The Potential of a Novel Concept of an Integrated Bio and Chemical Formulate Based on an Entomopathogenic Bacteria, Bacillus thuringiensis, and a Chemical Insecticide to Control Tomato Leafminer, Tuta absoluta ‘(Meyrick)’ (Lepidoptera: Gelechiidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticides
2.3. Bio and Chemical Insecticide Combinations
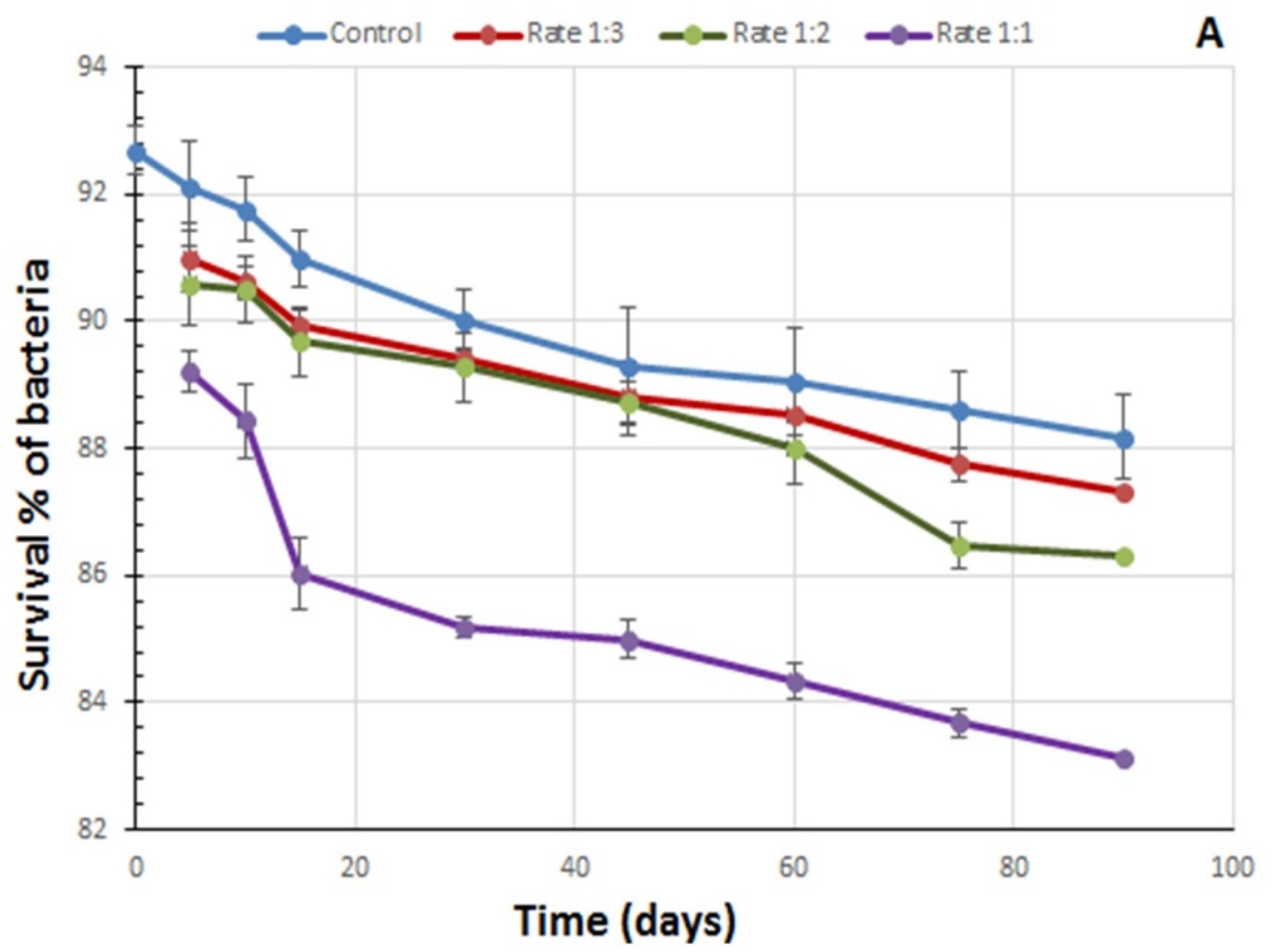
2.4. Bacterial Viability
2.5. Plate Counting and Turbidity Measurement Techniques
2.6. Baclight Viability Technique
2.7. Preparation of Live/Dead Cultures of Bacteria
2.8. Staining and Fluorescence Measurement
2.9. Physicochemical Properties of the Original and the Highest Mixing Ratio of Lab-Made Combined Formulate
2.9.1. Wettability Test–CIPAC F-MT 15.2
2.9.2. Suspensibility and Persistent Foam Test–CIPAC-F-MT15, MT184, and MT172
2.10. Bioassay
2.10.1. Original Insecticide Suspensions
2.10.2. Suspensions of the Lab-Made Fortified Formulates
2.10.3. Leaf-Dip Bioassay
2.11. Statistical Analysis
3. Results
3.1. Influence of Lambda-Cyhalothrin on the Viability of B. thuringiensis Strains in the Lab-Made Combined Formulates
3.2. Effect of the Proposed Combination on the Physicochemical Properties of the Tested Formulation
3.3. Potency of the Original Insecticide Formulations on the Second Instar Larvae of T. absoluta
3.4. Potency of the Lab-Made Fortified Formulates on the Second Instar Larvae of T. absoluta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brévault, T.; Sylla, S.; Diatte, M.; Bernadas, G.; Diarra, K. Tuta absoluta Meyrick (Lepidoptera, Gelechiidae), A new threat to tomato production in Sub-Saharan Africa. Afr. Entomol. 2014, 22, 441–444. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J. Biological invasion of European tomato crops by Tuta absoluta, ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- EPPO. Tuta absoluta Continues to Spread Around the Mediterranean Basin. 2011. Available online: https://gd.eppo.int/reporting/article-193 (accessed on 20 May 2019).
- Van Wilgen, B.W.; Measey, J.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological Invasions in South Africa: An Overview. In Biological Invasions in South Africa; Invading Nature—Springer Series in Invasion Ecology; VanWilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Cham, Switzerland, 2020; Volume 14. [Google Scholar]
- Tarusikirwa, V.L.; Machekano, H.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives. Insects 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Rwomushana, I.; Beale, T.; Chipabika, G.; Day, R.; Gonzalez-Moreno, P.; Lamontagne-Godwin, J.; Makale, F.; Pratt, C.; Tambo, J. Evidence Note, Tomato leafminer (Tuta absoluta), impacts and coping strategies for Africa. CABI Work. Pap. 2019, 12, 56. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanco, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; del Rosario Martínez-Aguirre, M.; Rison, J.L.; Haxaire-Lutun, M.O.; Nauen, R.; Tsagkarakou, A.; Bielza, P. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leafminer T. absoluta in the European/Asian region. J. Pest Sci. 2018, 91, 421–435. [Google Scholar] [CrossRef]
