Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives
Abstract
1. Introduction
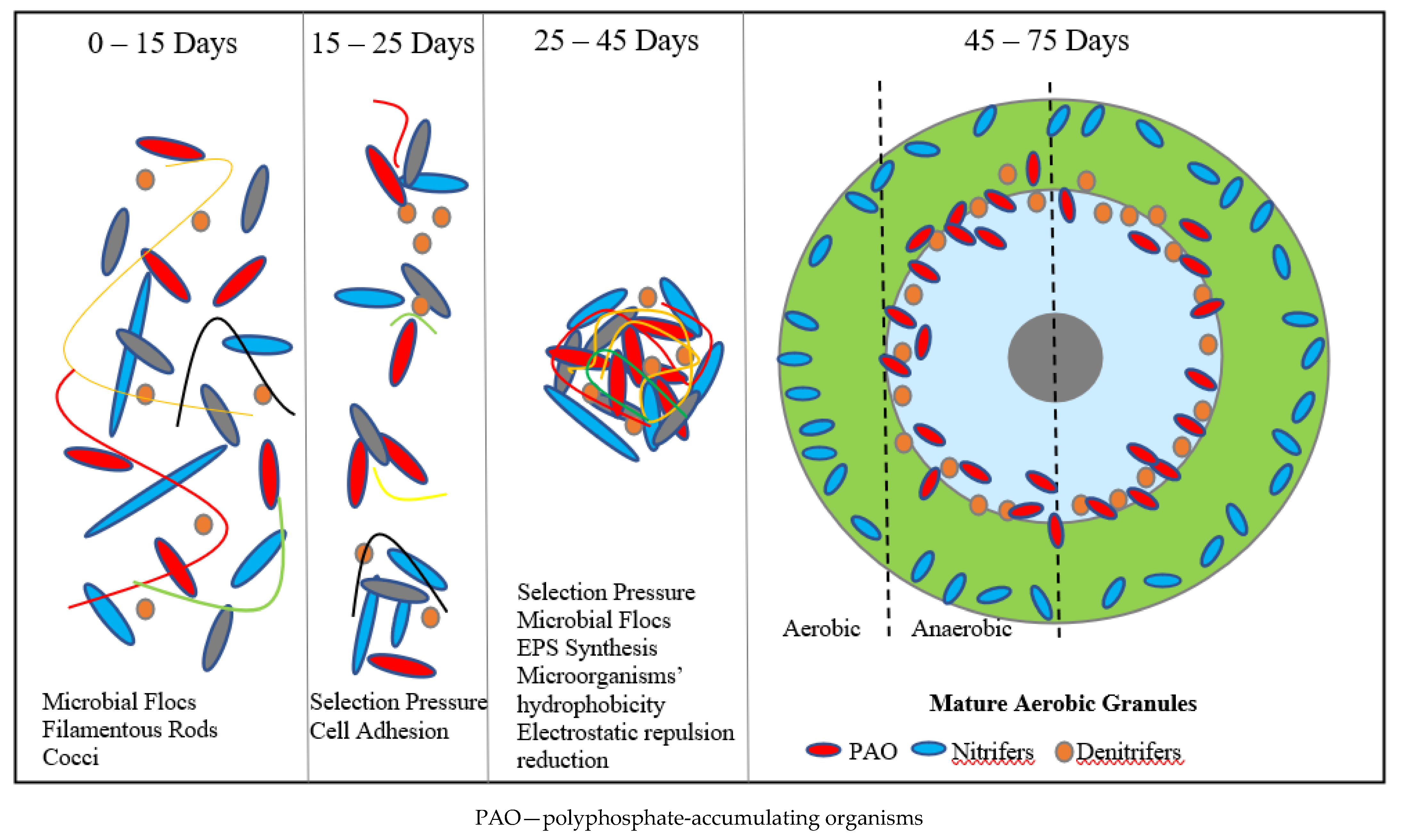
2. AGS Characteristics and Applications
3. Basics of Anaerobic Digestion
4. Anaerobic Digestion of AGS
5. AGS-Related Determinants of Anaerobic Digestion
6. Pre-Treatment Influence on AGS Anaerobic Digestion
7. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, Y.T.; Aziz, H.A.; Al-Khatib, I.A.; Abdel Rahman, R.O.; Cora-Hernandez, M.G.R. Water Quality Engineering and Wastewater Treatment. Water 2021, 13, 330. [Google Scholar] [CrossRef]
- Karolinczak, B.; Miłaszewski, R.; Dąbrowski, W. Cost Optimization of Wastewater and Septage Treatment Process. Energies 2020, 13, 6406. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.; Wibisono, Y.; Jaafar, J.; Indra Mahlia, T.M.; Khan, A.L.; Aslam, M. Recent Progress in Integrated Fixed-Film Activated Sludge Process for Wastewater Treatment: A Review. J. Environ. Manag. 2020, 268, 110718. [Google Scholar] [CrossRef]
- Wilén, B.M.; Liébana, R.; Persson, F.; Modin, O.; Hermansson, M. The Mechanisms of Granulation of Activated Sludge in Wastewater Treatment, Its Optimization, and Impact on Effluent Quality. Appl. Microbiol. Biotechnol. 2018, 102, 5005–5020. [Google Scholar] [CrossRef]
- Lettinga, G.; van Velsen, A.F.M.; Hobma, S.W.; de Zeeuw, W.; Klapwijk, A. Use of the Upflow Sludge Blanket (USB) Reactor Concept for Biological Wastewater Treatment, Especially for Anaerobic Treatment. Biotechnol. Bioeng. 1980, 22, 699–734. [Google Scholar] [CrossRef]
- Mishima, K.; Nakamura, M. Self-Immobilization of Aerobic Activated Sludge–A Pilot Study of the Aerobic Upflow Sludge Blanket Process in Municipal Sewage Treatment. Water Sci. Technol. 1991, 23, 981–990. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Calderón-Franco, D.; Sarelse, R.; Christou, S.; Pronk, M.; van Loosdrecht, M.C.M.; Abeel, T.; Weissbrodt, D.G. Metagenomic Profiling and Transfer Dynamics of Antibiotic Resistance Determinants in a Full-Scale Granular Sludge Wastewater Treatment Plant. Water Res. 2022, 219, 118571. [Google Scholar] [CrossRef]
- Rosa-Masegosa, A.; Muñoz-Palazon, B.; Gonzalez-Martinez, A.; Fenice, M.; Gorrasi, S.; Gonzalez-Lopez, J. New Advances in Aerobic Granular Sludge Technology Using Continuous Flow Reactors: Engineering and Microbiological Aspects. Water 2021, 13, 1792. [Google Scholar] [CrossRef]
- Sarvajith, M.; Nancharaiah, Y.V. Enhancing Biological Nitrogen and Phosphorus Removal Performance in Aerobic Granular Sludge Sequencing Batch Reactors by Activated Carbon Particles. J. Environ. Manag. 2022, 303, 114134. [Google Scholar] [CrossRef]
- Sarkar, G.S.; Rathi, A.; Basu, S.; Arya, R.K.; Halder, G.N.; Barman, S. Removal of Endocrine-Disrupting Compounds by Wastewater Treatment. In Advanced Industrial Wastewater Treatment and Reclamation of Water; Springer: Cham, Switzerland, 2022; pp. 129–151. [Google Scholar] [CrossRef]
- Peng, T.; Wang, Y.; Wang, J.; Fang, F.; Yan, P.; Liu, Z. Effect of Different Forms and Components of EPS on Sludge Aggregation during Granulation Process of Aerobic Granular Sludge. Chemosphere 2022, 303, 135116. [Google Scholar] [CrossRef]
- Pronk, M.; de Kreuk, M.K.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M.C.M. Full Scale Performance of the Aerobic Granular Sludge Process for Sewage Treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Bengtsson, S.; de Blois, M.; Wilén, B.M.; Gustavsson, D. A Comparison of Aerobic Granular Sludge with Conventional and Compact Biological Treatment Technologies. Environ. Technol. 2018, 40, 2769–2778. [Google Scholar] [CrossRef]
- Cai, F.; Lei, L.; Li, Y.; Chen, Y. A Review of Aerobic Granular Sludge (AGS) Treating Recalcitrant Wastewater: Refractory Organics Removal Mechanism, Application and Prospect. Sci. Total Environ. 2021, 782, 146852. [Google Scholar] [CrossRef]
- Corsino, S.F.; Devlin, T.R.; Oleszkiewicz, J.A.; Torregrossa, M. Aerobic Granular Sludge: State of the Art, Applications, and New Perspectives. In Advances in Wastewater Treatment; IWA Publishing: London, UK, 2019. [Google Scholar] [CrossRef]
- Muñoz-Palazón, B.; Hurtado-Martinez, M.; Gonzalez-Lopez, J. Simultaneous Nitrification and Denitrification Processes in Granular Sludge Technology. In Nitrogen Cycle; CRC Press: Boca Raton, FL, USA, 2021; pp. 222–244. [Google Scholar] [CrossRef]
- Niermans, R.; Giesen, A.; van Loosdrecht, M.; de Bruin, B. Full-Scale Experiences with Aerobic Granular Biomass Technology for Treatment of Urban and Industrial Wastewater. In Proceedings of the 2014 Water Environment Federation, Austin, TX, USA, 18–21 May 2014; pp. 2347–2357. [Google Scholar]
- Campo, R.; Lubello, C.; Lotti, T.; Di Bella, G. Aerobic Granular Sludge–Membrane BioReactor (AGS–MBR) as a Novel Configuration for Wastewater Treatment and Fouling Mitigation: A Mini-Review. Membranes 2021, 11, 261. [Google Scholar] [CrossRef]
- Sepúlveda-Mardones, M.; Campos, J.L.; Magrí, A.; Vidal, G. Moving Forward in the Use of Aerobic Granular Sludge for Municipal Wastewater Treatment: An Overview. Rev. Environ. Sci. Bio/Technol. 2019, 18, 741–769. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.Z.; Morgan, G.; Iorhemen, O.T. A Review of the State of Development of Aerobic Granular Sludge Technology over the Last 20 Years: Full-Scale Applications and Resource Recovery. Case Stud. Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Li, J.; Ding, L.B.; Cai, A.; Huang, G.X.; Horn, H. Aerobic Sludge Granulation in a Full-Scale Sequencing Batch Reactor. Biomed Res. Int. 2014, 2014, 268789. [Google Scholar] [CrossRef]
