1. Introduction
Agriculture plays a critical role in the economy of many countries [
1]. However, the agricultural sector is facing several challenges, one being the high incidence of disease and pests. Chemical control agents are commonly used to solve this problem with much success. However, their consistent usage poses a serious impact on both the environment and human health [
2], along with an increasing incidence of chemical resistance in plant pathogens. These concerns about chemical control agents highlight the need to search for new and safe plant protectants [
3] with minimal side effects. In this context, natural control agents are increasingly being studied, and are considered safe and sustainable alternatives to chemical control agents.
Among natural control agents, soil microbial communities can be considered as a potential source, since these microbes are observed to have a role in suppressing plant diseases [
4]. These communities are very diverse, complex, and important assemblies of organisms in the biosphere [
5], and are considered an important source for the search for novel antimicrobial agents and molecules with biotechnological importance. Soil microbial communities, however, are influenced by soil composition, and salinity is one of the most important factors shaping microbial community composition [
5]. The reason for this is that saline habitats have high ionic contents (mainly NaCl), and to grow in such conditions, saline-inhabiting microbes have to adapt to cope with the stress conditions generated by high ionic content. To do so, they synthesize unique molecules and physiological pathways that differ from those found in microbes from non-saline habitats. Thus, they are reported to produce several novel compounds, such as exopolysaccharides and enzymes, e.g., alpha-amylases, endoglucanases, or lipases, that exhibit unique properties and promising perspectives for biotechnological exploitation [
4].
Among natural control agents, new antimicrobial agents with different modes of action from existing antimicrobial agents are currently needed [
3]. In this regard, bacteria producing varying bioactive pigments can be considered a potential tool. These natural pigments do not just consist of color, but are a mixture of diverse chemical components with multifaceted biological activities [
6]. Chemically, pigments produced by bacteria are pyrole, carotenoid, phenozyne, quinine, xanthophylls, and quinone derivatives. Hence, these can be considered as promising new antimicrobial sources against various pathogenic and antibiotic-resistant microbes [
7].
The cosmetics, food, textile, and pharmaceutical industries are studying microbial pigments more often as safe and sustainable alternatives to various chemical counterparts [
8]. These pigments are used as plant growth promoters [
9], antioxidative agents, antibacterial agents [
10,
11], protectors against light damage [
12], enhancers of the immune response, antiprotozoal agents, anticancer agents [
11], potential antidiabetic drugs [
13], and for the treatment of eye-related diseases. Utilizing pigment-producing bacteria as a biocontrol agent against plant pathogens has been relatively less explored. This study aims to isolate bioactive pigment-producing bacteria from saline agricultural fields and to evaluate their potential to exhibit antimicrobial activities against different plant pathogenic microorganisms.
2. Materials and Methods
2.1. Sample Collection
Soil samples were collected from three different saline agricultural fields of the village Kassoki in Gujrat, Pakistan, at a latitude and longitude of 32.658080° N and 74.303055° E, respectively. Soil was clayey and usually used to grow fodder and wheat. At each sampling site, surface and subsurface soil samples were collected to isolate the maximum number of bacteria with the desired characteristics. Surface samples were collected by directly picking the surface soil off the field after carefully removing surface dirt, and subsurface soil was collected after digging in the sampling site up to 1 foot deep. About 300 g of soil was collected with a clean spatula and placed in an appropriately sized clean zip-lock bag. Samples were immediately stored at 4 °C until assayed, as described below.
2.2. Isolation of Bioactive Pigment-Producing Bacteria
A serial dilution method using three different bacterial growth media, namely TSA (tryptic soy agar) medium, GYM (glucose yeast extract malt extract medium) agar medium and R2A (Reasoner 2A) agar medium, supplemented with 2% NaCl, was used for the initial isolation of bacteria. About 10 g of all types of soil samples collected were mixed together in 100 mL sterile normal saline solution. From that, up to 10−6 serial dilutions were prepared and 50 µL of each serial dilution was spread on above mentioned media plates in triplicates. The inoculated plates were incubated at 30 ± 2 °C for up to 10 days and checked for bacterial growth until desire sized colonies appeared.
