Abstract
Discarded Waste-SCR (selective catalytic reduction) catalysts not only harm the natural environment, but also hinder the recovery of valuable metals such as V, W, and Ti, thus resulting in a waste of resources. Therefore, it has become imperative to dispose of Waste-SCR catalysts in large amounts. This study aims to develop a complete, eco-friendly, and economical process of dealing with Waste-SCR catalysts. In this process, the recovery of V and W from Waste-SCR catalysts was achieved in several steps. In addition, the leaching conditions of the acid-base process were optimized, while the structure and composition of the leaching residual were studied in depth. Sodium titanates generated by alkali leaching were then applied for regeneration of the TiO2 photocatalyst. It is worth noting that Fe-doped TiO2 photocatalysts were also prepared, while the required iron source was obtained through acid leaching. Regenerated photocatalyst achieved an excellent performance in the photocatalytic degradation of dye pollutants, which is attributable to small particle size and low forbidden band width. This contributes a new idea to the recycling of solid waste, and the economical treatment of wastewater.
1. Introduction
Currently, the SCR catalyst has been extensively applied in thermal power plants, but its service life is usually 2–3 years [], at most. In many cases, Waste-SCR catalysts are discarded even when there is no effective means to dispose of them. The discarded solid waste not only occupies a large amount of land resource, but it also hinders valuable metals such as V, W, and Ti contained in the catalysts from being effectively recycled, thus resulting in the waste of resources []. In recent years, study has been conducted on the recovery of Waste-SCR catalysts. In general, the recovery of Waste-SCR catalysts can be achieved through a dry process or wet process. The dry recovery process is usually reliant on the molten salt reaction of a substance with strong alkalinity (such as NaOH, Na2CO3), the Waste-SCR catalyst converting vanadium and tungsten into soluble vanadate and tungstate, and TiO2 generating insoluble titanate, thereby realizing the separation of V, W, and Ti [,]. Nevertheless, TiO2 recovered using this method tends to show mixed crystals of anatase and rutile, thus resulting in low purity. In addition, the roasting temperature is high in the dry process, and energy loss is substantial. Consequently, it is difficult to recycle for commercial purposes, TiO2 obtained through the dry process.
The wet process relies on acid or alkali to leach the spent SCR catalyst, adjust the physical and chemical conditions of the solution, separate the different components, and further recycle and process them into the desired products. To date, there have been some studies conducted on the leaching of Waste-SCR with acid [,]. In particular, organic acids derived from green sources have attracted extensive attention due to their less severe secondary pollution, and higher leaching efficiency, than inorganic acids. However, acid metallurgy also shows a significant disadvantage in that the valuable metal, W, cannot be effectively leached. Therefore, wet alkaline leaching has gradually drawn the attention of people. By means of high-temperature pressurization, the leaching efficiency of V and W can exceed 90% []. Nevertheless, alkaline metallurgy also has its own weaknesses. The leached vanadium and tungsten are mainly recovered by using a stepwise precipitation method. Since they are both transition metals with similar properties, behaviors, and orientations in solution, the range of pH value required to precipitate V and W is also similar, which significantly increases the difficulty of V and W separation []. In short, it is imperative to develop an effective solution to the eco-friendly recycling of valuable metals from Waste-SCR catalysts.
In addition to the recovery of valuable metals, it is also worth paying attention to the reutilization of the TiO2 carrier, with a content of up to 80–90% in the spent SCR catalyst. TiO2 has attracted extensive attention as a photocatalyst because of its excellent photoelectric properties, stable chemical properties, low cost, and non-toxicity [,,,]. However, there remain some constraints on the practical applications of TiO2 photocatalysts. Since they are mainly produced from expensive chemical reagents, such as TiCl4 [], TiOSO4 [,], and Ti(OC4H9)4 (TBOT) [,], the production cost is fairly high. In addition, the low-efficiency utilization of solar energy results from their wide band gap (3.2 eV for anatase and 3.0 eV for rutile). In view of the above problems, the TiO2 derived from spent SCR catalysts was applied in this study as a secondary raw material for photocatalysts.
Herein, both V and W were efficiently leached in steps through the acid-base wet process, with sustainable TiO2 photocatalyst produced. Notably, Fe-doped photocatalyst was also obtained by doping the FeC2O4 precipitated in the acid leaching process into TiO2 for improved solar energy utilization and reduced band gap, which has been demonstrated as effective in improving photocatalyst efficiency. The optimal process conditions of phased leaching for V, W, and Ti were determined, and the leaching residues were analyzed in depth. Finally, the physical and chemical properties and photocatalytic performance of regenerated photocatalysts were examined.
2. Materials and Methods
The experimental raw materials (Waste-SCR catalysts) were obtained from a thermal power plant in Jiangsu, China. To ensure the consistency of this experiment, the Waste-SCR used in the experiment was collected from the same batch. The equipment used in the experimental process mainly includes a constant temperature oil bath and high-pressure reactor. Oxalic acid leaching was carried out in a constant temperature oil bath, and NaOH leaching was performed in a high-pressure reactor. The elemental composition of the samples was analyzed by using Axios X-ray fluorescence spectrometer (XRF, panalytical company of the Netherlands, Almelo, The Netherlands), and optima 5300dv inductively coupled plasma atomic emission spectrometer (ICP-AES, Pekin Elmer company of the United States, Waltham, MA, USA). The phase structure was analyzed by using empyrean X-ray diffractometer (XRD, panalytical company of the Netherlands). Fei Talos f200s (TEM, Thermofisher, Waltham, MA, USA) was applied to conduct surface morphology analysis. To carry out the analysis of pore structure, an autosorb−1 automatic physicochemical adsorption instrument was employed (BET, quantachrome company of the United States, Boynton Beach, FL, USA). The diffuse reflectance spectra were recorded by using an ultraviolet−visible (UV−vis) spectrophotometer (DRS, PE lambda 750, Waltham, MA, USA). Fourier transform infrared spectroscopy was applied to analyze the functional groups on the surface of the sample. (FT-IR, Bruker TENSOR II, Rheinstetten, Germany).