- Lin, H.; Ma, R.; Hu, Y.; Lin, J.; Sun, S.; Jiang, J.; Li, T.; Liao, Q.; Luo, J. Reviewing Bottlenecks in Aerobic Granular Sludge Technology: Slow Granulation and Low Granular Stability. Environ. Pollut. 2020, 263, 114638. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, F.; Wang, L.; Sun, J. Overcoming the Instability of Aerobic Granular Sludge under Nitrogen Deficiency through Shortening Settling Time. Bioresour. Technol. 2019, 289, 121620. [Google Scholar] [CrossRef]
- Leal, C.; Val del Río, A.; Mesquita, D.P.; Amaral, A.L.; Castro, P.M.L.; Ferreira, E.C. Sludge Volume Index and Suspended Solids Estimation of Mature Aerobic Granular Sludge by Quantitative Image Analysis and Chemometric Tools. Sep. Purif. Technol. 2020, 234, 116049. [Google Scholar] [CrossRef]
- Zielinski, M.; Debowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Hidaka, T.; Tsushima, I.; Tsumori, J. Comparative Analyses of Microbial Structures and Gene Copy Numbers in the Anaerobic Digestion of Various Types of Sewage Sludge. Bioresour. Technol. 2018, 253, 315–322. [Google Scholar] [CrossRef]
- De Kreuk, M.K.; McSwain, B.S.; Bathe, S.; Tay, J.; Schwarzenbeck, S.T.L.; Wilderer, P.A. Discussion Outcomes. In Aerobic Granular Sludge, Water and Environmental Management Series; IWA Publishing: Munich, Germany, 2005; pp. 165–169. [Google Scholar]
- Xiao, X.; Ma, F.; You, S.; Guo, H.; Zhang, J.; Bao, X.; Ma, X. Direct Sludge Granulation by Applying Mycelial Pellets in Continuous-Flow Aerobic Membrane Bioreactor: Performance, Granulation Process and Mechanism. Bioresour. Technol. 2022, 344, 126233. [Google Scholar] [CrossRef]
- Amin Vieira da Costa, N.P.; Libardi, N.; Ribeiro da Costa, R.H. How Can the Addition of Extracellular Polymeric Substances (EPS)-Based Bioflocculant Affect Aerobic Granular Sludge (AGS)? J. Environ. Manag. 2022, 310, 114807. [Google Scholar] [CrossRef]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.; Tay, J.H. Influence of Operation Mode and Wastewater Strength on Aerobic Granulation at Pilot Scale: Startup Period, Granular Sludge Characteristics, and Effluent Quality. Water Res. 2019, 160, 81–96. [Google Scholar] [CrossRef]
- Wagner, J.; Weissbrodt, D.G.; Manguin, V.; Ribeiro da Costa, R.H.; Morgenroth, E.; Derlon, N. Effect of Particulate Organic Substrate on Aerobic Granulation and Operating Conditions of Sequencing Batch Reactors. Water Res. 2015, 85, 158–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, X.; Nuramkhaan, M.; Lei, Z.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J.; Tay, J.H. Rapid Granulation of Aerobic Granular Sludge: A Mini Review on Operation Strategies and Comparative Analysis. Bioresour. Technol. Rep. 2019, 7, 100206. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic Granular Sludge Technology: Mechanisms of Granulation and Biotechnological Applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, G.; Zhang, X.; Zhou, L. Influences of Extracellular Polymeric Substances on the Dewaterability of Sewage Sludge during Bioleaching. PLoS ONE 2014, 9, e102688. [Google Scholar] [CrossRef]
- Khan, M.Z.; Mondal, P.K.; Sabir, S. Aerobic Granulation for Wastewater Bioremediation: A Review. Can. J. Chem. Eng. 2013, 91, 1045–1058. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Mendes Barros, A.R.; Milen Firmino, P.I.; Bezerra dos Santos, A. Aerobic Granular Sludge: Cultivation Parameters and Removal Mechanisms. Bioresour. Technol. 2018, 270, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; de Blois, M.; Wilén, B.M.; Gustavsson, D. Treatment of Municipal Wastewater with Aerobic Granular Sludge. Crit. Rev. Environ. Sci. Technol. 2018, 48, 119–166. [Google Scholar] [CrossRef]
- Pronk, M.; Giesen, A.; Thompson, A.; Robertson, S.; Van Loosdrecht, M. Aerobic Granular Biomass Technology: Advancements in Design, Applications and Further Developments. Water Pract. Technol. 2017, 12, 987–996. [Google Scholar] [CrossRef]
- Lee, D.J.; Chen, Y.Y.; Show, K.Y.; Whiteley, C.G.; Tay, J.H. Advances in Aerobic Granule Formation and Granule Stability in the Course of Storage and Reactor Operation. Biotechnol. Adv. 2010, 28, 919–934. [Google Scholar] [CrossRef]
- Franca, R.D.G.; Pinheiro, H.M.; van Loosdrecht, M.C.M.; Lourenço, N.D. Stability of Aerobic Granules during Long-Term Bioreactor Operation. Biotechnol. Adv. 2018, 36, 228–246. [Google Scholar] [CrossRef]
- Han, X.; Jin, Y.; Yu, J. Rapid Formation of Aerobic Granular Sludge by Bioaugmentation Technology: A Review. Chem. Eng. J. 2022, 437, 134971. [Google Scholar] [CrossRef]
- Bassin, J.P. Aerobic Granular Sludge Technology. In Advanced Biological Processes for Wastewater Treatment; Springer: Cham, Switzerland, 2018; pp. 75–142. [Google Scholar] [CrossRef]
