0.12 c |
| Moisture Content (%) | 56.80 ± 2.52 a | 62.38 ± 3.10 ab | 74.19 ± 1.85 c | 68.02 ± 4.30 b | 59.07 ± 2.90 a |
| Organic Carbon (%) | 20.63 ± 1.20 a | 36.70 ± 0.60 d | 28.01 ± 1.23 bc | 25.92 ± 0.75 b | 31.35 ± 1.02 cd |
| Total Nitrogen (%) | 0.70 ± 0.02 a | 1.36 ± 0.05 d | 1.02 ± 0.01 c | 0.86 ± 0.03 b | 1.28 ± 0.07 d |
| C:N Ratio | 29.47 | 26.98 | 27.46 | 30.13 | 24.49 |
| Total Solids (%) | 7.23 ± 0.10 b | 12.04 ± 0.06 d | 8.16 ± 0.30 c | 4.60 ± 0.26 a | 10.24 ± 0.22 cd |
| Volatile Solids (%) | 9.14 ± 0.09 b | 16.08 ± 0.14 cd | 9.54 ± 0.18 c | 8.12 ± 0.06 a | 13.77 ± 0.40 c |
| COD (mg/L) | 8302.10 ± 10.53 e | 4283.20 ± 8.27 d | 3107.05 ± 21.04 b | 2690.81 ± 15.02 a | 3710.52 ± 9.15 c |
| Cellulose (%) | 4.90 ± 0.08 a | 13.56 ± 0.14 d | 9.10 ± 0.23 bc | 8.35 ± 0.06 b | 9.90 ± 0.08 c |
| Hemicellulose (%) | 3.05 ± 0.02 a | 7.15 ± 0.06 e | 5.10 ± 0.04 d | 3.77 ± 0.02 b | 4.14 ± 0.05 c |
| Lignin (%) | 6.10 ± 0.04 b | 12.75 ± 0.12 d | 8.24 ± 0.08 c | 5.09 ± 0.11 a | 8.53 ± 0.26 c |
| Reducing Sugars (g/L) | 42.60 ± 3.73 a | 121.61 ± 2.90 d | 89.10 ± 2.10 b | 81.04 ± 3.04 b | 105.29 ± 1.55 c |
| Total Ash (%) | 2.44 ± 0.02 a | 3.07 ± 0.01 d | 2.54 ± 0.02 b | 3.16 ± 0.03 d | 2.92 ± 0.04 c |
| Properties | Conventional Reactor | Electro-Assisted Anaerobic Reactor | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PA | SL | KN | PG | PA | SL | KN | PG | ||
| pH | Before | 6.93 ± 0.02 a | 7.12 ± 0.05 a | 7.08 ± 0.03 a | 7.11 ± 0.02 a | 6.97 ± 0.06 a | 7.14 ± 0.02 a | 7.06 ± 0.6 a | 7.12 ± 0.03 a |
| After | 5.28 ± 0.04 b | 5.83 ± 0.03 b | 5.91 ± 0.05 b | 5.49 ± 0.04 b | 5.10 ± 0.02 b | 5.62 ± 0.04 b | 5.80 ± 0.08 b | 5.38 ± 0.06 b | |
| EC (dS/m) | Before | 4.96 ± 0.05 a | 4.28 ± 0.06 a | 4.45 ± 0.04 a | 4.64 ± 0.03 a | 4.95 ± 0.04 a | 4.26 ± 0.07 a | 4.46 ± 0.05 a | 4.64 ± 0.05 a |
| After | 3.56 ± 0.02 b | 3.37 ± 0.03 b | 3.65 ± 0.05 b | 3.40 ± 0.06 b | 3.24 ± 0.8 b | 3.17 ± 0.04 b | 3.67 ± 0.03 b | 3.15 ± 0.04 b | |