The experimental steps were as follows: First, the Waste-SCR catalysts (denoted as Waste-SCR) were pulverized and ground. Subsequently, Waste-SCR was added into the oxalic acid solution that had reached the preset temperature. After the reaction was complete, the suspension was naturally cooled to room temperature. The acid leaching solution was collected by filtration and then analyzed by ICP. The filter residue, denoted as Residue-A, was washed and dried for 12 h. The NaOH solution and oxalic acid residues were poured into a high-pressure reactor, mixed well, and sealed. After the heating program was set, stirring was performed when the preset temperature was reached. After the reaction, the reactor was cooled down to room temperature. The alkali leaching solution was collected by filtration and then analyzed by ICP. The filter residue, denoted as Residue-B, was washed and dried for 12 h. The leaching efficiency was calculated through the following equation:
where, x represents the leaching rate of the element; denotes the dilution ratio of the sample; V indicates the volume of the leaching filtrate; C refers to the concentration of each element tested by ICP-AES; m stands for the mass of the raw material sample added; and w denotes the mass percentage of each element in the raw material.
The oxalic acid leaching solution was exposed to sufficient sunlight, and the FeC2O4 precipitate was collected after one night. Of course, incident daylight was variable and intermittent. When required, the solutions were agitated by using a stirrer motor and paddle, or a hotplate stirrer and magnetic follower, before being thermostatted in a water bath. H2SO4 aqueous solution was treated as the pickling agents for Residue-B. After pickling, the precipitate was centrifuged and washed until becoming neutral. After drying, it was denoted as a sample precursor. The sample precursor was divided into two parts, with one directly calcined in a muffle furnace at 400 °C, and the other thoroughly ground for at least 1 h, after mixing with the FeC2O4 precipitate and then calcined.
The photocatalytic abilities of the regenerated TiO2 products were evaluated by measuring the degradation of Rhodamine B (RhB) aqueous solutions at 25 °C. A 350 W xenon lamp (BBZM-II, Langxi Bobei Lighting Electric Factory, Xuancheng, China) was used as the source of lighting. The specific experimental procedures are detailed as follows: Firstly, 0.05 g of photocatalysts were dissolved into 100 mL of dye solutions. Secondly, the solutions were continuously stirred for 30 min in the dark to reach adsorption equilibrium. Thirdly, the solutions were exposed to sunlight. Fourthly, the 4 mL of suspensions were collected by using a 5 mL pipetting gun at intervals. Fifthly, the suspensions were centrifuged at 4000 rpm for 15 min and filtered through a 0.22 μm filter. Finally, the supernatants were analyzed using a UV-Vis spectrophotometer (UV-2401PC, Zhejiang Huacong Instrument Co., Ltd., Hangzhou, China) to evaluate the photodegradability of the samples. The photocatalytic degradation rate was calculated through the following formula:
where represents the initial absorbance of the degraded product; A represents the absorbance of the degraded product at the end of catalysis; η represents the degradation rate of the degraded product at the end of catalysis.
3. Results
3.1. Characterization of Waste-SCR
The results of XRF analysis for Waste-SCR are shown in Table 1. The Waste-SCR is mainly composed of the carrier TiO2 and the active agent WO3, accounting for 85.49% and 4.38%, respectively. The active component V2O5 accounts for 0.65%. In addition, there are small amounts of various impurity elements such as Fe, Al, Si, etc. The XRD pattern of Waste-SCR is shown in Figure 1, from which it can be seen that the diffraction peaks of Waste-SCR are completely consistent with the diffraction peaks of anatase TiO2. At the same time, there are no diffraction peaks observed for V2O5, WO3, and other impurities, which may be due to the fact that the content of components other than TiO2 being less than 5%, and are widely distributed on the surface of the TiO2 support.

Table 1.
Chemical compositions (wt %) of Waste-SCR.
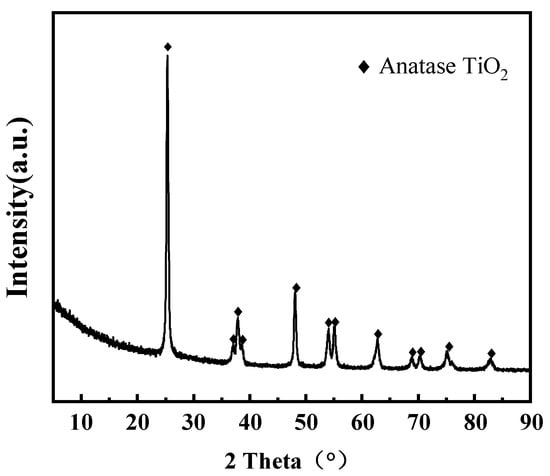
Figure 1.
XRD spectra of Waste-SCR catalysts.
3.2. Mechanism and Optimization of Leaching Process
3.2.1. Leaching Process Mechanism
In the Waste-SCR catalyst, element V exists in the form of V2O5. Due to the strong reducibility and high coordination ability of oxalic acid, V can be involved in redox and dissolution coordination reactions with oxalic acid [,]. As shown in Equation (3), stable VO(C2O4)22− complexes are generated. However, the valence state of W and Ti elements remains unchanged in the reducing acid solution. The leaching process is confined to dissolving WO3 and TiO2. A small amount of dissolved WO3 and TiO2 exist in acidic solutions as WO22+ and TiO2+, respectively. In the oxalic acid system, WO22+ and TiO2+ tend to be combined with the complexing agent oxalate, thus forming complexes such as WO2(C2O4)22− and TiO(C2O4)22−. Equations (4) and (5) show the dissolution reaction of W and Ti.