- Dulekgurgen, E.; Artan, N.; Orhon, D.; Wilderer, P.A. How Does Shear Affect Aggregation in Granular Sludge Sequencing Batch Reactors? Relations between Shear, Hydrophobicity, and Extracellular Polymeric Substances. Water Sci. Technol. 2008, 58, 267–276. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.W.; Li, Y.; Zhou, J.Q.; Liu, Y. The Influence of Short-Term Starvation on Aerobic Granules. Process Biochem. 2006, 41, 2373–2378. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.F.; Tay, J.H. Improved Stability of Aerobic Granules by Selecting Slow-Growing Nitrifying Bacteria. J. Biotechnol. 2004, 108, 161–169. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, S.H.; Yu, X.; Wei, B. Variations of Both Bacterial Community and Extracellular Polymers: The Inducements of Increase of Cell Hydrophobicity from Biofloc to Aerobic Granule Sludge. Bioresour. Technol. 2011, 102, 6421–6428. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, Q.; Han, H.; Yin, H.; Chen, M.; Guo, C.; Li, J.; Qiu, H. Functional Analysis of Extracellular Polymeric Substances (EPS) during the Granulation of Aerobic Sludge: Relationship among EPS, Granulation and Nutrients Removal. Environ. Res. 2022, 208, 112692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lens, P.N.L.; Shi, W.; Zhang, R.; Zhang, Z.; Guo, Y.; Bao, X.; Cui, F. Enhancement of Aerobic Granulation and Nutrient Removal by an Algal–Bacterial Consortium in a Lab-Scale Photobioreactor. Chem. Eng. J. 2018, 334, 2373–2382. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Zhao, L.; Liu, X.; Wan, C. Understanding the Role of Cations and Hydrogen Bonds on the Stability of Aerobic Granules from the Perspective of the Aggregation and Adhesion Behavior of Extracellular Polymeric Substances. Sci. Total Environ. 2021, 795, 148659. [Google Scholar] [CrossRef]
- Zou, J.; Yu, F.; Pan, J.; Pan, B.; Wu, S.; Qian, M.; Li, J. Rapid Start-up of an Aerobic Granular Sludge System for Nitrogen and Phosphorus Removal through Seeding Chitosan-Based Sludge Aggregates. Sci. Total Environ. 2021, 762, 144171. [Google Scholar] [CrossRef]
- Karakas, I.; Sam, S.B.; Cetin, E.; Dulekgurgen, E.; Yilmaz, G. Resource Recovery from an Aerobic Granular Sludge Process Treating Domestic Wastewater. J. Water Process Eng. 2020, 34, 101148. [Google Scholar] [CrossRef]
- Moura, L.L.; Duarte, K.L.S.; Santiago, E.P.; Mahler, C.F.; Bassin, J.P. Strategies to Re-Establish Stable Granulation after Filamentous Outgrowth: Insights from Lab-Scale Experiments. Process Saf. Environ. Prot. 2018, 117, 606–615. [Google Scholar] [CrossRef]
- Ouyang, L.; Huang, W.; Huang, M.; Qiu, B. Polyaniline Improves Granulation and Stability of Aerobic Granular Sludge. Adv. Compos. Hybrid Mater. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Lashkarizadeh, M. Operating pH and Feed Composition as Factors Affecting Stability of Aerobic Granular Sludge. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2015. [Google Scholar]
- Franca, R.D.G.; Pinheiro, H.M.; Lourenço, N.D. Recent Developments in Textile Wastewater Biotreatment: Dye Metabolite Fate, Aerobic Granular Sludge Systems and Engineered Nanoparticles. Rev. Environ. Sci. Bio/Technol. 2020, 19, 149–190. [Google Scholar] [CrossRef]
- Stes, H.; Caluwé, M.; Dockx, L.; Cornelissen, R.; de Langhe, P.; Smets, I.; Dries, J. Cultivation of Aerobic Granular Sludge for the Treatment of Food-Processing Wastewater and the Impact on Membrane Filtration Properties. Water Sci. Technol. 2021, 83, 39–51. [Google Scholar] [CrossRef]
- Tavares Ferreira, T.J.; Luiz de Sousa Rollemberg, S.; Nascimento de Barros, A.; Machado de Lima, J.P.; Bezerra dos Santos, A. Integrated Review of Resource Recovery on Aerobic Granular Sludge Systems: Possibilities and Challenges for the Application of the Biorefinery Concept. J. Environ. Manag. 2021, 291, 112718. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.-Y.; Qin, Y.; Zhang, Z.-M.; Yuan, S.-C.; Chen, Y.; Zhu, J.-Y.; Qin, Y.; Zhang, Z.-M.; Yuan, S.-C. Reactivation of Hypersaline Aerobic Granular Sludge after Low-Temperature Storage. Tecnol. Cienc. Agua 2017, 8, 61–70. [Google Scholar] [CrossRef]
- Muñoz-Palazón, B. Biological and Technical Study of Aerobic Granular Sludge Systems for Treating Urban Wastewater Effect of Temperature. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2020. [Google Scholar]
- Giesen, A.; de Bruin, L.M.M.; Niermans, R.P.; van der Roest, H.F. Advancements in the Application of Aerobic Granular Biomass Technology for Sustainable Treatment of Wastewater. Water Pract. Technol. 2013, 8, 47–54. [Google Scholar] [CrossRef]
- Dall’Agnol, P.; Libardi, N.; Muller, J.M.; Xavier, J.A.; Domingos, D.G.; Da Costa, R.H.R. A Comparative Study of Phosphorus Removal Using Biopolymer from Aerobic Granular Sludge: A Factorial Experimental Evaluation. J. Environ. Chem. Eng. 2020, 8, 103541. [Google Scholar] [CrossRef]
- Ladnorg, S.; Junior, N.L.; Dall’Agnol, P.; Domingos, D.G.; Magnus, B.S.; Wichern, M.; Gehring, T.; Da Costa, R.H.R. Alginate-like Exopolysaccharide Extracted from Aerobic Granular Sludge as Biosorbent for Methylene Blue: Thermodynamic, Kinetic and Isotherm Studies. J. Environ. Chem. Eng. 2019, 7, 103081. [Google Scholar] [CrossRef]
- Lemaire, R.; Yuan, Z.; Blackall, L.L.; Crocetti, G.R. Microbial Distribution of Accumulibacter Spp. and Competibacter Spp. in Aerobic Granules from a Lab-Scale Biological Nutrient Removal System. Environ. Microbiol. 2008, 10, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The Effects of Shear Force on the Formation, Structure and Metabolism of Aerobic Granules. Appl. Microbiol. Biotechnol. 2001, 57, 227–233. [Google Scholar] [CrossRef]
- Weber, S.D.; Ludwig, W.; Schleifer, K.H.; Fried, J. Microbial Composition and Structure of Aerobic Granular Sewage Biofilms. Appl. Environ. Microbiol. 2007, 73, 6233–6240. [Google Scholar] [CrossRef]