| OC (%) | Before | 28.08 ± 1.42 a | 24.10 ± 1.80 a | 23.25 ± 0.96 a | 26.02 ± 0.18 a | 28.67 ± 1.27 a | 24.32 ± 1.93 a | 23.28 ± 1.02 a | 25.99 ± 0.26 a |
| After | 17.10 ± 2.70 b | 17.64 ± 2.15 b | 19.02 ± 1.68 b | 17.51 ± 0.93 b | 14.49 ± 0.62 b | 15.92 ± 2.18 b | 17.24 ± 1.40 b | 15.82 ± 1.01 b | |
| TN (%) | Before | 1.03 ± 0.03 a | 0.86 ± 0.02 a | 0.79 ± 0.04 a | 0.98 ± 0.05 a | 1.03 ± 0.02 a | 0.86 ± 0.03 a | 0.78 ± 0.06 a | 0.99 ± 0.03 a |
| After | 0.79 ± 0.02 b | 0.74 ± 0.03 b | 0.71 ± 0.05 b | 0.76 ± 0.07 b | 0.75 ± 0.04 b | 0.72 ± 0.04 b | 0.67 ± 0.02 b | 0.74 ± 0.05 b | |
| TS (%) | Before | 9.65 ± 0.08 a | 7.70 ± 0.05 a | 5.91 ± 0.06 a | 8.76 ± 0.08 a | 9.64 ± 0.51 a | 7.70 ± 0.06 a | 5.92 ± 0.08 a | 8.74 ± 0.08 a |
| After | 5.12 ± 0.10 b | 5.30 ± 0.04 b | 4.54 ± 0.12 b | 5.82 ± 0.09 | 4.61 ± 0.13 b | 5.10 ± 0.05 b | 4.20 ± 0.16 b | 5.06 ± 0.14 b | |
| VS (%) | Before | 12.60 ± 0.18 a | 9.32 ± 0.08 a | 8.64 ± 0.09 a | 11.41 ± 0.15 a | 12.61 ± 0.17 a | 9.34 ± 0.09 a | 8.63 ± 0.10 a | 11.46 ± 0.23 a |
| After | 5.70 ± 0.20 b | 5.68 ± 0.13 b | 5.45 ± 0.17 b | 5.93 ± 0.24 b | 5.01 ± 0.20 b | 4.95 ± 0.15 b | 4.80 ± 0.27 b | 5.58 ± 0.16 b | |
| COD (mg/L) | Before | 6210.27 ± 12.09 a | 5710.50 ± 36.24 a | 5491.29 ± 28.04 a | 6014.07 ± 30.11 a | 6292.65 ± 17.38 a | 5704.58 ± 28.10 a | 5496.46 ± 21.09 a | 6006.31 ± 27.20 a |
| After | 2208.61 ± 9.18 b | 3325.50 ± 15.02 b | 3410.18 ± 11.46 b | 2874.05 ± 8.90 b | 1730.13 ± 20.55 b | 2777.06 ± 18.07 b | 2845.10 ± 9.74 b | 2010.26 ± 12.04 b | |
| Cellulose (%) | Before | 9.24 ± 0.07 a | 7.01 ± 0.08 a | 6.63 ± 0.04 a | 7.39 ± 0.06 a | 9.23 ± 0.07 a | 7.00 ± 0.09 a | 6.63 ± 0.05 a | 7.40 ± 0.07 a |
| After | 7.40 ± 0.16 b | 5.90 ± 0.20 b | 6.05 ± 0.09 b | 6.21 ± 0.03 b | 7.16 ± 0.18 b | 5.75 ± 0.21 b | 5.66 ± 0.10 b | 5.93 ± 0.02 b | |