V2O5 + 5H2C2O4 = 2H2VO(C2O4)2 + 3H2O + 2CO2
WO3 + 2H2C2O4 = H2WO2(C2O4)2 + H2O
TiO2 + 2H2C2O4 = H2TiO(C2O4)2 + H2O
In the alkaline leaching process, WO3 reacts in strong alkaline solution to form soluble tungstate []. TiO2 does not form soluble matter in the alkaline solution system, which reacts with NaOH to form insoluble Na2TiO3 []. The reaction formula of W, Ti, and NaOH can be expressed as Equations (6) and (7).
2NaOH + TiO2 = Na2TiO3 + H2O
2NaOH + WO3 = Na2WO4 + H2O
Figure 2 shows a thermodynamic analysis of Equations (1), (4) and (5). According to Figure 2a, all the values of standard Gibbs free energy are negative, suggesting a greater spontaneous trend for the leaching of vanadium in oxalic acid solution, and the leaching of tungsten and titanium in NaOH solution. In addition, Equation (4) shows a larger absolute value of Gibbs free energy change than Equation (5), indicating that WO3 is more likely to react with NaOH than TiO2. As shown in Figure 2b, the enthalpy changes for the three leaching reactions are all negative, implying that they are all exothermic. Consequently, it is thermodynamically feasible to recover V by means of leaching with oxalic acid, and recover W and Ti by means of leaching with NaOH.
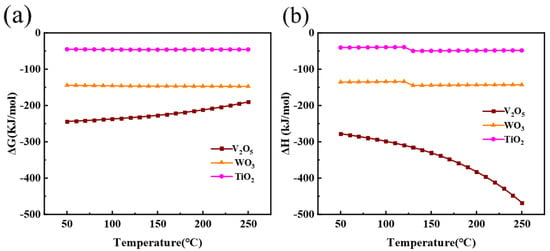
Figure 2.
Thermodynamic calculations during oxalic acid leaching and alkali leaching. (a) Relationship between Gibbs free energy ΔG and temperature; (b) Relationship between enthalpy change ΔH and temperature.
3.2.2. Optimization of the Leaching Process
Figure 3a shows the effect produced by oxalic acid concentration on Residue-A under the following conditions: a reaction time of 3 h; a reaction temperature of 60 °C; and a H2C2O4 -Waste-SCR ratio of 10 mL/g. When H2C2O4 concentration increased from 0.5 mol/L to 1.0 mol/L, the leaching rate rose from 61.8% to 69.3% for V, and from 4.3% to 10.4% for W. When the oxalic acid concentration exceeded 1.0 mol/L, the leaching rate of V barely changed, while that of W continued increasing slowly with the rise of oxalic acid concentration. The leaching rate of Ti was consistently below 0.5%. In order to facilitate subsequent operations and recover high-purity V, we needed to control the leaching rate of W, while ensuring the efficient leaching of V. Therefore, the optimal H2C2O4 concentration was set to 1 mol/L. According to the XRD patterns in Figure 3b, Residue-A shows only the diffraction peak of anatase TiO2 at different H2C2O4 concentrations.
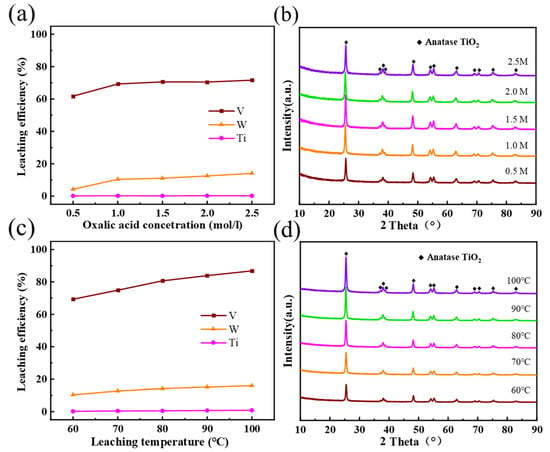
Figure 3.
(a) Effect of H2C2O4 concentration on the V, W, and Ti leaching efficiencies. (b) XRD patterns of Residue-A at different H2C2O4 concentration. (c) Effect of reaction temperature on the V, W, and Ti leaching efficiencies. (d) XRD patterns of Residue-A at different reaction temperatures.
Figure 3c shows the effect caused by reaction temperature on Residue-A under the following conditions: an oxalic acid concentration of 1.0 mol/L; a reaction time of 3 h; and a H2C2O4 -Waste-SCR ratio of 10 mL/g. The leaching rate of V increased rapidly with temperature rise, from 69.3% at 60 °C to 86.8% at 100 °C. By comparison, the leaching rate of W and Ti reached their maximum at 100 °C, which is 16.0% and 0.89%, respectively. This is because the ion diffusion rate between oxalic acid and the spent catalyst increases with temperature rise, which accelerates the dissolution of the waste catalyst. Nevertheless, when the leaching temperature reached above 100 °C, the reaction solution was close to boiling. Consequently, oxalic acid volatilized in large amounts and thermal decomposition occurred []. Thus, 100 °C was chosen as the optimal reaction temperature. As can be seen from the XRD patterns in Figure 3d, only anatase TiO2 was detected at different temperatures, which evidences that the leaching of oxalic acid made no difference to the phase structure of the sample within the selected concentration and temperature ranges.
Figure 4a shows the effect of NaOH concentration on Residue-B under the following conditions: a reaction time of 3 h; a reaction temperature of 150 °C; and a NaOH -Residue-A ratio of 10 mL/g. When NaOH concentration reached 2 mol/L, the leaching of W was merely 52.6%. With the increase of alkali concentration, leaching rate increased rapidly for W. When alkali concentration reached 8 mol/L, the W leaching rate was 98.6%, which is close to 100%. At this time, the W leaching rate hardly changed, given a continued increase of alkali concentration; therefore, the optimal concentration of NaOH was set to 8 mol/L. Figure 4b shows the XRD patterns of slag B under different NaOH concentrations. From this figure, it can be clearly seen that the diffraction peak of anatase TiO2 is gradually weakened with the rise of concentration. Residue-B is in amorphous state at a higher concentration than 8 mol/L, which indicates that the transformation of TiO2 into amorphous titanate is almost complete.