- Song, Z.; Ren, N.; Zhang, K.; Tong, L. Influence of Temperature on the Characteristics of Aerobic Granulation in Sequencing Batch Airlift Reactors. J. Environ. Sci. 2009, 21, 273–278. [Google Scholar] [CrossRef]
- Lee, S.; Basu, S.; Tyler, C.; Pitt, P.A. A Survey of Filamentous Organisms at the Deer Island Treatment Plant. Env. Technol. 2008, 24, 855–865. [Google Scholar] [CrossRef]
- Rossetti, S.; Tomei, M.C.; Nielsen, P.H.; Tandoi, V. “Microthrix Parvicella”, a Filamentous Bacterium Causing Bulking and Foaming in Activated Sludge Systems: A Review of Current Knowledge. FEMS Microbiol. Rev. 2005, 29, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Y.; Yu, H.Q.; Sun, Y.J.; Huang, X. Characteristics of Aerobic Granules Rich in Autotrophic Ammonium-Oxidizing Bacteria in a Sequencing Batch Reactor. Chem. Eng. J. 2009, 147, 102–109. [Google Scholar] [CrossRef]
- Kim, D.J.; Seo, D. Selective Enrichment and Granulation of Ammonia Oxidizers in a Sequencing Batch Airlift Reactor. Process Biochem. 2006, 41, 1055–1062. [Google Scholar] [CrossRef]
- Al-Hashimi, M.A.I.; Abbas, T.R.; Jumaha, G.F. Aerobic Granular Sludge: An Advanced Technology to Treat Oil Refinery and Dairy Wastewaters. Eng. Technol. J. 2017, 35, 216–221. [Google Scholar]
- Bumbac, C.; Ionescu, I.A.; Tiron, O.; Badescu, V.R. Continuous Flow Aerobic Granular Sludge Reactor for Dairy Wastewater Treatment. Water Sci. Technol. 2015, 71, 440–445. [Google Scholar] [CrossRef]
- Ren, Y.; Ferraz, F.M.; Yuan, Q. Landfill Leachate Treatment Using Aerobic Granular Sludge. J. Environ. Eng. 2017, 143, 04017060. [Google Scholar] [CrossRef]
- Ho, K.L.; Chen, Y.Y.; Lin, B.; Lee, D.J. Degrading High-Strength Phenol Using Aerobic Granular Sludge. Appl. Microbiol. Biotechnol. 2010, 85, 2009–2015. [Google Scholar] [CrossRef]
- Carucci, A.; Milia, S.; De Gioannis, G.; Piredda, M. Acetate-Fed Aerobic Granular Sludge for the Degradation of 4-Chlorophenol. J. Hazard. Mater. 2009, 166, 483–490. [Google Scholar] [CrossRef]
- Wang, S.G.; Liu, X.W.; Zhang, H.Y.; Gong, W.X.; Sun, X.F.; Gao, B.Y. Aerobic Granulation for 2,4-Dichlorophenol Biodegradation in a Sequencing Batch Reactor. Chemosphere 2007, 69, 769–775. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, J.M.; Fang, F. Biodegradation of Methyl T-Butyl Ether by Aerobic Granules under a Cosubstrate Condition. Appl. Microbiol. Biotechnol. 2008, 78, 543–550. [Google Scholar] [CrossRef]
- Schwarzenbeck, N.; Borges, J.M.; Wilderer, P.A. Treatment of Dairy Effluents in an Aerobic Granular Sludge Sequencing Batch Reactor. Appl. Microbiol. Biotechnol. 2005, 66, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Moy, B.; Kong, Y.H.; Tay, J.H. Formation, Physical Characteristics and Microbial Community Structure of Aerobic Granules in a Pilot-Scale Sequencing Batch Reactor for Real Wastewater Treatment. Enzyme Microb. Technol. 2010, 46, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.; Anuar, A.N.; Ujang, Z.; Rosman, N.H.; Harun, H.; Chelliapan, S. Livestock Wastewater Treatment Using Aerobic Granular Sludge. Bioresour. Technol. 2013, 133, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Ujang, Z.; Yahya, A. Aerobic Granular Sludge Formation for High Strength Agro-Based Wastewater Treatment. Bioresour. Technol. 2011, 102, 6778–6781. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.; Li, X.; Yuan, Y. Performance of Aerobic Granular Sludge in a Sequencing Batch Bioreactor for Slaughterhouse Wastewater Treatment. Bioresour. Technol. 2015, 190, 487–491. [Google Scholar] [CrossRef]
- Rosman, N.H.; Nor Anuar, A.; Othman, I.; Harun, H.; Sulong, M.Z.; Elias, S.H.; Mat Hassan, M.A.H.; Chelliapan, S.; Ujang, Z. Cultivation of Aerobic Granular Sludge for Rubber Wastewater Treatment. Bioresour. Technol. 2013, 129, 620–623. [Google Scholar] [CrossRef]
- Adav, S.S.; Chen, M.Y.; Lee, D.J.; Ren, N.Q. Degradation of Phenol by Aerobic Granules and Isolated Yeast Candida Tropicalis. Biotechnol. Bioeng. 2007, 96, 844–852. [Google Scholar] [CrossRef]
- Suja, E.; Nancharaiah, Y.V.; Venugopalan, V.P. P-Nitrophenol Biodegradation by Aerobic Microbial Granules. Appl. Biochem. Biotechnol. 2012, 167, 1569–1577. [Google Scholar] [CrossRef]
- Kiran Kumar Reddy, G.; Sarvajith, M.; Nancharaiah, Y.V.; Venugopalan, V.P. 2,4-Dinitrotoluene Removal in Aerobic Granular Biomass Sequencing Batch Reactors. Int. Biodeterior. Biodegradation 2017, 119, 56–65. [Google Scholar] [CrossRef]
- De Kreuk, M.K.; Kishida, N.; van Loosdrecht, M.C.M. Aerobic Granular Sludge—State of the Art. Water Sci. Technol. 2007, 55, 75–81. [Google Scholar] [CrossRef]
- Ni, B.J.; Xie, W.M.; Liu, S.G.; Yu, H.Q.; Wang, Y.Z.; Wang, G.; Dai, X.L. Granulation of Activated Sludge in a Pilot-Scale Sequencing Batch Reactor for the Treatment of Low-Strength Municipal Wastewater. Water Res. 2009, 43, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Luiz De Souza Rollemberg, S.; Queiroz De Oliveira, L.; Igor, P.; Firmino, M.; Bezerra, A.; Santos, D. Aerobic Granular Sludge Technology in Domestic Wastewater Treatment: Opportunities and Challenges. Eng. Sanit. Ambient. 2020, 25, 439–449. [Google Scholar] [CrossRef]
- Bay Area Clean Water Agencies (BACWA)—AECOM. Nereda® Aerobic Granular Sludge Demonstration. 2017. Available online: https://bacwa.org/wp-content/uploads/2017/04/BACWA-AECOM-March-17th-2017-Nereda-Demonstration-Opportunity-3.pdf (accessed on 4 July 2022).