- Lietti, M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Picanco, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera, Gelechiidae). Agric. For. Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Fragoso, D.D.B.; Magalhaes, L.C. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae). Int. J. Pest Manag. 2001, 47, 247–251. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Michaelides, G.; Seraphides, N.; Pitsillou, M.; Sfenthourakis, S. Susceptibility of Cypriot Tuta absoluta populations to four targeted insecticides and control failure likelihood. J. Appl. Entomol. 2019, 143, 508–517. [Google Scholar] [CrossRef]
- Grant, C.; Jacobson, R.; Ilias, A.; Berger, M.; Vasakis, E.; Bielza, P.; Zimmer, C.T.; Williamson, M.S.; Ffrench-Constant, R.H.; Vontas, J.; et al. The evolution of multiple-insecticide resistance in UK populations of tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2019, 75, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Rocha, K.; Alarcón, L.; Miegwart, M.; Sauphanor, B. Metabolic mechanisms involved in the resistance of field populations of Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae) to Spinosad. Pesticide Biochem. Physiol. 2012, 102, 45–50. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Cifuentes, D.; Field, L.M.; Gorman, K.; Rapisarda, C.; Williamson, M.S.; Bass, C. Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem. Mol. Biol. 2012, 42, 506–513. [Google Scholar] [CrossRef]
- Bala, I.; Mukhtar, M.M.; Saka, H.K.; Abdullahi, N.; Ibrahim, S.S. Determination of Insecticide Susceptibility of Field Populations of Tomato Leaf Miner (Tuta absoluta) in Northern Nigeria. Agriculture 2019, 9, 7. [Google Scholar] [CrossRef]
- Das, S.K. Scope and Relevance of using Pesticide Mixtures in Crop Protection, A Critical Review. Inter. J. Environ. Sci. Toxicol. Res. 2014, 2, 119–125. [Google Scholar]
- OEPP/EPPO. Insecticide co-formulated mixtures. EPPO Bull. 2012, 42, 353–357. [Google Scholar] [CrossRef]
- Phokela, A.; Singh, S.P.; Mehrotra, K.N. Effect of synergists on pyrethroids toxicity in adults of Helicoverpa armigera (Hubner). Pesticide Res. J. 1999, 11, 62–64. [Google Scholar]
- Walung, R.; Scott, P. Rational use of mixture in crops. Environ. Entomol. 2003, 11, 85–89. [Google Scholar]
- Khan, H.A.A.; Akram, W.; Shad, S.A.; Lee, J. Insecticide mixtures could enhance the toxicity of insecticides in a resistant dairy population of Musca domestica L. PLoS ONE 2013, 8, e60929. [Google Scholar]
