The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Materials
2.2.1. Microalgae Biomass
2.2.2. Dairy Wastewater
2.2.3. Culture Medium
2.2.4. Biohydrogen Production Medium
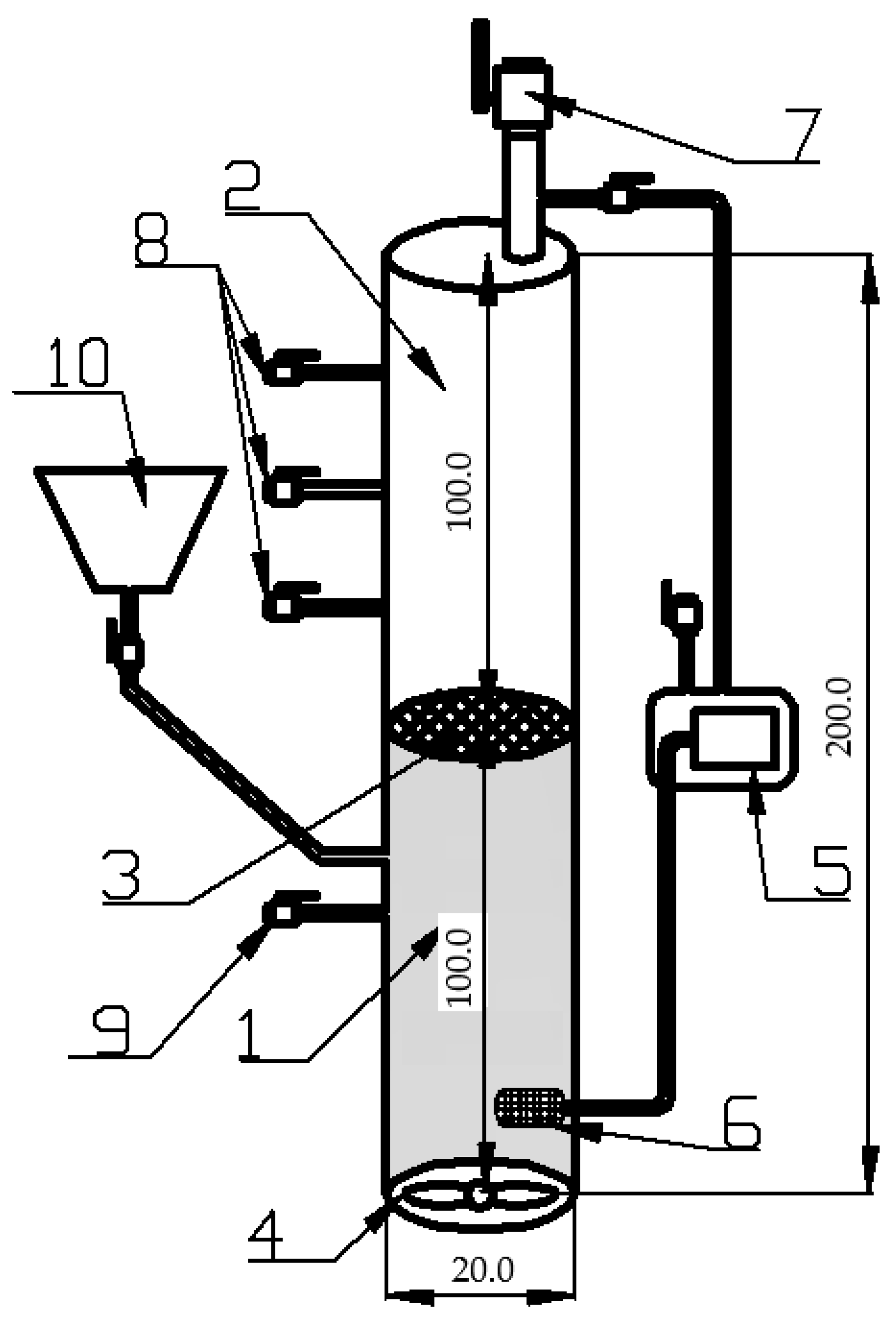