- Guo, H.; van Lier, J.B.; de Kreuk, M. Digestibility of Waste Aerobic Granular Sludge from a Full-Scale Municipal Wastewater Treatment System. Water Res. 2020, 173, 115617. [Google Scholar] [CrossRef] [PubMed]
- Świątczak, P.; Cydzik-Kwiatkowska, A. Performance and Microbial Characteristics of Biomass in a Full-Scale Aerobic Granular Sludge Wastewater Treatment Plant. Environ. Sci. Pollut. Res. 2018, 25, 1655–1669. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2020, 14, 150. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Pavičić, J.; Mavar, K.N.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.u.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Bot, F.; Plazzotta, S.; Anese, M. Treatment of Food Industry Wastewater With Ultrasound: A Big Opportunity for the Technology. Ultrasound Adv. Food Process. Preserv. 2017, 391–408. [Google Scholar] [CrossRef]
- Debowski, M.; Zielinski, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M. Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R). Energies 2020, 13, 6626. [Google Scholar] [CrossRef]
- Chinnici, G.; Selvaggi, R.; D’Amico, M.; Pecorino, B. Assessment of the Potential Energy Supply and Biomethane from the Anaerobic Digestion of Agro-Food Feedstocks in Sicily. Renew. Sustain. Energy Rev. 2018, 82, 6–13. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of Methane Fermentation as a Valorisation Method for Food Waste Products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Jankowska, E.; Zieliński, M.; Dȩbowski, M.; Oleśkowicz-Popiel, P. Anaerobic Digestion of Microalgae for Biomethane Production. Second Third Gener. Feed. Evol. Biofuels 2019, 405–436. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Cybulska, K.; Kołosowska, I.; Kramkowski, K.; Karpińska, M.; Roszkowicz-Ostrowska, K.; Kowalczyk, P. Improvement of Biogas Yield by Pre-Treating Poultry Waste with Bacterial Strains. Energies 2021, 14, 5601. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Yolanda Moratilla Soria, B. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Świątek, M.; Lewicki, A.; Szymanowska, D.; Kubiak, P. The Effect of Introduction of Chicken Manure on the Biodiversity and Performance of an Anaerobic Digester. Electron. J. Biotechnol. 2019, 37, 25–33. [Google Scholar] [CrossRef]
- Kazimierowicz, J. Organic Waste Used in Agricultural Biogas Plants. J. Ecol. Eng. 2014, 15, 88–92. [Google Scholar] [CrossRef]
- Gayathiri, G.; Kiruthiga, P.; Karthikeyan, R.; Anand, A.V.; Sivamurugan, V.; Saradhadevi, K.M. Enzymatic Production of Organic Acids via Microbial Fermentative Processes. In Biomass, Biofuels, Biochemicals: Biochemicals and Materials Production from Sustainable Biomass Resources; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–54. [Google Scholar] [CrossRef]
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Czekała, W. Biogas as a Sustainable and Renewable Energy Source. Energy Environ. Sustain. 2022, 201–214. [Google Scholar] [CrossRef]
- Lim, H.G.; Lee, J.H.; Noh, M.H.; Jung, G.Y. Rediscovering Acetate Metabolism: Its Potential Sources and Utilization for Biobased Transformation into Value-Added Chemicals. J. Agric. Food Chem. 2018, 66, 3998–4006. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Singh, S.P.; Srivastava, S.K.; Singh, S.P.; Iqbal, H.M.N.; Varjani, S. Different Stages of Microbial Community during the Anaerobic Digestion of Food Waste. J. Food Sci. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M. Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Andriani, D.; Rajani, A.; Kusnadi; Santosa, A.; Saepudin, A.; Wresta, A.; Atmaja, T.D. A Review on Biogas Purification through Hydrogen Sulphide Removal. IOP Conf. Ser. Earth Environ. Sci. 2020, 483, 012034. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.K.; Kalia, V.C.; Lee, J.K. Integrating Strategies for Sustainable Conversion of Waste Biomass into Dark-Fermentative Hydrogen and Value-Added Products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on Microbial Community in Anaerobic Digestion with Emphasis on Environmental Parameters: A Systematic Review. Chemosphere 2021, 270, 128618. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Influence of Feedstock Mix Ratio on Microbial Dynamics during Acidogenic Fermentation for Polyhydroxyalkanoates Production. J. Environ. Manag. 2022, 303, 114132. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in Biogas Production: Pretreatment and Codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of Pretreatment Strategies for Enhancing Sewage Sludge Disintegration and Subsequent Anaerobic Digestion: Current Advances, Full-Scale Application and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Ampese, L.C.; Sganzerla, W.G.; Di Domenico Ziero, H.; Mudhoo, A.; Martins, G.; Forster-Carneiro, T. Research Progress, Trends, and Updates on Anaerobic Digestion Technology: A Bibliometric Analysis. J. Clean. Prod. 2022, 331, 130004. [Google Scholar] [CrossRef]
- Grosser, A.; Grobelak, A.; Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemière, S.; Guyoneaud, R.; Attard, E.; Celary, P. Effects of Silver Nanoparticles on Performance of Anaerobic Digestion of Sewage Sludge and Associated Microbial Communities. Renew. Energy 2021, 171, 1014–1025. [Google Scholar] [CrossRef]
- Markowski, M.; Białobrzewski, I.; Zieliński, M.; Debowski, M.; Krzemieniewski, M. Optimizing Low-Temperature Biogas Production from Biomass by Anaerobic Digestion. Renew. Energy 2014, 69, 219–225. [Google Scholar] [CrossRef]
- Novais, R.M.; Gameiro, T.; Carvalheiras, J.; Seabra, M.P.; Tarelho, L.A.C.; Labrincha, J.A.; Capela, I. High PH Buffer Capacity Biomass Fly Ash-Based Geopolymer Spheres to Boost Methane Yield in Anaerobic Digestion. J. Clean. Prod. 2018, 178, 258–267. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.H.; Zheng, Y.; Liu, J.B.; Xiong, W.P.; Zhang, Y.R.; Lu, Y.; Xue, W.J.; Fan, C.Z. Organic Loading Rate and Hydraulic Retention Time Shape Distinct Ecological Networks of Anaerobic Digestion Related Microbiome. Bioresour. Technol. 2018, 262, 184–193. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Microwave Radiation Influence on Dairy Waste Anaerobic Digestion in a Multi-Section Hybrid Anaerobic Reactor (M-SHAR). Processes 2021, 9, 1772. [Google Scholar] [CrossRef]
- Wade, M.J. Not Just Numbers: Mathematical Modelling and Its Contribution to Anaerobic Digestion Processes. Processes 2020, 8, 888. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielińska, M.; Gusiatin, M.Z.; Wojnowska-Baryła, I.; Kulikowska, D. Valorization of Full-Scale Waste Aerobic Granular Sludge for Biogas Production and the Characteristics of the Digestate. Chemosphere 2022, 303, 135167. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.; Saracevic, E.; Svardal, K.; Krampe, J. Anaerobic Biodegradation and Dewaterability of Aerobic Granular Sludge. J. Chem. Technol. Biotechnol. 2019, 94, 2908–2916. [Google Scholar] [CrossRef]
- Cecconi, F.; Garrido-Baserba, M.; Eschborn, R.; Damerel, J.; Rosso, D. Oxygen Transfer Investigations in an Aerobic Granular Sludge Reactor. Environ. Sci. Water Res. Technol. 2020, 6, 679–690. [Google Scholar] [CrossRef]