- Martin, T.; Ochou, O.G.; Vaissayre, M.; Fournier, D. Organophosphorus insecticides synergise pyrethroids in the resistant strain of cotton bollworm, Helicoverpa armigera (Lepidoptera, Noctuidae) from West Africa. J. Econ. Entomol. 2003, 92, 468–474. [Google Scholar] [CrossRef]
- US-EPA. Pesticide Product Label, Voliam Xpress Insecticide; USEPA: Washington, DC, USA, 2011. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/000100-01320-20111205.pdf (accessed on 3 December 2019).
- US-EPA. Pesticide Product Label, Voliam Flexi Insecticide; USEPA: Washington, DC, USA, 2014. Available online: https://www3.epa.gov/pesticides/chem_search/ppls/000100-01319-20140108.pdf (accessed on 3 December 2019).
- Reda, M.; Mashtoly, T.A.; El-Zemaity, M.S.; Abolmaaty, A.; Abdelatef, G.M. Phylogenic and antagonistic characteristics of novel Bacillus cereus isolates against desert locust, Schistocerca gregaria Forskal (Orthoptera: Acrididae). Egypt. J. Biol. Pest Control. 2018, 28, 1–7. Available online: https://ejbpc.springeropen.com/articles/10.1186/s41938-018-0056-x (accessed on 20 June 2020). [CrossRef]
- Mashtoly, T.A.; El-Zemaity, M.S.; Abolmaaty, A.; Abdelatef, G.M.; Marzouk, A.A.; Reda, M. Phylogenetic characteristics of Novel Bacillus weihenstephanensis and Pseudomonas sp to desert locust Schistocerca gregaria (Orthoptera: Acrididae). Egypt J. Biol. Pest Ctrl. 2019, 29, 85. [Google Scholar] [CrossRef]
- Mashtoly, T.A.; El-Zemaity, M.S.; Hussien, M.I.; Alm, S.R. LC and LD50 values of Bacillus thuringiensis serovar japonensis strain Buibui toxin to oriental beetle and northern masked chafer larvae (Coleoptera, Scarabaeidae). J. Econ. Entomol. 2009, 102, 1891–1895. [Google Scholar] [CrossRef]
- Mashtoly, T.A.; Abolmaaty, A.; Thompson, N.; El-Zemaity, M.S.; Hussien, M.I.; Alm, S.R. Enhanced toxicity of Bacillus thuringiensis japonensis strain Buibui toxin to oriental beetle and northern masked chafer (Coleoptera: Scarabaeidae) larvae with Bacillus sp. NFD2. J. Econ. Entomol. 2010, 103, 1444–1454. [Google Scholar] [CrossRef]
- Sánchez–Yáñez, J.M.; Rico, J.L.; Ulíbarri, G. Bacillus thuringiensis (Bt) is more than a special agent for biological control of pests. J. Appl. Biotechnol. Bioeng. 2022, 9, 33–39. [Google Scholar] [CrossRef]
- Roh, J.Y.; Choi, J.Y.; Li, M.S.; Jin, B.R.; Je, Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007, 17, 547–559. [Google Scholar]
- Chakroun, M.; Banyuls, N.; Bel, Y.; Escriche, B.; Ferré, J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 329–350. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Bravo, A.; Gómez, I.; Conde, J.; Muñoz-Garay, C.; Sánchez, J.; Miranda, R.; Zhuang, M.; Gill, S.S.; Soberón, M. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 2004, 1667, 38–46. [Google Scholar] [CrossRef]