| Hemicellulose (%) | Before | 5.10 ± 0.04 a | 4.09 ± 0.05 a | 3.40 ± 0.03 a | 3.62 ± 0.02 a | 5.10 ± 0.03 a | 4.08 ± 0.07 a | 3.41 ± 0.04 a | 3.60 ± 0.06 a |
| After | 3.68 ± 0.10 b | 3.47 ± 0.07 b | 2.78 ± 0.06 b | 2.85 ± 0.02 b | 3.41 ± 0.08 b | 3.10 ± 0.06 b | 2.49 ± 0.07 b | 2.54 ± 0.03 b | |
| Lignin (%) | Before | 9.40 ± 0.07 a | 7.16 ± 0.06 a | 5.59 ± 0.03 a | 7.33 ± 0.08 a | 9.43 ± 0.09 a | 7.17 ± 0.05 a | 5.60 ± 0.05 a | 7.32 ± 0.11 a |
| After | 5.92 ± 0.12 b | 5.15 ± 0.10 b | 4.27 ± 0.02 b | 5.05 ± 0.05 b | 5.52 ± 0.10 b | 4.70 ± 0.11 b | 3.75 ± 0.03 b | 4.60 ± 0.08 b | |
| RS (g/L) | Before | 82.10 ± 6.80 a | 64.90 ± 4.22 a | 61.84 ± 10.21 a | 73.92 ± 8.85 a | 82.11 ± 5.97 a | 65.85 ± 4.30 a | 61.82 ± 12.15 a | 73.95 ± 8.29 a |
| After | 22.08 ± 2.09 b | 31.32 ± 5.03 b | 34.01 ± 3.17 b | 28.10 ± 4.34 b | 14.06 ± 1.49 b | 20.12 ± 5.09 b | 26.40 ± 2.86 b | 19.38 ± 2.01 b | |
| Biogas Properties | Conventional Reactor | Electro-assisted Anaerobic Reactor | ||||||
|---|---|---|---|---|---|---|---|---|
| PA | SL | KN | PG | PA | SL | KN | PG | |
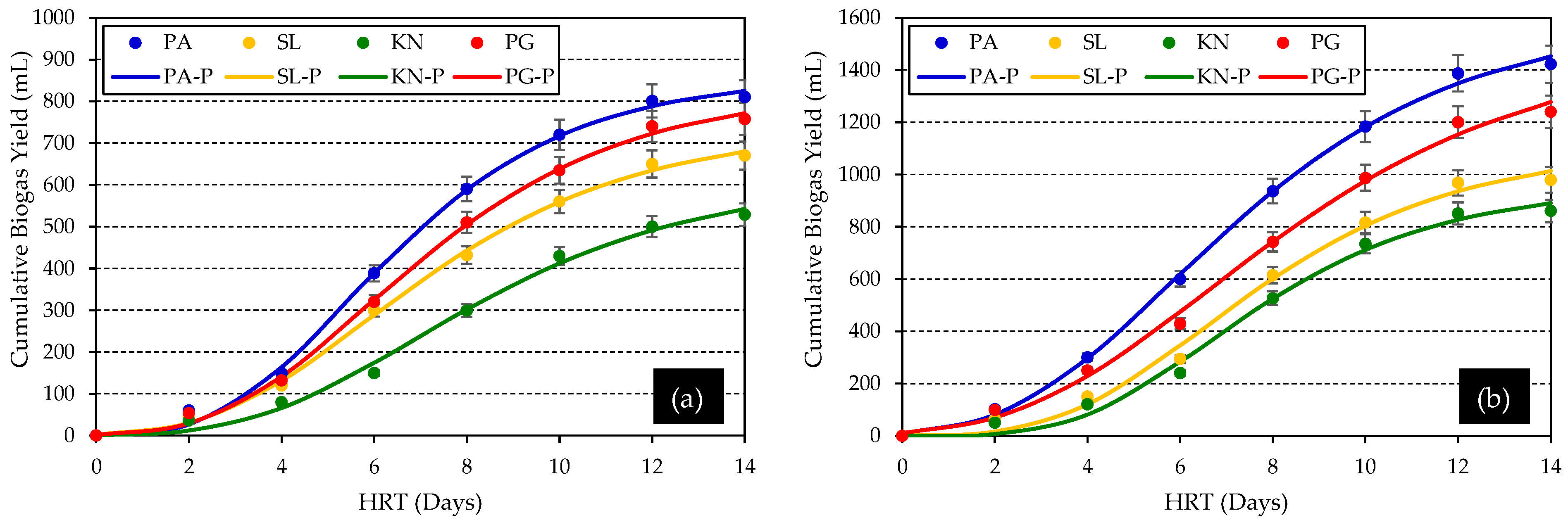
| Biogas Yield (mL) | 810.50 ± 2.54 a | 670.30 ± 1.90 a | 529.10 ± 2.10 a | 758.53 ± 1.52 a | 1422.76 ± 3.10 b | 979.18 ± 1.30 b | 860.43 ± 0.52 b | 1240.10 ± 0.80 b |
| Biogas Yield (mL/gVS) | 44.34 ± 1.20 c | 20.84 ± 0.70 a | 27.73 ± 0.34 b | 46.71 ± 1.06 c | 77.83 ± 2.20 d | 30.45 ± 1.87 b | 45.10 ± 0.40 c | 76.36 ± 1.93 d |
| CH4 (%) | 59.10 ± 0.08 a | 57.12 ± 0.15 a | 55.55 ± 0.06 a | 58.02 ± 0.02 a | 62.21 ± 0.13 b | 58.35 ± 0.05 b | 54.08 ± 0.10 b | 62.15 ± 0.07 b |
| CO2 (%) | 40.86 ± 0.02 a | 42.84 ± 0.05 a | 44.47 ± 0.03 a | 41.93 ± 0.09 a | 37.74 ± 0.01 b | 41.62 ± 0.06 b | 45.85 ± 0.03 b | 37.81 ± 0.09 b |
| H2S (%) | 0.03 ± 0.01 a | 0.05 ± 0.01 a | 0.02 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.02 ± 0.01 b | 0.06 ± 0.01 b | 0.03 ± 0.01 a |