- Luiz de Sousa Rollemberg, S.; Queiroz de Oliveira, L.; Nascimento de Barros, A.; Igor Milen Firmino, P.; Bezerra dos Santos, A. Pilot-Scale Aerobic Granular Sludge in the Treatment of Municipal Wastewater: Optimizations in the Start-up, Methodology of Sludge Discharge, and Evaluation of Resource Recovery. Bioresour. Technol. 2020, 311, 123467. [Google Scholar] [CrossRef] [PubMed]
- Bernat, K.; Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I.; Karczewska, M. Physicochemical Properties and Biogas Productivity of Aerobic Granular Sludge and Activated Sludge. Biochem. Eng. J. 2017, 117, 43–51. [Google Scholar] [CrossRef]
- Guo, H.; Felz, S.; Lin, Y.; van Lier, J.B.; de Kreuk, M. Structural Extracellular Polymeric Substances Determine the Difference in Digestibility between Waste Activated Sludge and Aerobic Granules. Water Res. 2020, 181, 115924. [Google Scholar] [CrossRef]
- Ali, M.; Wang, Z.; Salam, K.W.; Hari, A.R.; Pronk, M.; Van Loosdrecht, M.C.M.; Saikaly, P.E. Importance of Species Sorting and Immigration on the Bacterial Assembly of Different-Sized Aggregates in a Full-Scale Aerobic Granular Sludge Plant. Environ. Sci. Technol. 2019, 53, 8291–8301. [Google Scholar] [CrossRef]
- Val Del Río, Á.; Palmeiro-Sanchez, T.; Figueroa, M.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Anaerobic Digestion of Aerobic Granular Biomass: Effects of Thermal Pre-Treatment and Addition of Primary Sludge. J. Chem. Technol. Biotechnol. 2014, 89, 690–697. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; Val del Río, A.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Comparison of the Anaerobic Digestion of Activated and Aerobic Granular Sludges under Brackish Conditions. Chem. Eng. J. 2013, 231, 449–454. [Google Scholar] [CrossRef]
- Liu, Y.; Nilsen, P.J.; Maulidiany, N.D. Thermal Pretreatment to Enhance Biogas Production of Waste Aerobic Granular Sludge with and without Calcium Phosphate Precipitates. Chemosphere 2019, 234, 725–732. [Google Scholar] [CrossRef]
- Kehrein, P.; Van Loosdrecht, M.; Osseweijer, P.; Posada, J. Exploring Resource Recovery Potentials for the Aerobic Granular Sludge Process by Mass and Energy Balances—Energy, Biopolymer and Phosphorous Recovery from Municipal Wastewater. Environ. Sci. Water Res. Technol. 2020, 6, 2164–2179. [Google Scholar] [CrossRef]
- Val del Río, A.; Morales, N.; Isanta, E.; Mosquera-Corral, A.; Campos, J.L.; Steyer, J.P.; Carrère, H. Thermal Pre-Treatment of Aerobic Granular Sludge: Impact on Anaerobic Biodegradability. Water Res. 2011, 45, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Breitenmoser, L.; Gross, T.; Huesch, R.; Rau, J.; Dhar, H.; Kumar, S.; Hugi, C.; Wintgens, T. Anaerobic Digestion of Biowastes in India: Opportunities, Challenges and Research Needs. J. Environ. Manag. 2019, 236, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Dubey, B.K. A Critical Review on Operating Parameters and Strategies to Improve the Biogas Yield from Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- De Sanctis, M.; Altieri, V.G.; Piergrossi, V.; Di Iaconi, C. Aerobic Granular-Based Technology for Water and Energy Recovery from Municipal Wastewater. N. Biotechnol. 2020, 56, 71–78. [Google Scholar] [CrossRef]
- Thwaites, B.J.; Stuetz, R.; Short, M.; Reeve, P.; Alvarez-Gaitan, J.P.; Dinesh, N.; Philips, R.; van den Akker, B. Analysis of Nitrous Oxide Emissions from Aerobic Granular Sludge Treating High Saline Municipal Wastewater. Sci. Total Environ. 2021, 756, 143653. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Amorim, C.L.; Ramos, M.A.; Mesquita, D.P.; Inocêncio, P.; Ferreira, E.C.; Van Loosdrecht, M.; Castro, P.M.L. Variability in the Composition of Extracellular Polymeric Substances from a Full-Scale Aerobic Granular Sludge Reactor Treating Urban Wastewater. J. Environ. Chem. Eng. 2020, 8, 104156. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Zhou, X.; Wang, X.; Zhu, J. Effect of Different Vegetable Wastes on the Performance of Volatile Fatty Acids Production by Anaerobic Fermentation. Sci. Total Environ. 2020, 748, 142390. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Li, S.; Zhang, S.; Han, G. The Removal of Erythromycin and Its Effects on Anaerobic Fermentation. Int. J. Environ. Res. Public Heal. 2022, 19, 7256. [Google Scholar] [CrossRef]
- Toja Ortega, S.; Pronk, M.; de Kreuk, M.K. Anaerobic Hydrolysis of Complex Substrates in Full-Scale Aerobic Granular Sludge: Enzymatic Activity Determined in Different Sludge Fractions. Appl. Microbiol. Biotechnol. 2021, 105, 6073–6086. [Google Scholar] [CrossRef]
- Keerthana, S.; Kalaiselvi, P.; Maheshwari, M.; Kalaiselvi, T. Isolation and Screening of Lignin and Cellulose Degrading Proficient Microbial Strains from Diverse Biotic Substrates Based on Qualitative Traits. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 475–483. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K.; Meda, V. A Review of Torrefaction Technology for Upgrading Lignocellulosic Biomass to Solid Biofuels. BioEnergy Res. 2021, 14, 645–669. [Google Scholar] [CrossRef]
- Kisielewska, M.; Rusanowska, P.; Dudek, M.; Nowicka, A.; Krzywik, A.; Dębowski, M.; Joanna, K.; Zieliński, M. Evaluation of Ultrasound Pretreatment for Enhanced Anaerobic Digestion of Sida Hermaphrodita. Bioenergy Res. 2020, 13, 824–832. [Google Scholar] [CrossRef]
- Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic Corn Stover Biomass Pre-Treatment by Deep Eutectic Solvents (DES) for Biomethane Production Process by Bioresource Anaerobic Digestion. Sustainability 2021, 13, 10504. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A Comprehensive Review on the Pretreatment of Lignocellulosic Wastes for Improved Biogas Production by Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2021, 19, 3429–3456. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Xue, Y. Nickel-Loaded Shrimp Shell Biochar Enhances Batch Anaerobic Digestion of Food Waste. Bioresour. Technol. 2022, 352, 127092. [Google Scholar] [CrossRef]
- Li, X.; Xiong, N.; Wang, X.; Dai, X.; Guo, Y.; Dong, B. New Insight into Volatile Sulfur Compounds Conversion in Anaerobic Digestion of Excess Sludge: Influence of Free Ammonia Nitrogen and Thermal Hydrolysis Pretreatment. J. Clean. Prod. 2020, 277, 123366. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Zhu, T.; Li, J.; Feng, Y.; Zhao, H.; Liu, S. A Metabolomic View of How Low Nitrogen Strength Favors Anammox Biomass Yield and Nitrogen Removal Capability. Water Res. 2018, 143, 387–398. [Google Scholar] [CrossRef]
- Perendeci, N.A. Boosting Biogas Production and Process Stability by Pretreatment. AIP Conf. Proc. 2021, 2447, 020002. [Google Scholar] [CrossRef]
- Buhlmann, C.H.; Mickan, B.S.; Jenkins, S.N.; Tait, S.; Kahandawala, T.K.A.; Bahri, P.A. Ammonia Stress on a Resilient Mesophilic Anaerobic Inoculum: Methane Production, Microbial Community, and Putative Metabolic Pathways. Bioresour. Technol. 2019, 275, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.P.; Sharaf, A.; Zhang, L.; Liu, Y. Overcoming Ammonia Inhibition in Anaerobic Blackwater Treatment with Granular Activated Carbon: The Role of Electroactive Microorganisms. Environ. Sci. Water Res. Technol. 2019, 5, 383–396. [Google Scholar] [CrossRef]