Selective Isolation of Bioactive Pigment-Producing Bacteria
To selectively isolate bioactive pigment-producing bacteria, a seeded agar overlay method was used. In this method, culture plates, having substantial sized bacterial colonies, that were initially inoculated with serially diluted soil suspension were inoculated again with an isolate of Bacillus subtilis (B. subtilis) that was sensitive to the number of antibiotics. For this, freshly prepared B. subtilis culture in an antibiotic assay agar was overlaid over the isolation plates, followed by incubation for 3 h to allow any antibiotic in the bacterial isolation medium layer (agar medium used for isolation) to diffuse up into the seeded layer. Plates were then incubated again overnight at room temperature. After 24 h 1.5 mL of a 2 mg/mL aqueous solution of 2,3,5 triphenyltetrazolium chloride (MTT) was spread over the agar overlay and incubated again at 30 ± 2 °C for 3 h. Clear inhibition zones appear above colonies where an antibiotic has killed the inoculating B. subtilis colonies. Only three pigmented colonies show bioactivity, and hence, were selected for further analysis. Selected colonies were purified through a rigorous procedure as pure cultures and were checked again to confirm bioactivity against B. subtilis cultures. All the isolates were maintained later in GYM agar medium, as they showed good pigment production in said medium.
2.3. Screening Isolates for Antimicrobial Activity
Bioactive-pigmented bacteria isolated as a result of above mentioned procedure were screened to possess antimicrobial activity against phytopathogenic microbes.
2.3.1. Phytopathogenic Microorganisms Tested
Plant pathogenic fungi and bacteria used in this study were obtained from First Fungal Culture Bank of Pakistan (FCBP) and American Type Culture Collection (ATCC). Fungal and bacterial pathogens used in the study with their accession numbers were Fusarium oxysporum (F. oxysporum) (FCBP-PTF-0082), Fusarium solani (F. solani) (ATCC 12823), Aspergillus flavus (A. flavus) (FCBP-0064), Aspergillus niger (A. niger) (FCBP-0198), Alternaria alternata (A. alternata) (FCBP-PTF-0003), Psuedomonas syringae (P. syringae) (FCBP, 009) and Xanthomonas axonopodis (X. axonopodis) (FCBP, 001).
2.3.2. Screening Bioactive Pigment-Producing Bacteria for Antifungal Activity
The antagonistic activity of purified bioactive pigment-producing bacteria against fungal phytopathogens was determined by the conventional dual culture method [
14]. A 5 mm fungal plug was placed at the center of the plate, and tested bacterial cultures were spot inoculated at about 25 mm from the center of the plate. A plate with a fungal disk alone served as negative control and a plate having fungal plug and an amphotericin-impregnated disk served as a positive control. Inoculated plates were incubated at 30 ± 2 °C for 5–7 days. After incubation zones of inhibition were recorded by measuring the distance between growth of the fungal mycelium and the growth of tested bacteria. Experiment was performed in triplicate and was repeated without running triplicate for confirmation. The following formula was used to determine percent inhibition of the radial growth (PIRG):
In this formula,
R1 = radius of growth of fungal mycelium in negative
R2 = radius of fungal mycelium towards treatment [
15].
2.3.3. Screening Bioactive Pigment-Producing Bacteria for Antibacterial Activity
The antibacterial potential of bioactive pigment-producing bacteria was evaluated by a previously reported agar disk diffusion method [
16]. Phytpathogenic bacterial cultures were refreshed in nutrient broth, culture densities were adjusted according to Mcfarland 0.5, and bacterial lawns were prepared by evenly spreading above mentioned phytopathogenic bacterial culture broths on TSA plates. Sterile filter paper disks impregnated with freshly prepared cultures of isolated bacteria with pre-set densities were placed on bacterial lawns. The antagonistic activity was recorded by measuring halo zones after incubation at 30 ± 2 °C for 24 h. Negative and positive controls used were filter paper disks impregnated with 1% DMSO and Streptomycin (10 μg/mL), respectively. The average diameters of clear zones were measured and recorded in mm.
2.4. Strain Identification
Isolates were identified using 16S rRNA gene sequence analysis. Bacterial genomic DNA was extracted using PureLink™ Microbiome DNA following the manufacturer’s instruction. Bacterial universal primers i.e., 27F and 1492R were used to amplify the required sequence for identification [
17]. PureLink™ Quick PCR Purification Kit (Invitrogen) was used to purify PCR products. Amplified and purified products were sequenced commercially by Macrogen (South Korea) using 27F primer. To determine the closest matching strain EzBioCloud database and Mega BLAST algorithm by National Centre for Biotechnology Information (NCBI) (
http://www.ncbi.nlm.nih.gov/BLAST, accessed on 30 July 2020) were used [
18].