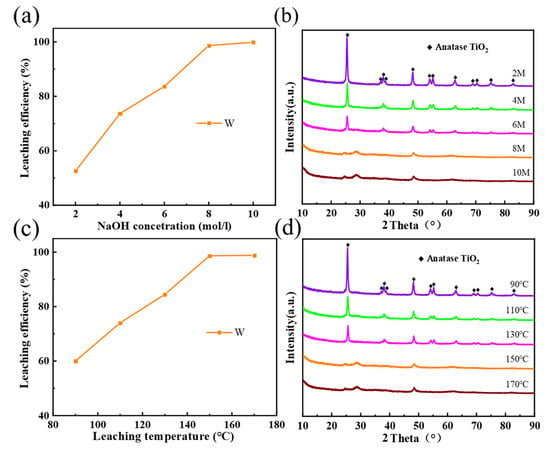
Figure 4.
(a) Effect of NaOH concentration on the W leaching efficiencies. (b) XRD patterns of Residue-B at different NaOH concentrations. (c) Effect of reaction temperature on the W leaching efficiencies. (d) XRD patterns of Residue-B at different reaction temperatures.
Figure 4c shows the effect of reaction temperature on Residue-B under the following conditions: a NaOH concentration of 8.0 mol/L; a reaction time of 3 h; and a NaOH -Residue-A ratio of 10 mL/g. As reaction temperature rose, the leaching rate of W increased rapidly. At 90 °C, the leaching rate of W reached merely 60.0%. When the temperature further increased to 150 °C, the leaching rate of W reached 99.6%. As the temperature continued its upward trend, the leaching rate of W increased, thus adding to unnecessary energy consumption and equipment burden; therefore, the optimal leaching temperature was set to 150 °C. Figure 4d shows the XRD patterns of Residue-B at different reaction temperatures. With the increase of reaction temperature, the diffraction peak of anatase TiO2 in Residue-B was gradually weakened, indicating a gradual decrease in the content of anatase TiO2 crystals in slags. When the temperature reached above 150 °C, the characteristic peak of TiO2 diffraction vanished, and amorphous titanate was generated.
3.2.3. Composition and Structure Analysis of Residues
To better understand the leaching process, the residues were also analyzed before and after leaching. Table 2 shows the chemical composition of Residue-A and Residue-B. It can be seen from this table that the TiO2 content is 89.42% and 88.28% for Residue-A and Residue-B, respectively, both of which are slightly higher compared to Waste-SCR. The content of V2O5 and Fe2O3 fell below 0.11% after leaching with oxalic acid, which suggests that oxalic acid exerted a significant leaching effect on both V and Fe. In addition, the content of WO3 was merely 0.10%, and the molding aids (Al2O3, SiO2, CaO) were effectively removed after the leaching of sodium hydroxide, indicating that the residue was completely purified.

Table 2.
Chemical compositions (wt %) of Residue-A and Residue-B.
Figure 5 shows the FT-IR spectra of different leached residues. The bands observed in the range of 796−438 cm−1 are assigned to Ti−O and Ti−O−Ti groups []. The absorption band at 887 cm−1 can be observed in Reside-B, which is attributed to the vibration of Ti-O-Na []. It is shown that NaOH reacts with the main phase TiO2, which destroys the Ti-O-Ti bond. In addition, the bands observed at 2923, 2852, 2347, and 1633 cm−1 can be attributed to the strong interaction between -OH groups and Ti4+ []. The absorption peaks at 1122 cm−1 and 1063 cm−1 are attributed to the characteristic peaks of SO42− and the stretching vibration peaks of ν(S-O), formed by the interaction of SO42− species and TiO2 surface, respectively []. After leaching with oxalic acid, the characteristic peaks of SO42- disappeared, implying that oxalic acid is effective in removing the sulfate that blocked the pores, rather than the SO42− species acting on the surface of TiO2. However, after leaching with sodium hydroxide, the ν(S-O) stretching vibration peak vanished, indicating that the surface structure of TiO2 was affected by NaOH, and the surface structure was reorganized. Consequently, SO42− was completely removed.
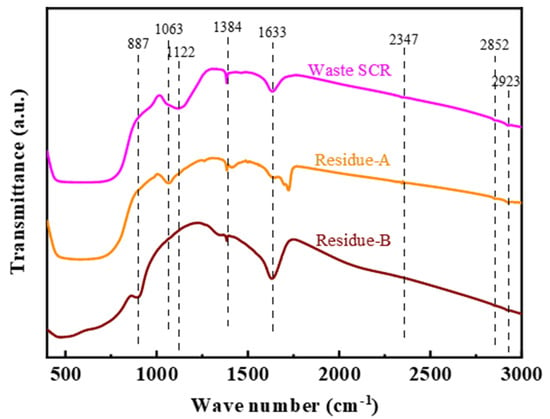
Figure 5.
FT-IR results for Waste-SCR, Residue-A, and Residue-B.
Figure 6a shows the N2 adsorption-desorption isotherms of the slags. According to the classification of IUPAC, all slags exhibited type IV adsorption isotherms, indicating that the slags were mesopores []. The N2 adsorption-desorption isotherms of Waste-SCR and H2C2O4 residue were basically identical, with a narrow hysteresis loop shown by both. However, after alkali leaching, the hysteresis loop of the slags was elongated. NaOH residues manifested the H3 hysteresis loop of a typical flake granular material. Figure 6b shows the BJH pore size distribution of the slags. According to this figure, the pore size of Waste-SCR and H2C2O4 residue is concentrated in the range of 5–46 nm, which falls within the typical mesoporous range. The pore size of NaOH residue is concentrated in the range of 3–5 nm, which falls within the range of small mesopores. The pore structure parameters of different residues are listed in Table 3.