- Yan, W.; Mukherjee, M.; Zhou, Y. Direct Interspecies Electron Transfer (DIET) Can Be Suppressed under Ammonia-Stressed Condition—Reevaluate the Role of Conductive Materials. Water Res. 2020, 183, 116094. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Deng, D.; Li, R.; Guo, C.; Ma, J.; Chen, M. Investigation of Extracellular Polymeric Substances (EPS) in Four Types of Sludge: Factors Influencing EPS Properties and Sludge Granulation. J. Water Process Eng. 2021, 40, 101924. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A. Biopolymers in Aerobic Granular Sludge—Their Role in Wastewater Treatment and Possibilities of Re-Use in Line with Circular Economy. Energies 2021, 14, 7219. [Google Scholar] [CrossRef]
- Wang, Z.-W.; Liu, Y. Biodegradability of Extracellular Polymeric Substances Produced by Aerobic Granules. Wastewater Purif. 2007, 209–222. [Google Scholar] [CrossRef]
- Sanz, J.L.; Köchling, T. Next-Generation Sequencing and Waste/Wastewater Treatment: A Comprehensive Overview. Rev. Environ. Sci. Biotechnol. 2019, 18, 635–680. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Comparison of Ultrasonic and Hydrothermal Cavitation Pretreatments of Cattle Manure Mixed with Straw Wheat on Fermentative Biogas Production. Waste and Biomass Valorization 2019, 10, 747–754. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Chang, S.W.; Ngo, H.H.; Guo, W.; Nghiem, L.D.; Banu, J.R.; Jeon, B.H.; Nguyen, D.D. Influence of Thermal Hydrolysis Pretreatment on Physicochemical Properties and Anaerobic Biodegradability of Waste Activated Sludge with Different Solids Content. Waste Manag. 2019, 85, 214–221. [Google Scholar] [CrossRef]
- Farhat, A.; Asses, N.; Ennouri, H.; Hamdi, M.; Bouallagui, H. Combined Effects of Thermal Pretreatment and Increasing Organic Loading by Co-Substrate Addition for Enhancing Municipal Sewage Sludge Anaerobic Digestion and Energy Production. Process Saf. Environ. Prot. 2018, 119, 14–22. [Google Scholar] [CrossRef]
- Sun, D.; Qiao, M.; Xu, Y.; Ma, C.; Zhang, X. Pretreatment of Waste Activated Sludge by Peracetic Acid Oxidation for Enhanced Anaerobic Digestion. Environ. Prog. Sustain. Energy 2018, 37, 2058–2062. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, R.; Wu, Y. Hydrothermal Carbonization and Liquefaction of Sludge for Harmless and Resource Purposes: A Review. Energy Fuels 2020, 34, 13268–13290. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Di Maggio, R.; Toscano, A.; Andreottola, G. Effect of Hydrodynamic Cavitation on Flocs Structure in Sewage Sludge to Increase Stabilization for Efficient and Safe Reuse in Agriculture. Bioremediat. J. 2021, 26, 41–52. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Ji, Y.; Li, M.; Dong, B.; Qian, G.; Zhou, J.; Dai, X. Effects of Chemical Pretreatments on Microplastic Extraction in Sewage Sludge and Their Physicochemical Characteristics. Water Res. 2020, 171, 115379. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, J.; de Oliveira, D.; Maldonado, R.R.; Kamimura, E.S.; Furigo, A. Enzymatic Pretreatment and Anaerobic Co-Digestion as a New Technology to High-Methane Production. Appl. Microbiol. Biotechnol. 2020, 104, 4235–4246. [Google Scholar] [CrossRef] [PubMed]



| Operating Conditions | Impacts on Granulation Process | References |
|---|---|---|
| Additives Metal Cations | Ca2+, Mg2+, Fe2+/Fe3+ intensify granulation by neutralizing negatively charged sludge particles and enhancing adsorption/bridging interactions. | [23,33] |
| Aerobic starvation | Granulation is initiated by the lack of nutrients, increasing shear force and an increase in the hydrophobicity of the bacteria. | [34] |
| Coagulant or inert carrier | Effect on the neutralization of negatively charged particles, which promotes aggregation and adsorption of the flocs. The large surface area of coagulants and inert carriers increases the granulation efficiency. | [21,33] |
| Extracellular polymeric substances (EPS) | EPS aggregates bacterial cells and other solid particles to a granule precursor. The high content of EPS in the system allows the granules to withstand high values of hydraulic and pollutant loading. | [23,35] |
| Food to microorganism F/M ratio | A high F/M ratio facilitates the formation of large granules. Finding the right F/M ratio is essential for achieving a fast and stable granulation. | [36,37] |
| Hydraulic retention time (HRT) | Increasing HRT reduces OLR, which limits the granulation efficiency, hinders sedimentation and leads to a decrease in biomass concentration in the technological system, as well as the size and stability of granules. | [36,38] |
| Hydrodynamic shear force | It regulates the growth of fibres, the porosity and density of the granules as well as the stability of granulation. Higher hydrodynamic shear provides better compaction and density of the granules. | [21,39] |
| Organic loading rate (OLR) | High OLR allows for quick and efficient granulation, while delayed and difficult granulation formation was observed with low OLR. | [36,40] |
| Seeding sludge | Type of seed pellets may contain cations and other properties that can help speed up the granulation process. It also acts as a nucleus that promotes the attraction of sludge flocs. | [21,33] |
| Settling period | Removes poorly settling, flocculent sludge, enabling the deposition of appropriate granules and the selection of appropriate species of microorganisms. | [21,33] |
| Sludge retention time SRT | Prolonged SRT causes deterioration of aerobic granulation, discharge of aging granular sludge and retention of appropriate newly synthesized granules is required for the stability of the aerobic granular sludge process, while shorter SRT results in a reduction of the size of the sludge flocs. | [4,37] |
| Temperature | Granulation was successfully carried out in the temperature range of 8–30 °C. It was proven that low temperatures caused an increase in fiber content, causing leaching of bacterial cells and instability of granules. | [4,41] |
| Volumetric exchange ratio | High volumetric exchange rates increase the granulation, facilitating the formation and improving the sedimentation properties of the granules. | [36,40] |
| Parameter | Unit | Value | Reference | |
|---|---|---|---|---|
| AGS | CAS | |||
| Shape | - | Compact and spherical granular structure | Irregular and flocculent structure | [39] |
| Size | μm | >200 | 50–300 | [41] |
| Settling velocity | m/h | 10–130 | 2–10 | [60] |
| Specific gravity | - | 1.010–1.017 | 0.997–1.01 | [7] |
| 1.004–1.100 | 1.002–1.106 | [55] | ||
| Water content | % | 94–97 | 99 | [55] |
| Sludge Volume Index | mL/g | [39,61] | ||
| 5 min | 30–60 | – | ||
| 30 min | 30–60 | 110–160 | ||
| Redox microenvironments | - | Aerobic, anoxic, and anaerobic microbial layers | Minimum feasibility for anaerobic zones | [7,62] |
| EPS synthesis | - | High EPS content in aerobic granules as compared to CAS | Lower EPS content | [7,63] |
| OLR | - | Capable of withstanding high OLR | Poor removal performance at high OLR | [7,63] |
| Resistance to shock and fluctuating OLR | - | Able to remove pollutants under shock or fluctuating OLR | Poor removal under shock or fluctuating OLR | [7] |
| Tolerance to toxic compounds | - | Higher tolerance to toxic pollutants | Lower tolerance to toxic pollutants | [7,63] |
| Type of Sewage/Waste | Initial Concentration [mg/dm3] | Reactor Configuration and Operation Conditions | Removal Efficiency [%] | Final Concentration [mg/dm3] * | Reference |
|---|---|---|---|---|---|