2.3. Experimental Stations
2.3.1. Bioreactor for Wastewater Treatment
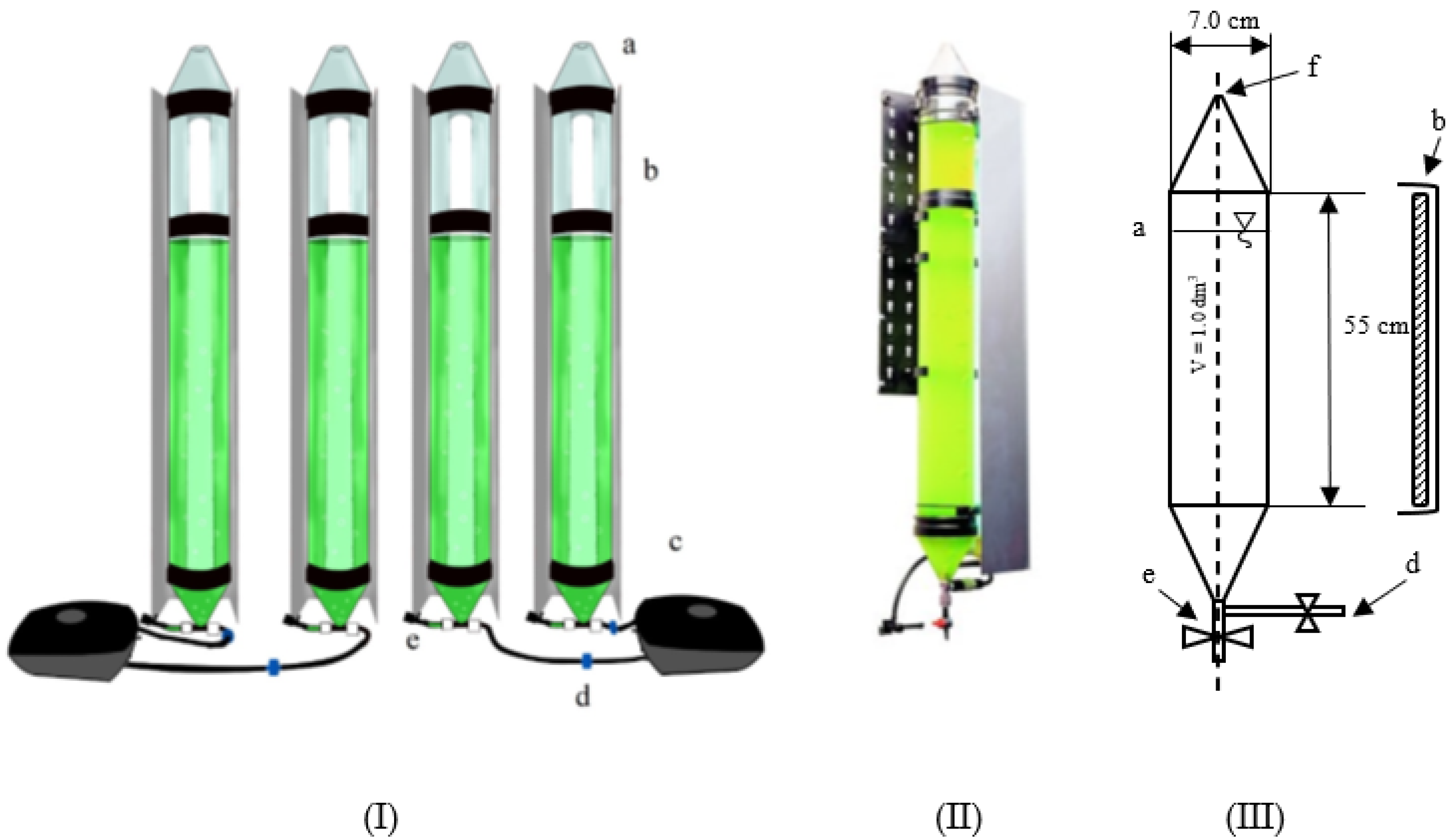
2.3.2. Microalgae Biomass Cultivation in PBR
2.3.3. Biomass Separation Station