2.5. Crude Bioactive Pigment Extract Preparation
Bioactive pigment-producing bacteria were separately assessed to check optimum pigment production in different culture media at different incubation temperatures and media. In the present case, all three bioactive pigment-producing bacteria showed good growth and maximum pigment production in a solid GYM medium at 30 ± 2 °C. To get crude extract in the desired quantity for analysis, bacteria were inoculated in bulk on GYM agar plates and incubated at 30 ± 2 °C for 7–10 days. Growth in plates was monitored regularly and checked for the presence of any contamination, and contaminated plates were subsequently eliminated. After achieving maximum growth, crude pigment extract was prepared by cutting culture-containing media in all plates using a clean spatula and dumping it into a single sterile beaker. After that, analytical grade ethyl acetate was added enough to cover all media with bacterial cultures and sonicated for half an hour at 30 ± 2 °C. Crude extract was filtered out by vacuum filtration. The process was repeated until no further pigment was extracted from the culture medium.
2.6. Antifungal Activity of Crude Bioactive Pigment Extract
The antifungal activity of crude bioactive pigment extract of isolated strains was determined using the agar disk diffusion assay [
19]. The phytopathogenic fungal spore suspensions (having turbidity set according to McFarland 0.5) were prepared and swabbed on SDA (Sabouraud Dextrose agar) plates. A bioactive pigment-producing bacterial crude extract was prepared at a concentration of 4 mg/mL in 1% DMSO. Sterile 5 mm filter paper disks impregnated with 5 μL of sample extracts were placed on prepared seeded plates. DMSO (1%) was used as negative control and Amphotericin B (4 mg/mL) was used as positive control. Zones of inhibition were recorded in mm after 48 h of incubation at 30 ± 2 °C. Crude bioactive pigment extracts with zones of inhibition greater than 10 mm were further analyzed to determine minimum inhibitory concentrations (MICs).
Determination of Minimum Inhibitory Concentration (MIC) against Fungal Phytopathogens
MIC was determined by a broth dilution method [
20]. Spore suspensions were prepared for all phytopathogenic fungal strains with turbidity set according to McFarland 0.5. Prepared suspensions were used to inoculate 5 mL SDB (Sabouraud dextrose broth) incubated at 30 ± 2 °C for 48 h. Bioactive pigment extracts (2000 μg/mL) of isolated strains of bacteria were prepared in 1% DMSO and two-fold serial dilutions of the extracts were prepared for MIC (100–0.781 μg/mL). Fungal growth in SDB was checked after 48 h of incubation at 30 ± 2 °C in the presence of crude bioactive extract (having final concentrations of 100 μg/mL, 50 μg/mL, 25 μg/mL, 12.5 μg/mL, 6.25 μg/mL, 3.125 μg/mL, 1.562 μg/mL, and 0.781 μg/mL). To check MIC, 10 μL of broth culture from each tube was spread on SDA plates and growth was checked after 48 h of incubation at 30 ± 2 °C. MIC was recorded as the lowest concentration of the crude pigment extract that inhibited the visible growth of phytopathogens completely after 48 h.
2.7. Antifungal Activity on Mycelial Radial Growth (MRG)
A previously reported procedure known as the poisoned food technique was used to check the antifungal activity of crude bioactive pigment extract on mycelial growth [
20]. For assessment, crude pigment extract at a concentration of 20 mg/mL (in 1% DMSO) was mixed with warm (45–50 °C) SDA medium to get a final concentration of 100 μg/mL. This mixture of crude extract and SDA was then poured into sterilized petri dishes. A similar process was used to prepare negative and positive control plates using 1% DMSO and Amphotericin B. A 5 mm fungal disk was then placed carefully at the center of each petri plate, sealed with parafilm, and incubated at 30 ± 2 °C until the control mycelium fully covered the plates. Assay was performed in triplicate, colony radii were measured, and the below mentioned formula was used to calculate the percent inhibition of the mycelial radial growth:
Here, C represents the colony diameter in negative control, and T is the colony diameter in treatment [
21].