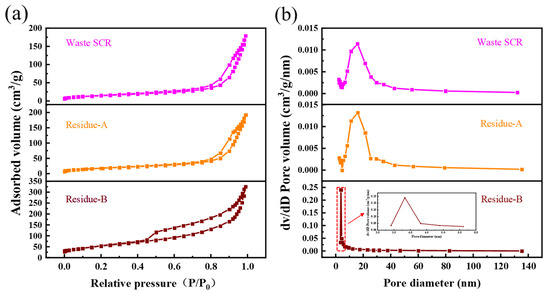
Figure 6.
BET results for Waste-SCR, Residue-A, and Residue-B: (a) N2 adsorption-desorption isotherms; and (b) BJH pore size distribution.

Table 3.
Pore structure parameters for Waste-SCR, Residue-A, and Residue-B.
These results demonstrate that the acid leaching process will not undermine the original pore structure, and only removes part of the particles that block the pore. In contrast, in the strong alkali system, the Ti-O-Ti bonds in anatase TiO2 collapse, and these broken Ti-O bonds can be attacked by Na+ []. As a result, Ti-O-Na bonds are formed and the pore structure of the slag is altered. In addition, impurities in the pores are released and fully exposed to the strong alkaline system for dissolution; therefore, the residues are effectively purified. Ultimately, amorphous sodium titanate is generated.
3.3. Characterization and Activity Test of Regenerated Photocatalysts
3.3.1. Acquisition of Regenerated Photocatalyst Dopants
A photochemical decomposition method was employed to obtain Fe dopants. In the process of H2C2O4 leaching, it is known through calculation that the leaching rate of impurity Fe2O3 is comparable to that of V2O5, reaching up to 85.3%, as shown in Table 2. Fe2O3 undergoes redox reaction in oxalic acid solution, thus forming the complex Fe(C2O4)22− existent in the solution []. Equation (8) shows the redox reaction of Fe with oxalic acid. However, the stability constant of the Fe(C2O4)22− complex is only 104.52, and the stability in solution is low []. FeC2O4 can be slowly precipitated from oxalic acid leaching solution after exposure to sunlight for a long time. Figure 7 shows the XRD pattern of precipitation of oxalic acid leaching solution after standing. The main components of precipitation are determined as FeC2O4·2H2O and CaC2O4·H2O. After digestion of the precipitate, the content of FeC2O4·2H2O was measured by ICP to be 71.04%. We then further purified treated as-obtained Na2TiO3 with dilute sulfuric acid, to obtain Re-TiO2 precursor. Finally, after ferrous oxalate doping and calcination, the regenerated TiO2 samples could be applied in environmental pollutant.
Fe2O3 + 5H2C2O4 = 2H2Fe(C2O4)2 + 3H2O + 2CO2
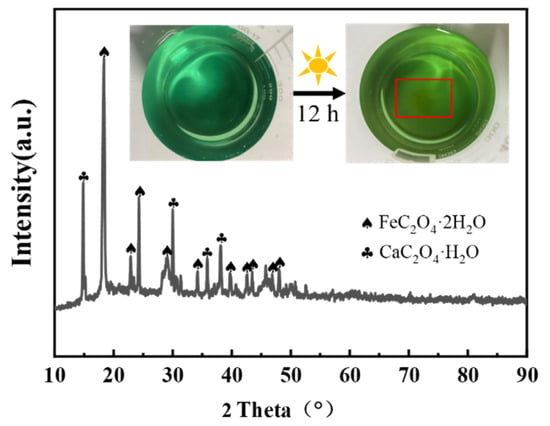
Figure 7.
XRD spectra of precipitates from H2C2O4 leachate after standing.
3.3.2. Characterization of Regenerated Photocatalysts
Table 4 shows the chemical composition of regenerated photocatalysts. The percentage of TiO2 contained in Sample-A and Sample-B is 98.16% and 97.38%, respectively, which means that the samples obtained in our work meet the requirements for commercial purposes. In addition, the main impurity in Sample-B is Fe2O3, with the content of other impurities being less than 0.5%.

Table 4.
Chemical compositions (wt %) of Sample-A and Sample-B.
The morphology and structure of Waste-SCR and Sample-B were characterized by TEM, as shown in Figure 8. They are comprised of nano spherical particles. The crystal size of the Waste-SCR varies from 20 to 40 nm. By contrast, the crystal size of Sample-B ranges only from 6 to 12 nm. A smaller particle size is conducive to the generation of photogenerated carriers and the adsorption of reactants on the surface, thereby improving the photocatalytic performance. In addition, XPS analysis was also conducted to explore the chemical oxidation of regenerated photocatalysts. As shown in Figure 9, there are two intense peaks observable for the Ti2p core at the binding energies of 464.4 eV and 458.7 eV in Sample-A and Sample-B, which is attributable to the peaks of 2p1/2 and 2p3/2, respectively, for TiO2 []. In the O (1s) spectrum, the peaks of Sample-A at 530.0 and 531.8 eV are attributable to the lattice oxygen and the hydroxyl groups [], respectively. Compared with Sample-A, the lattice oxygen of Sample-B shifts by 0.25 eV towards higher electron binding energies. This phenomenon is probably attributed to the formation of Ti-O-Fe bonds in the crystal lattice []. In Sample-B, Fe 2p XPS peaks appear at 709.7 and 723.0 eV, which is ascribed to the binding energies of Fe 2p3/2 and Fe 2p1/2, respectively. It is indicated that the dopant of Fe is in the chemical state of Fe3+ in the sample [].