- Gómez, I.; Sanchez, J.; Muñoz-Garay, C.; Matus, V.; Gill, S.S.; Soberón, M.; Bravo, A. Bacillus thuringiensis Cry1A toxins are versatile-proteins with multiple modes of action, Two distinct pre-pores are involved in toxicity. Biochem. J. 2014, 459, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Balzan, M.V.; Moonen, A.C. Management strategies for the control of Tuta absoluta (Lepidoptera, Gelechiidae) damage in open-field cultivations of processing tomato in Tuscany (Italy). EPPO Bull. 2012, 42, 217–225. [Google Scholar] [CrossRef]
- Giustolin, T.A.; Vendramim, J.D.; Alves, S.B.; Vieira, S.A.; Pereira, R.M. Susceptibility of Tuta absoluta (Meyrick) (Lep., Gelechiidae) reared on two species of Lycopersicon to Bacillus thuringiensis var. kurstaki. J. Appl. Entomol. 2001, 125, 551–556. [Google Scholar] [CrossRef]
- Mallia, D. Guidelines for the Control and Eradication of Tuta absoluta; Ministry of Resources and Rural Affairs, Plant Health Department: Attard, Malta, 2009. Available online: http//www.agric.gov.mt/plant-health-deptprofile?1=1 (accessed on 12 October 2012).
- González-Cabrera, J.; Mollá, O.; Montón, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) for controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae). Biocontrol 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Mashtoly, T.A.; Abolmaaty, A.; El-Zemaity, M.S.; Hussien, M.I.; Alm, S.R. Enhanced toxicity of Bacillus thuringiensis subspecies kurstaki and aizawai to black cutworm larvae (Lepidoptera, Noctuidae) with Bacillus sp. NFD2 and Pseudomonas sp. FNFD1. J. Econ. Entomol. 2011, 104, 41–46. [Google Scholar] [CrossRef]
- Valent BioScience Biorational Crop Protection-Dipel®. The World’s Leading Biological-Insecticides. 2020. Available online: https://www.valentbiosciences.com/cropprotection/products/dipel/ (accessed on 5 November 2018).
- Valent BioScience Biorational Crop Protection-XenTari®. Pure, Potent and Targeted-Control. 2020. Available online: https://www.valentbiosciences.com/cropprotection/products/xentari/ (accessed on 5 November 2018).
- Valent BioScience XenTari-DiPel Program Technical Bulletin. 2017. Available online: https://www.valentbiosciences.com/cropprotection/wp-content/uploads/sites/2/2017/06/xentari-protoxin-blend-technical-bulletin-ag-5398.pdf (accessed on 5 November 2018).
- OEPP/EPPO. Insecticide co-formulated mixtures. Bull. OEPP/EPPO 2018, 42, 353–357. [Google Scholar]
- EPPO. Efficacy evaluation of plant protection products—Insecticide co-formulated mixtures. EPPO Bull. 2012, 42, 353–357. [Google Scholar] [CrossRef]
- IRAC. IRAC Susceptibility Test Methods 022. 2012. Available online: https://irac-online.org/methods/tuta-absoluta-larvae/ (accessed on 15 August 2015).