2.8. Antibacterial Activity of Crude Bioactive Pigment Extract against Bacterial Phytopathogens
The antibacterial activity of crude bioactive pigment extract was determined using the agar disk diffusion assay [
16]. Stock solution (4 mg/mL) of crude bioactive pigment extract was prepared in 1% DMSO. Test bacterial cultures were refreshed in TSB in test tubes and their turbidity was set according to McFarland 0.5. Test cultures were then spread on TSA plates and sterile filter paper disks impregnated in crude extract were placed carefully on inoculated plates. Plates were checked for clear zones after incubation at 30 ± 2 °C for 24 h. DMSO (1%) and ampicillin (4 mg/mL) impregnated disks served as a negative and positive control respectively. Samples having zones of inhibition greater than 10 mm were further screened for MIC.
2.9. Statistical Analysis
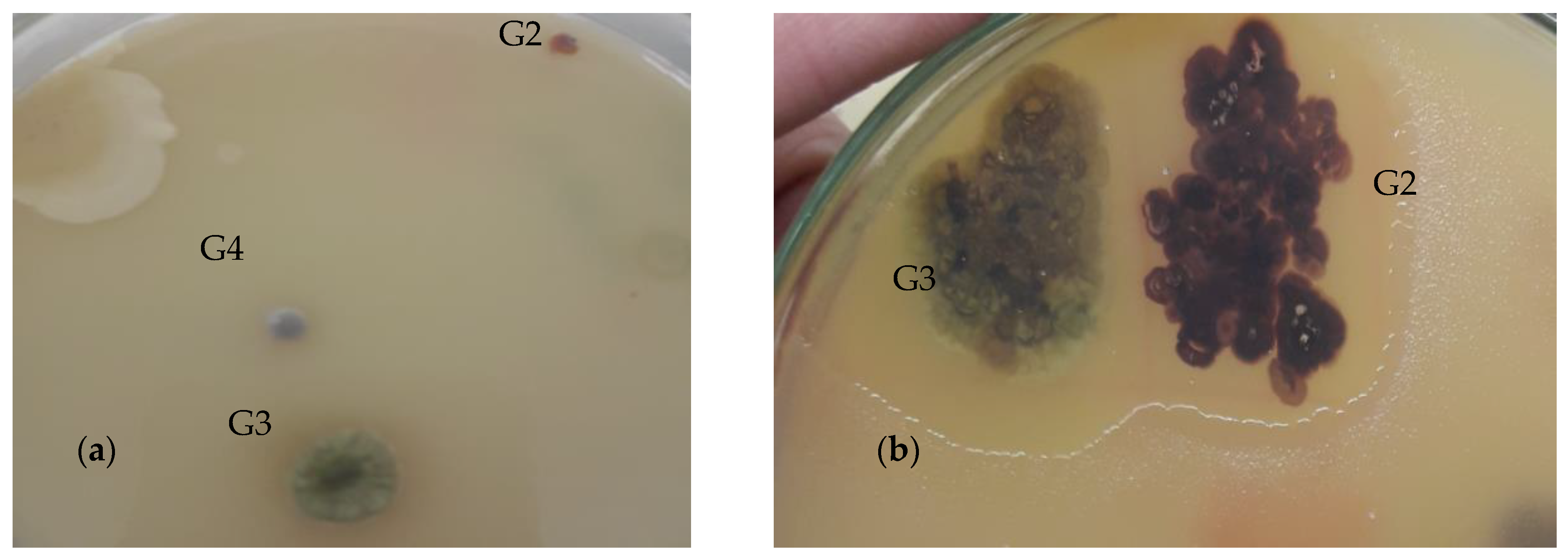
The experiments were performed in triplicate, and the obtained results were analyzed statistically by Kruskel–Wallis test, followed by Dunn’s post hoc test for data that did not follow Normal distribution and by One way Analysis of Variance (ANOVA) followed by Dunnett’s Multiple Comparison test for data that followed Normal distribution. In these analyses, bioactive-pigmented bacterial treatments named as Nonomurae salmonae (G2), Streptomyces chromofuscus (G3), and Actinocorallia libanotica (G4) are Explanatory variables while F. oxysporum, F. solani, A. flavus, A. alternata, and A. niger are Response variables i.e., changing bacterial treatments affect growth inhibition of F. oxysporum, F. solani, A. flavus, A. alternate, and A. niger. All the analyses reported here were performed using GraphPad Prism 5 (5.01). Data have been expressed as mean ± SD.
4. Discussion
The current research was initiated to search for novel, new, and sustainable plant protectants. In this regard, bacteria have been increasingly investigated as a sustainable and safe alternative to chemical plant protectants. Bioactive pigment-producing bacteria can be considered a relatively new and less-investigated source in this context.