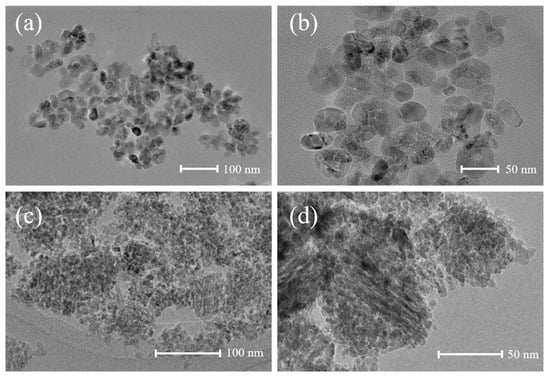
Figure 8.
(a) TEM image of Waste-SCR at 100 nm. (b) TEM image of Waste-SCR at 50 nm. (c) TEM image of Sample-B at 100 nm. (d) TEM image of Sample-B at 50 nm.
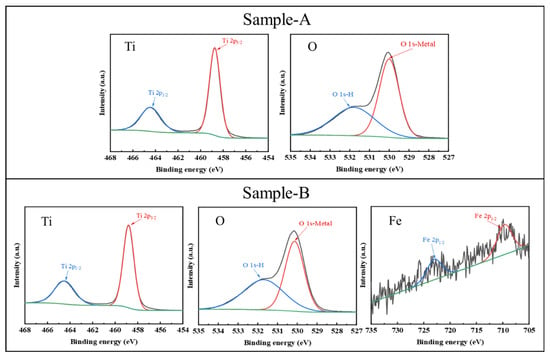
Figure 9.
XPS spectra of Sample-A and Sample-B for Ti 2p, O 1s, and Fe 2p peaks.
The optical properties of the regenerated catalysts were characterized by UV-VIS absorption spectroscopy. As shown in Figure 10a, Sample-A exhibits an increased absorbance in the broad UV−vis region, compared to the pure P25. After doping with iron, the regenerated catalyst exhibited a further red shift, which can be attributed to the Fe3+ doping that led to a more efficient transfer and separation of photogenerated electrons and holes. According to the Tauc Plot method, band gap energy can be estimated from the plot of (Ahν)1/2 versus photo energy (hν), as shown in Figure 10b. The band gap value of P25 is 2.82 eV. Relative to P25, the band gap value of Sample-A is 2.59 eV, which suggests a narrow band gap. After doping with iron, the band gap of the regenerated catalyst was further reduced to 2.43 eV, which is attributed to the bond formed between Fe-O-Ti and excited photon energies reduction. This resulted in low band gap energy [].
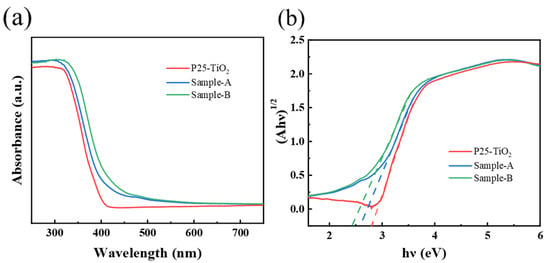
Figure 10.
(a) The UV-vis DRS spectra of P25-TiO2 and Sample-A and Sample-B; (b) band gap energy spectra of P25-TiO2 and Sample-A and Sample-B.
3.3.3. Evaluation of Photocatalytic Activities
Photocatalytic efficiency of the regenerated photocatalysts and P25-TiO2 for degrading dyes (RhB) was evaluated through the simulation of sun-light irradiation, as shown in Figure 11. P25-TiO2, Sample-A and Sample-B photodegraded 73.2%, 82.3%, and 94.7% of 10 mg/L RhB, respectively, under stimulated sun-light for 40 min. When RhB concentration increased to 20 mg/L, more than 84.2% of dye was photodegraded in the presence of Sample-B for 60 min, in comparison with 65.4% and 72.9% of the dye degraded by P25-TiO2 and Sample-A for 60 min, respectively. On this basis, the apparent rate constant (κapp) for the photocatalytic oxidation of RhB with different products was evaluated using a pseudo-first-order model, which is expressed as follows:
where, κapp represents the apparent rate constant; Ct denotes the solution-phase concentration of dyes; C0 refers to the initial concentration at t = 0; and t stands for the reaction time. The values of apparent rate constant κapp are also listed in Figure 11.
ln(C0/Ct) = κapp t
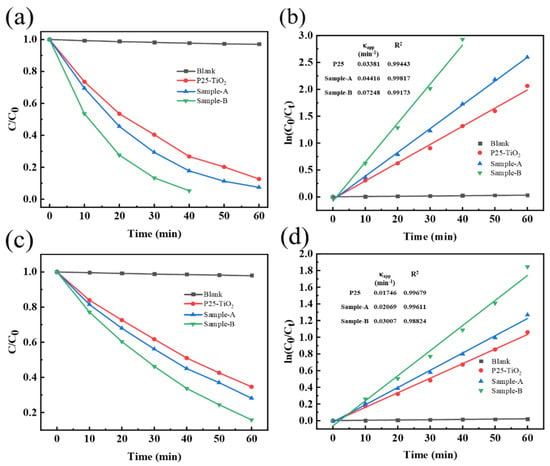
Figure 11.
Photodegradation of: (a) 10 mg/L RhB; and (c) 20 mg/L RhB under sun-light irradiation. Photocatalytic reaction kinetics of: (b) 10 mg/L RhB; and (d) 20 mg/L RhB as a function of reaction time.
As shown in the table as an inset of Figure 11, the slope of Sample-B (0.0725 min−1) is 2.14 and 1.64, respectively, times that of P25-TiO2 and Sample-A for the photodegradation of 10 mg/L RhB. Meanwhile, the slope of Sample-B at a dye concentration of 20 mg/l reached a level that is 1.72 and 1.45 times higher, compared to P25-TiO2 and Sample-A, respectively. According to photodegradation results, the samples doped with Fe produced a better photocatalytic performance.