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, 111809. [Google Scholar] [CrossRef]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the protocol for the LIVE/DEAD BacLight Bacterial Viability Kit for rapid determination of bacterial load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef]
- CIPAC Collaborative International Pesticide Analytical Council, Ltd. Handbook K, “MT 186 Bulk Density”. 2003. Available online: www.cipac.org (accessed on 20 May 2017).
- SAS Institute. SAS Version 9.4; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- FAO/WHO. Specification Guidelines for Microbial Pesticides (Revised); Section 9; World Health Organization: Geneva, Switzerland, 2018. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/JMPS_Manual_2016/Manual-Revised_Section_9_Final_-_2018_12_05.pdf (accessed on 14 January 2018).
- WHO. WHO Specifications and Evaluations for Public Health Pesticides, Bacillus thuringiensis Subspecies Israelensis Strain AM; World Health Organization: Geneva, Switzerland, 2012; pp. 52–65. Available online: https://www.who.int/whopes/quality/Bti_eval_spec_October_2012.pdf (accessed on 14 January 2018).
- WHO. WHO Specifications and Evaluations for Public Health Pesticides, Lambda-Cyhalothrin; World Health Organization: Geneva, Switzerland, 2015. Available online: https://www.who.int/pq-vector-control/prequalified-lists/LAMBDA-CYHALOTHRIN.pdf?ua=1 (accessed on 14 January 2018).
- Finney, D.J. (Ed.) Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Sun, Y.P. Toxicity index-an improved method of comparing the relative toxicity of insecticides. J. Econ. Entomol. 1950, 43, 45–53. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- US Geological. Estimated Agricultural Use for λ-cyhalothrin. 2018. Available online: https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2018&map=CYHALOTHRINLAMBDA&hilo=L&disp=Cyhalothrin-Lambda (accessed on 23 July 2022).
- Afify, A.E.M.M.; El-Beltagi, H.S. Effect of insecticide cyanophos on liver function in adult male rats. Fresenius Environ. Bull. 2011, 20, 1084–1088. [Google Scholar]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of pyrethroid neurotoxicity, Implications for cumulative risk assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- WHO. Cyhalothrin, Environmental Health Criteria; WHO: Geneva, Switzerland, 1990; Volume 99.
- Zibaee, I.; Bandani, R.A.; Sabahi, G. The expression profile of detoxifying enzyme of tomato leaf miner, Tuta absoluta Meyrik (Lepidoptera: Gelechiidae) to chlorpyrifos. Arthrology 2016, 5, 77–86. [Google Scholar]
- Lazarte, J.N.; Valacco, M.P.; Moreno, S.; Salerno, G.L.; Berón, C.M. Molecular characterization of a Bacillus thuringiensis strain from Argentina, toxic against Lepidoptera and Coleoptera, based on its whole-genome and Cry protein analysis. J. Invertebr. Pathol. 2021, 107563. [Google Scholar] [CrossRef]
- IRAC. Mode of Action Classification Scheme Version 10.3. June 2022. Available online: https://irac-online.org/?s=MODE+OF+ACTION+CLASSIFICATION+SCHEME+VERSION+10.3 (accessed on 28 July 2022).