| Water Vapours (%) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Variables | Conventional Reactor | Electro-Assisted Anaerobic Reactor | ||||||
|---|---|---|---|---|---|---|---|---|
| PA | SL | KN | PG | PA | SL | KN | PG | |
| y (mL) | 825.20 ± 5.28 | 680.60 ± 4.50 | 543.74 ± 3.08 | 772.40 ± 6.25 | 1452.81 ± 7.90 | 1013.05 ± 3.10 | 891.22 ± 4.36 | 1278.09 ± 2.53 |
| P (mL) | 859.78 | 738.57 | 618.24 | 831.65 | 1599.01 | 1105.78 | 957.41 | 1501.15 |
| λ (days) | 5.37 | 5.79 | 6.82 | 5.80 | 5.81 | 6.46 | 6.55 | 6.57 |
| μm (mL/day) | 0.36 | 0.30 | 0.28 | 0.31 | 0.28 | 0.32 | 0.35 | 0.24 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Properties | Control | FPW Digestate (5% w/w Soil) | |||
|---|---|---|---|---|---|
| PA | SL | KN | PG | ||
| pH | 7.33 ± 0.02 a | 7.59 ± 0.03 b | 7.61 ± 0.04 b | 7.62 ± 0.02 b | 7.60 ± 0.02 b |
| Electrical Conductivity (EC: dS/m) | 2.12 ± 0.04 a | 2.28 ± 0.05 b | 2.28 ± 0.02 b | 2.30 ± 0.05 b | 2.28 ± 0.04 b |
| Organic Carbon (OC: %) | 1.36 ± 0.10 a | 2.08 ± 0.08 b | 2.16 ± 0.05 b | 2.22 ± 0.04 bc | 2.15 ± 0.07 b |
| Total Nitrogen (N: %) | 0.13 ± 0.02 a | 0.17 ± 0.01 b | 0.17 ± 0.01 b | 0.16 ± 0.03 bc | 0.17 ± 0.01 b |
| C:N Ratio | 10.46 | 12.44 | 12.99 | 13.59 | 12.88 |
| Total Phosphorus (P: %) | 0.41 ± 0.04 a | 0.43 ± 0.02 a | 0.45 ± 0.03 a | 0.43 ± 0.04 a | 0.49 ± 0.01 b |
| Total Potassium (K: 0) | 0.23 ± 0.02 a | 0.24 ± 0.02 a | 0.27 ± 0.01 b | 0.26 ± 0.03 a | 0.25 ± 0.02 a |
| Properties | Control (Arable Soil) | FPW Digestate (5% w/w Soil) | |||
|---|---|---|---|---|---|
| PA | SL | KN | PG | ||
| Plant Height (above ground: cm) | 24.40 ± 1.20 a | 30.20 ± 0.73 b | 34.14 ± 1.04 c | 36.50 ± 0.82 c | 32.90 ± 1.10 b |
| Plant Spread (cm2) | 58.15 ± 2.75 a | 62.04 ± 3.03 a | 68.35 ± 1.18 bc | 70.80 ± 3.79 c | 65.26 ± 2.52 b |
| Number of Leaves | 12.18 ± 0.10 a | 14.50 ± 0.18 b | 16.02 ± 0.18 c | 16.12 ± 0.05 c | 15.70 ± 0.26 bc |