| Dairy wastewater | COD: 2800; TN: 40; TP: 30 | 12 L SBR; Cycle period: 8 h | COD: 90; TN: 80; TP: 67 | COD: 28; TN: 8; TP: 9.9 | [79] |
| Household wastewater | COD: 506; BOD5: 224; TN: 49.4; AN: 39 TP: 6.7; | Two SBR tanks with height of 7.5 m and working volume of 9600 m3 each; Cycle: 6.5 h in dry season, 3 h in rainy season; VER: 65% | COD: 88; BOD5: 96; TN: 86; AN: 97; TP: 87 | COD: 60.7; BOD5: 9; TN: 6.9; AN: 1.2; TP: 0.9 | [13] |
| Household sewage (40%) + industrial wastewater (60%) | COD: 1000; AN: 60 | SBR; Height: 100 cm and Diameter: 20 cm; Cycle period: 4 h; VER: 50% | COD: 80; TN: 98 | COD: 200; AN: 1.2 | [80] |
| Livestock wastewater | COD: 3600; TN: 650; TP: 380 | 4 L SBR; Cycle period: 4 h; VER: 50%; 27–30 °C | COD: 74; TN: 73; TP: 70 | COD: 93.6; TN: 175.5; TP: 114 | [81] |
| Palm oil mill effluents | COD: 69500; AN: 45 | 3 L SBR; Cycle period: 3 h; VER: 50% | COD: 91.1; AN: 97.6 | COD: 69500; AN: 45 | [82] |
| Septic wastewater | COD: 971; AN: 80; TP: 19; SS: 670 | Three SBR tanks (7 m height) with maximum capacity of 5000 m3 d−1 | COD: 94; AN: 99; TP: 83.5; SS: 98 | COD: 58.3; AN: 0.8; TP: 3.1; SS: 13.4 | [61] |
| Slaughter house wastewater | COD: 1250 ± 150; AN: 120 ± 20; TP: 30 ± 5 | 20 L volume SBR; Cycle period: 6 h; VER: 50%; 18–22 °C | COD: 95.1; AN: 99.3; TP: 83.5 | COD: 6.1; AN: 0.8; TP: 5.0 | [83] |
| Rubber industry wastewater | COD: 1850; TN: 248; AN: 49 | 0.6 L SBR; Cycle period: 3 h; VER: 50%; 27 ± 1 °C | COD: 96.5; TN: 89.4 AN: 94.7 | COD: 64.75; TN: 2.6 AN: 94.7 | [84] |
| Synthetic wastewater with phenol | 200 | 1 L SBR, 12 h cycle, 2 cycles per day, 30 °C, 200 rpm | 100 | - | [85] |
| 1000 | 97 | 30 | |||
| Synthetic wastewater with p-Nitrophenol (PNP) | 200 | 1 L SBR, 75% VER; 24 h cycle with 23.5 h aeration, 1.6 cm/s SAV, 30 °C | 100 | - | [86] |
| Synthetic wastewater with 2,4-Dintrotoluene (2,4-DNT) | 10 | 1 L SBR, 70% VER; 24 h cycle with 23 h aeration, 1.2 cm/s SAV, 30 °C, 100 rpm | 90 | 1 | [87] |
| Location | Start-Up | Flow Rate [m3/d] | Wastewater | Removal Efficiency [%] | Reference |
|---|---|---|---|---|---|
| Deodoro, Brazil | 2016 | Phase I—64,800 Phase II—86,400 | Municipal | COD: >90; TN: >60; TP: >50 | [39,90] |
| Epe, Netherlands | 2011 | 8000 | Municipal and food-industry | COD: 96.9; BOD5: >99.4; TN: >94.7; AN: 99.8; TP: 97.2; total suspended solids (TSS): >98.5 | [20,39,91] |
| Gansbaai, South Africa | 2009 | 4000 | Municipal (with a high proportion of industrial slaughterhouse effluent) | COD: 94; TN: 90; TP: >80 | [39,90] |
| Garmerwolde, Netherlands | 2013 | 30,000 | Municipal | COD: 89.2; BOD5: 96.0; TN: 86.0; TP: 90.3; TSS: 96.4 | [92] |
| Kingaroy, Australia | 2016 | 2625 | Municipal | COD: >90; TN: 95; TP: >90 | [39,90] |
| Lubawa, Poland | 2017 | 3200 | Mainly municipal, 30–40% dairy effluent | COD: 97.0; BOD5: 98.2; TN: 87.0; AN: 99.4 TP: 95.4; | [93] |
| Ryki, Poland | 2015 | 5320 | Municipal | COD: >90; TN: >90; TP: >90 | [39,90] |
| Yancang, China | 2008 | 50,000 | Urban (30% domestic sewage and 70% industrial wastewater from printing and dyeing, chemical, textile and beverage) | COD: 85; TN: 59.6; AN: 95.8 | [22] |
| Wastewater | Methane [dm3/kg VS] | Biodegradability [%] | Reference |
|---|---|---|---|
| Municipal | 272.5–357.2 * | - | [135] |
| Municipal | AGS-EX: 194 ± 10; AGS-SD: 198 ± 10 | COD: >96 | [92] |
| Municipal | 260 | COD: 51.1 ± 4.2; VSS: 50.9 ± 4.2 | [132] |
| Synthetic | 285 | COD: 59.6 ± 4.4; VSS: 59.9 ± 4.4 | [132] |
| Liquid fraction of swine manure | 210 * | VS: 44; TSS: 32 | [138] |
| Municipal | 197 ± 11 | VS: 25.4 ± 1.3 | [136] |
| Synthetic wastewater | 235–310 | - | [140] |
| Urban wastewater | 215 | - | [131] |
| Liquid fraction of swine manure | 169 | BD: 33 | [142] |
| Factor | Characteristics | Potential Effects on AD | Reference |
|---|---|---|---|
| Structure | Biomass composed of compact and dense granules | Higher biomass retention, resistance to high organic loadings and toxicity, which is beneficial for AD. | [39,166] |
| Lignocellulosic content | Of the filamentous substances present in AGS, approximately 54% are lignocellulosic substances resilient to AD | Lowers the performance of AGS AD (as expressed by methane yields). The lignocellulosic material usually has to be pre-treated. | [135,156] |
| Vesicle protein content | Nitrogen is an essential nutrient for microbes, usually released as ammonia during hydrolysis and digestion of protein-containing feedstocks. Selecting the wrong parameters for sludge AD can lead to elevated nitrogen levels in the digester | Can cause ammoniacal nitrogen to accumulate in the bioreactor, resulting in increased toxicity of the medium and reduced metabolic capacity of fermenting bacteria, especially sensitive methane-producing Archea. | [138,159,163] |
| Fiber content | High fractions of fiber, especially lignin—often more than 18% TS | Lignin inhibits biodegradation and reduces methane yields in BMP tests. | [37,154] |
| EPS content | A major component of the AGS structure. Most of the biodegradable fraction of EPS is embedded in the granule interior and core, whereas the surface is mostly composed of structure-forming polymers | Can serve as a readily-digestible and biodegradable source of organic matter for anaerobes, thus, significantly improving biogas yields and methane fractions. | [141,165,166] |
| Wastewater | Pre-Treatment | Methane [dm3/kg VS] | Biodegradability [%] | Reference |
|---|---|---|---|---|
| Liquid fraction of swine manure | Hydrothermal depolymerization at 20 °C | 169 ± 7 | BD: 33 ± 1 | [142] |
| Hydrothermal depolymerization at 60 °C | 207 ± 10 | BD: 40 ± 2 | ||
| Hydrothermal depolymerization at 90 °C | 236 ± 6 | BD: 47 ± 1 | ||
| Hydrothermal depolymerization at 115 °C | 280 ± 12 | BD: 54 ± 2 | ||
| Hydrothermal depolymerization at 140 °C | 308 ± 14 | BD: 60 ± 3 | ||
| Hydrothermal depolymerization at 170 °C | 337 ± 5 | BD: 62 ± 1 | ||
| Hydrothermal depolymerization at 190 °C | 311 ± 5 | BD: 56 ± 1 | ||
| Hydrothermal depolymerization at 210 °C | 314 ± 18 | BD: 52 ± 3 | ||
| Model municipal wastewater | Hydrothermal depolymerization at 20 °C | 243 ± 1 | BD: 49 ± 0 | [142] |
| Hydrothermal depolymerization at 170 °C | 346 ± 7 | BD: 46 ± 1 | ||
| Hydrothermal depolymerization at 190 °C | 370 ± 15 | BD: 56 ± 2 | ||
| Hydrothermal depolymerization at 210 °C | 404 ± 23 | BD: 58 ± 3 | ||
| Liquid fraction of swine manure | Heat treatment at 133 °C | - | TSS: 47 | [138] |
| Synthetic wastewater | Steam explosion at 170 °C | 370–400 | - | [140] |
| Urban wastewater | Ultrasound disintegration | 300 | - | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierowicz, J.; Dębowski, M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability 2022, 14, 10904. https://doi.org/10.3390/su141710904
Kazimierowicz J, Dębowski M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability. 2022; 14(17):10904. https://doi.org/10.3390/su141710904
Chicago/Turabian StyleKazimierowicz, Joanna, and Marcin Dębowski. 2022. "Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives" Sustainability 14, no. 17: 10904. https://doi.org/10.3390/su141710904
APA StyleKazimierowicz, J., & Dębowski, M. (2022). Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability, 14(17), 10904. https://doi.org/10.3390/su141710904