Bioactive-pigmented bacteria from saline agricultural soil were purposely targeted in the current research because the soil is a rich source of beneficial bacteria with a high percentage still uncultured and uninvestigated. Furthermore, as a general perception, the probability of isolating plant-beneficial bacteria is greater in agricultural soil as compared to non-agricultural soils. It was deduced from previous reports that bacterial inhabitants of saline environments tend to produce different and diverse useful metabolites/compounds that differ in properties from those found in non-saline habitats [
4]. Extreme or stressed ecosystems are also reported to cause the evolution of novel secondary metabolic pathways that enhance the chances of finding new biological functions of bioactive compounds [
23].
As a result of above-mentioned selective isolation, three bioactive colored colonies were isolated and were identified as
Nonomurae salmonae,
Streptomyces chromofuscus, and
Actinocorallia libanotica, using 16S rRNA gene sequencing. All the isolates were found to be
Actinobacteria, belonging to three different genera, namely,
Actinocorallia,
Streptomyces, and
Nonomuraea. This is in congruence with previous studies that actinobacteria occur most commonly in soil [
24]. The reporting on the isolation of bioactive-pigmented actinobacteria only, in the present study, agrees with previous reports on actinobacteria as producers of instinctive pigments on the media, which are red, green, yellow, brown, and black in color [
25], and are an important and attractive source of bioactive secondary metabolites [
20,
26,
27]. Because of this ability, these bacteria are one of the most explored microbes among prokaryotes [
28].
Of all the three isolates of the current study,
Streptomyces chromofuscus showed good antimicrobial activity against plant pathogens as compared to other isolates. It showed broad-spectrum antifungal activity against plant pathogenic fungi. These results are in congruence with previous reports about
Streptomyces being an active producer of bioactive natural products as compared to other actinobacteria, and that more than 70% of the natural product having bioactive potential have been isolated from
Streptomyces [
26,
29,
30]. One of the previous studies reported that novel formulations of some of the strains of
Streptomyces in the form of encapsulation are being developed as biocontrol agents against different plant pathogens [
31]. Our results assessing the antimicrobial potential of
Streptomyces chromofuscus are very encouraging, showing a broad-spectrum antimicrobial potential of this isolate.
Here, we also report the isolation of two rare actinobacteria, namely,
Nonomurae salmonae and
Actinocorallia libanotica, from saline agricultural fields. Rare actinobacteria are actinobacteria that are difficult to isolate, are abundant in different habitats but need specialized protocols for isolation, and are believed to be a source of diverse new chemical compounds [
32]. Rare actinobacteria are considered as an unexplored source of novel bioactive metabolites and hence are increasingly being investigated [
33]. Our study provides useful preliminary information about the antimicrobial potential of isolated rare actinobacteria against plant pathogens.
Our study is the first to assess the antimicrobial potential in general and antibacterial potential in specific of
Nonomurae salmonae and
Actinocorallia libanotica against
P. syringae and
X. axonopodis. It was deduced from the results that
Nonomurae salmonae was active against
P. syringae only and
Actinocorallia libanotica was inactive against both of the bacterial pathogens. Furthermore, both isolates exhibited moderate to good antifungal activity. These results encourage us to further assess the active component involved in the activity and study different conditions required for the optimum production of the active component. Some previous studies on the members of the same genera of rare actinobacteria can be related to our results. One of the studies on
Nonomuraea sp. IB 2015I9-2 grown on different media reported bioactivity of its crude extract against
St. carnosus culture at varying concentrations [
34]. In another study, some members of genus
Nonomuraea were revealed to be a valuable source of novel metabolites for medical and industrial purposes [
35]. Hence, we can say that preliminary information on the bioactivity of
Nonomurae salmonae from our research is in congruence with previous findings on the members of the same genus.
A previous study on
Actinocorallia libanotica reported its antimicrobial activity as weak to moderate against
E. coli,
S. aureus,
M. luteus, and
B. cereus [
33]. This can be related to our results, where
Actinocorallia libanotica was observed to show no activity against different bacterial pathogens and moderate activity against fungal plant pathogens. Another study on
Actinocorallia aurantiaca reported the isolation of three new bioactive compounds, namely, aurantiadioic acids A-B and aurantoic acid A [
36]. The apparent inactivity or weak activity of rare actinomycetes in the present study can be attributed to the requirement of special conditions for the growth and production of the active metabolite. Detailed studies to assess the conditions under which these rare actinobacteria produce active metabolites are required.