| Fresh Weight of Leaves (g/plant) | 130.53 ± 5.04 a | 145.16 ± 3.16 b | 154.60 ± 4.27 c | 158.08 ± 2.85 c | 150.22 ± 3.09 bc |
| Fruit Yield (g/plant) | 116.26 ± 3.29 a | 129.03 ± 1.70 b | 137.00 ± 4.02 bc | 140.10 ± 2.13 c | 132.09 ± 4.35 b |
| Fruit Length (cm) | 17.10 ± 0.05 a | 20.42 ± 0.13 b | 24.97 ± 0.09 d | 25.05 ± 0.15 d | 22.18 ± 0.20 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Huqail, A.A.; Kumar, V.; Kumar, R.; Eid, E.M.; Taher, M.A.; Adelodun, B.; Abou Fayssal, S.; Mioč, B.; Držaić, V.; Goala, M.; et al. Sustainable Valorization of Four Types of Fruit Peel Waste for Biogas Recovery and Use of Digestate for Radish (Raphanus sativus L. cv. Pusa Himani) Cultivation. Sustainability 2022, 14, 10224. https://doi.org/10.3390/su141610224
AL-Huqail AA, Kumar V, Kumar R, Eid EM, Taher MA, Adelodun B, Abou Fayssal S, Mioč B, Držaić V, Goala M, et al. Sustainable Valorization of Four Types of Fruit Peel Waste for Biogas Recovery and Use of Digestate for Radish (Raphanus sativus L. cv. Pusa Himani) Cultivation. Sustainability. 2022; 14(16):10224. https://doi.org/10.3390/su141610224
Chicago/Turabian StyleAL-Huqail, Arwa A., Vinod Kumar, Rohit Kumar, Ebrahem M. Eid, Mostafa A. Taher, Bashir Adelodun, Sami Abou Fayssal, Boro Mioč, Valentino Držaić, Madhumita Goala, and et al. 2022. "Sustainable Valorization of Four Types of Fruit Peel Waste for Biogas Recovery and Use of Digestate for Radish (Raphanus sativus L. cv. Pusa Himani) Cultivation" Sustainability 14, no. 16: 10224. https://doi.org/10.3390/su141610224
APA StyleAL-Huqail, A. A., Kumar, V., Kumar, R., Eid, E. M., Taher, M. A., Adelodun, B., Abou Fayssal, S., Mioč, B., Držaić, V., Goala, M., Kumar, P., & Širić, I. (2022). Sustainable Valorization of Four Types of Fruit Peel Waste for Biogas Recovery and Use of Digestate for Radish (Raphanus sativus L. cv. Pusa Himani) Cultivation. Sustainability, 14(16), 10224. https://doi.org/10.3390/su141610224