The mechanism of enhanced photocatalytic activity can be summarized as: a small amount of Fe3+ can inhibit the recombination of photoexcited electrons and holes, and acts as a temporary photo-generated electron or hole-trapping site []. The reaction steps of regenerated samples can be expressed as follows []:
TiO2 + hν → h+ + e−
Fe3+ + e− → Fe2+
Fe3+ + h+ → Fe4+
Fe2+ + O2 → Fe3+ + O2•−
Fe4+ + OH− → •OH + Fe3+
Firstly, an electron−hole pair is created in regenerated TiO2 products under sun-light irradiation. Subsequently, part of the Fe3+ ions can capture photogenerated electrons to form Fe2+ ions, and another part of the Fe3+ ions can also react with holes to form Fe4+ ions. Fe4+ and Fe2+ are relatively unstable compared to Fe3+ with half-filled d orbitals (d5). Consequently, there is a tendency that Fe2+ and Fe4+ react with adsorbed O2 and surface hydroxyl groups (−OH), respectively, to form highly reactive species O2•− and •OH, and regenerate Fe3+. After several repeated O2•− and •OH attacks, dye degrades to produce CO2 and H2O.
3.4. Flow Sheet Establishment
Based on the experiments, a flow sheet was established, as shown in Figure 12. With regards to the oxalic acid leaching step, 86.8 % of the vanadium leaching efficiency was obtained with a H2C2O4 addition of 1 mol/L, at a leaching temperature of 100 °C. Meanwhile, 85.3% of iron impurity was also leached. Subsequently, the iron impurity was successfully precipitated as a value-added product of FeC2O4·2H2O, with a content of 71.04%. With regards to the NaOH leaching step, 98.6 % of the tungsten leaching efficiency was obtained with a NaOH addition of 8 mol/L, at a leaching temperature of 150°C. Residue-B was washed with dilute H2SO4 to obtain a regenerated photocatalyst precursor. Alternatively, the precursor was directly calcined to obtain Sample-A with 98.16% TiO2, or it was mixed with FeC2O4 and calcined to obtain Sample-B, with 97.38% TiO2 and 0.64% Fe2O3. Finally, the regenerated TiO2 samples could be directly used for efficient degradation of dyes under sunlight.
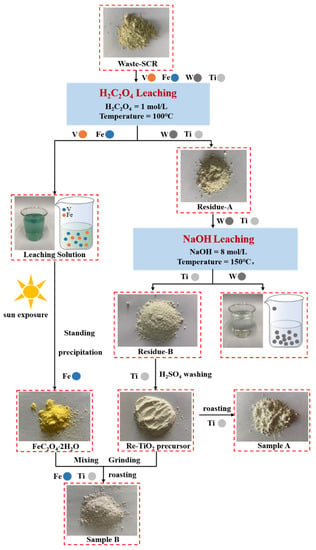
Figure 12.
Flow sheet for recovery of V and W, and regeneration of TiO2 photocatalyst.
4. Conclusions
In this paper, a clean, eco-friendly, and novel method was proposed to recycle V and W, and prepare TiO2 photocatalysts regenerated from Waste-SCR catalysts. With regards to the acid-alkaline leaching process, the optimum conditions of oxalic acid leaching were finalized as 100°C and 1.0 mol/L, which led to the leaching rate of 86.8% for V. The optimum conditions were 150°C and 8.0 mol/L for sodium hydroxide leaching, with the leaching rate of W reaching 98.6% under these conditions. Oxalic acid leaching made no difference to the phase structure of the residue. After alkali leaching, anatase TiO2 undergoes structural reorganization and transforms into amorphous titanates with a high specific surface area. In addition, sulfate impurities bound to the surface of TiO2 and molding aids were effectively removed, which led to effective purification. The content of the regenerated TiO2 product was higher than 97.3%, and thus met commercial requirements. The FeC2O4 precipitated during the acid leaching process was doped into the regenerated TiO2 product as an iron source, thereby improving the photocatalytic performance. Regenerated TiO2 photocatalysts exhibited a higher photocatalytic efficiency than P25-TiO2 to oxidize RhB under simulated solar irradiation. This experimental process contributes a complete, eco-friendly, and economical method to the recycling of Waste-SCR catalysts.
Author Contributions
Methodology, K.C. and L.X.; validation, K.C., Y.L. and L.X.; formal analysis, B.M. and Y.Y.; data curation, K.C.; writing—original draft preparation, K.C.; writing—review and editing, Y.Y.; visualization, B.M.; supervision, Y.L. and L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kompio, P.G.W.A.; Brueckner, A.; Hipler, F.; Manoylova, O.; Auer, G.; Mestl, G.; Gruenert, W. V2O5-WO3/TiO2 catalysts under thermal stress: Responses of structure and catalytic behavior in the selective catalytic reduction of NO by NH3. Appl. Catal. B-Environ. 2017, 217, 365–377. [Google Scholar] [CrossRef]
- Dai, Z.J.; Wang, L.L.; Tang, H.; Sun, Z.J.; Liu, W.; Sun, Y.; Su, S.; Hu, S.; Wang, Y.; Xu, K.; et al. Speciation analysis and leaching behaviors of selected trace elements in spent SCR catalyst. Chemosphere 2018, 207, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, Q.; Lin, Z.; Li, W.; Dong, C. Research progress of element recovery of waste De-NOx SCR catalyst from coal-fired power plants. Chem. Ind. Eng. Prog. 2016, 35, 3306–3312. [Google Scholar]
- Ismael, M.H.; El Hussaini, O.M.; El-Shahat, M.F. New method to prepare high purity anatase TiO2 through alkaline roasting and acid leaching from non-conventional minerals resource. Hydrometallurgy 2020, 195, 105399. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Liu, Q. Kinetics of vanadium leaching from a spent industrial V2O5/TiO2 catalyst by sulfuric acid. Ind. Eng. Chem. Res. 2014, 53, 2956–2962. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Liu, N.N.; Xu, X.Y.; Liu, Y. Recovery of vanadium and tungsten from spent selective catalytic reduction catalyst by alkaline pressure leaching. Physicochem. Probl. Miner. Processing 2020, 56, 407–420. [Google Scholar] [CrossRef]