| Source | df | MS | F-Statistic | p | Effect |
|---|---|---|---|---|---|
| The three rates of combination + Control (Lambda-cyhalothrin/B. thuringiensis) | 3 | 27.8 | 3.7 | 0.0612 | Not Significant |
| Error | 8 | 7.5 | |||
| Total | 11 |
| Parameter Tests | Original Formulations | Lab-Made Combined Formulations | |||||
|---|---|---|---|---|---|---|---|
| Icon® | Dipel® | XenTari® | Agree® | Icon:Dipel (1:1) | Icon:XenTari (1:1) | Icon:Agree (1:1) | |
| Wettability at 1 min. | Completely wetted | Completely wetted | Completely wetted | Completely wetted | Completely wetted | Completely wetted | Completely wetted |
| Persistent Foam after 1 min | 40 (+) (60 mL) † | 20 (+) (60 mL) † | 26 (+) (60 mL) † | 22 (+) (60 mL) † | 28 (+) (60 mL) † | 30 (+) (60 mL) † | 28 (+) (60 mL) † |
| Suspensibility% | 81 (ML 50) ‡ | 96 (ML 90) ‡ | 95 (ML 90) ‡ | 91 (ML 90) ‡ | 83 (NA) § | 80 (NA) § | 78 (NA) § |
| Treatments | N | Slope (SE) | LC50 ab | 95% FL | χ2(df) c | RP d | CM e |
|---|---|---|---|---|---|---|---|
| Dipel Pro® 54 DF | 720 | 3.59 (0.38) | 1566.8 b | 1196.81–1734.03 | 3.64(6) | 1.72 | 4.4 |
| XenTari® 54 DF | 720 | 2.73 (0.29) | 2119.1 c | 1782.23–2352.19 | 2.41(6) | 1.27 | 2.2 |
| Agree® 50 WDG | 720 | 2.49 (0.23) | 2694.4 d | 2432.44–2868.12 | 5.09(6) | 1 | 2.2 |
| Icon® 10 WP | 540 | 2.62 (0.22) | 826.9 a | 709.6–1043.5 | 3.32(4) | 3.25 | 3.3 |
| Treatments (Lab-Made Combined Formulates) | N | Slope (SE) | LC50 ab | 95% FL | χ2(df) c | Toxicity Index d | RP e | CM f |
|---|---|---|---|---|---|---|---|---|
| Dipel (Btk) + Icon (lambda cyhalothrin) | 450 | 2.24 (0.22) | 344.9 a | 296.11–423.55 | 2.31(3) | 77.5 | 7.81 | 3.3 |
| XenTari (Bta) + Icon (lambda cyhalothrin) | 450 | 2.49 (0.27) | 267.3 a | 206.16–354.07 | 2.62(3) | 100 | 10.08 | 3.3 |
| Agree (Bta) + Icon (lambda cyhalothrin) | 540 | 2.52(0.32) | 733.5 b | 610.05–782.21 | 3.43(4) | 36.4 | 3.67 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashtoly, T.A.; El-Beltagi, H.S.; Almujam, A.N.; Othman, M.N. The Potential of a Novel Concept of an Integrated Bio and Chemical Formulate Based on an Entomopathogenic Bacteria, Bacillus thuringiensis, and a Chemical Insecticide to Control Tomato Leafminer, Tuta absoluta ‘(Meyrick)’ (Lepidoptera: Gelechiidae). Sustainability 2022, 14, 10582. https://doi.org/10.3390/su141710582
Mashtoly TA, El-Beltagi HS, Almujam AN, Othman MN. The Potential of a Novel Concept of an Integrated Bio and Chemical Formulate Based on an Entomopathogenic Bacteria, Bacillus thuringiensis, and a Chemical Insecticide to Control Tomato Leafminer, Tuta absoluta ‘(Meyrick)’ (Lepidoptera: Gelechiidae). Sustainability. 2022; 14(17):10582. https://doi.org/10.3390/su141710582
Chicago/Turabian StyleMashtoly, Tamer A., Hossam S. El-Beltagi, Abdulrahman N. Almujam, and Muteb N. Othman. 2022. "The Potential of a Novel Concept of an Integrated Bio and Chemical Formulate Based on an Entomopathogenic Bacteria, Bacillus thuringiensis, and a Chemical Insecticide to Control Tomato Leafminer, Tuta absoluta ‘(Meyrick)’ (Lepidoptera: Gelechiidae)" Sustainability 14, no. 17: 10582. https://doi.org/10.3390/su141710582
APA StyleMashtoly, T. A., El-Beltagi, H. S., Almujam, A. N., & Othman, M. N. (2022). The Potential of a Novel Concept of an Integrated Bio and Chemical Formulate Based on an Entomopathogenic Bacteria, Bacillus thuringiensis, and a Chemical Insecticide to Control Tomato Leafminer, Tuta absoluta ‘(Meyrick)’ (Lepidoptera: Gelechiidae). Sustainability, 14(17), 10582. https://doi.org/10.3390/su141710582