- Han, G.; Wang, H.; Su, S.; Huang, Y.; Liu, B. Research progress and discussion on selective separation technology of dissolved tungsten and vanadium. Chin. J. Nonferrous Met. 2021, 31, 3380–3395. [Google Scholar]
- Kamegawa, T.; Yamashita, H. Development of nanostructured titania-based photocatalysts and their applications. J. Jpn. Pet. Inst. 2019, 62, 97–105. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Le, Q.V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Julkapli, N.M.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, D.; Du, J.; Xie, Y. Low temperature self-assembled growth of rutile TiO2/manganese oxide nanocrystalline films. Appl. Surf. Sci. 2017, 420, 489–495. [Google Scholar] [CrossRef]
- Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Sever Skapin, A. Hydrothermal synthesis of rare-earth modified titania: Influence on phase composition, optical properties, and photocatalytic activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Ao, W.; Wang, J.; Hou, X. A simple route to synthesize mesoporous titania from TiOSO4: Influence of the synthesis conditions on the structural, pigments and photocatalytic properties. Appl. Surf. Sci. 2016, 376, 227–235. [Google Scholar] [CrossRef]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, G.Q.; Zhang, Y.K.; Ma, Y.; Zhang, Q.K.; Pu, H.Q.; Xu, W.J.B.; Gao, D.K.; Wang, B.Z.; Qi, X.W. Preparation of RGO/TiO2 photocatalyst and the mechanism of its hydrothermal process. J. Chin. Chem. Soc. 2019, 66, 734–739. [Google Scholar] [CrossRef]
- Bruyere, V.I.E.; Rodenas, L.A.G.; Morando, P.J.; Blesa, M.A. Reduction of vanadium(v) by oxalic acid in aqueous acid solutions. J. Chem. Soc. Dalton Trans. 2001, 24, 3593–3597. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zhao, Z.-w. Thermodynamic analysis for separation of tungsten and vanadium in W(VI)-V(V)-H2O system. Chin. J. Nonferrous Met. 2014, 24, 1656–1662. [Google Scholar]
- Feng, Y.; Wang, J.; Wang, L.; Qi, T.; Xue, T.; Chu, J. Decomposition of acid dissolved titanium slag from Australia by sodium hydroxide. Rare Met. 2009, 28, 564–569. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Yang, Y. Separation and recovery of iron impurity from a vanadium-bearing stone coal via an oxalic acid leaching-reduction precipitation process. Sep. Purif. Technol. 2017, 180, 99–106. [Google Scholar] [CrossRef]
- Qu, A.; Xie, H.; Xu, X.; Zhang, Y.; Wen, S.; Cui, Y. High quantum yield graphene quantum dots decorated TiO2 nanotubes for enhancing photocatalytic activity. Appl. Surf. Sci. 2016, 375, 230–241. [Google Scholar] [CrossRef]
- Yan, X.; Sun, D.; Jiang, J.; Yan, W.; Jin, Y. Self-assembled twine-like Na2Ti3O7 nanostructure as advanced anode for sodium-ion batteries. J. Alloys Compd. 2017, 697, 208–214. [Google Scholar] [CrossRef]
- Becker, I.; Hofmann, I.; Mueller, F.A. Preparation of bioactive sodium titanate ceramics. J. Eur. Ceram. Soc. 2007, 27, 4547–4553. [Google Scholar] [CrossRef]
- Orsenigo, C.; Lietti, L.; Tronconi, E.; Forzatti, P.; Bregani, F. Dynamic investigation of the role of the surface sulfates in NOx reduction and SO2 oxidation over V2O5-WO3/TiO2 catalysts. Ind. Eng. Chem. Res. 1998, 37, 2350–2359. [Google Scholar] [CrossRef]
- Yang, S.; Xu, D.; He, S.; Yan, W.; Xiong, Y. Modified Fe-rich palygorskite as an efficient and low-cost heterogeneous fenton catalyst for NOx and SO2 removal. Energy Fuels 2020, 34, 8493–8502. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Lee, S.O.; Tran, T.; Jung, B.H.; Kim, S.J.; Kim, M.J. Dissolution of iron oxide using oxalic acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar] [CrossRef]
- Krishnamurty, K.V.; Harris, G.M. The chemistry of the metal oxalato complexes. Chem. Rev. 1961, 61, 213–246. [Google Scholar] [CrossRef]
- Sharma, A.; Thuan, D.V.; Pham, T.-D.; Tung, M.H.T.; Truc, N.T.T.; Vo, D.-V.N. Advanced surface of fibrous activated carbon immobilized with FeO/TiO2 for photocatalytic evolution of hydrogen under visible light. Chem. Eng. Technol. 2020, 43, 752–761. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, Y.; Wu, Y.; Zhang, Y.-N.; Zuo, T. Low-cost Y-doped TiO2 nanosheets film with highly reactive {001} facets from CRT waste and enhanced photocatalytic removal of Cr(VI) and methyl orange. ACS Sustain. Chem. Eng. 2016, 4, 1794–1803. [Google Scholar] [CrossRef]
- Meng, H.; Wang, B.; Liu, S.; Jiang, R.; Long, H. Hydrothermal preparation, characterization and photocatalytic activity of TiO2/Fe–TiO2 composite catalysts. Ceram. Int. 2013, 39, 5785–5793. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Wan, F.; Zheng, H.; Zhang, X. Synergistic effect of surface self-doping and Fe species-grafting for enhanced photocatalytic activity of TiO2 under visible-light. Appl. Surf. Sci. 2017, 396, 26–35. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhao, X.; Zhang, Q. Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J. Photochem. Photobiol. A Chem. 2006, 182, 121–127. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).