2.3.4. Hydrogen Production in PBR
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. Wastewater Treatment in the Bioreactor
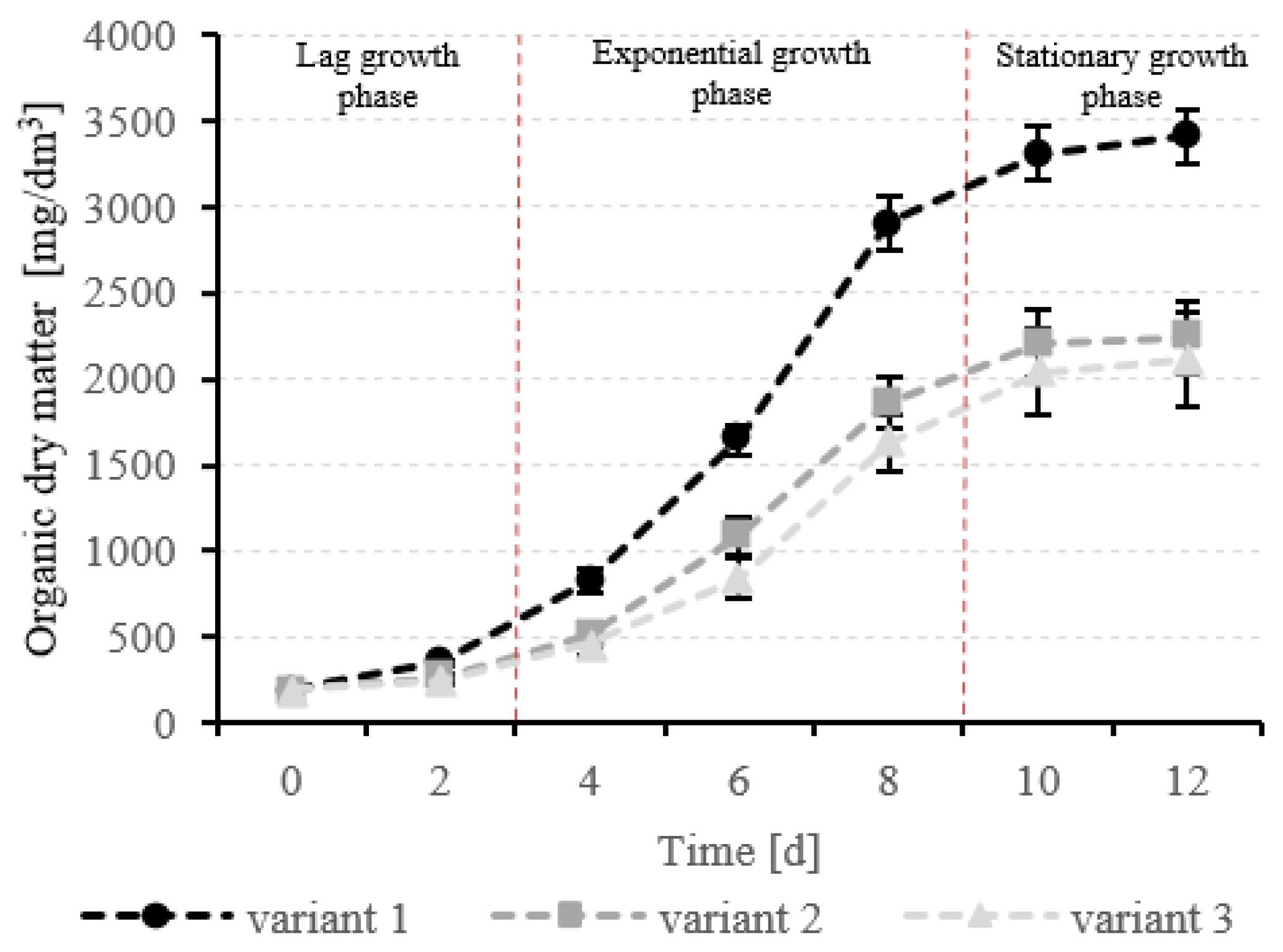
3.2. Production of T. Subcordiformis Biomass
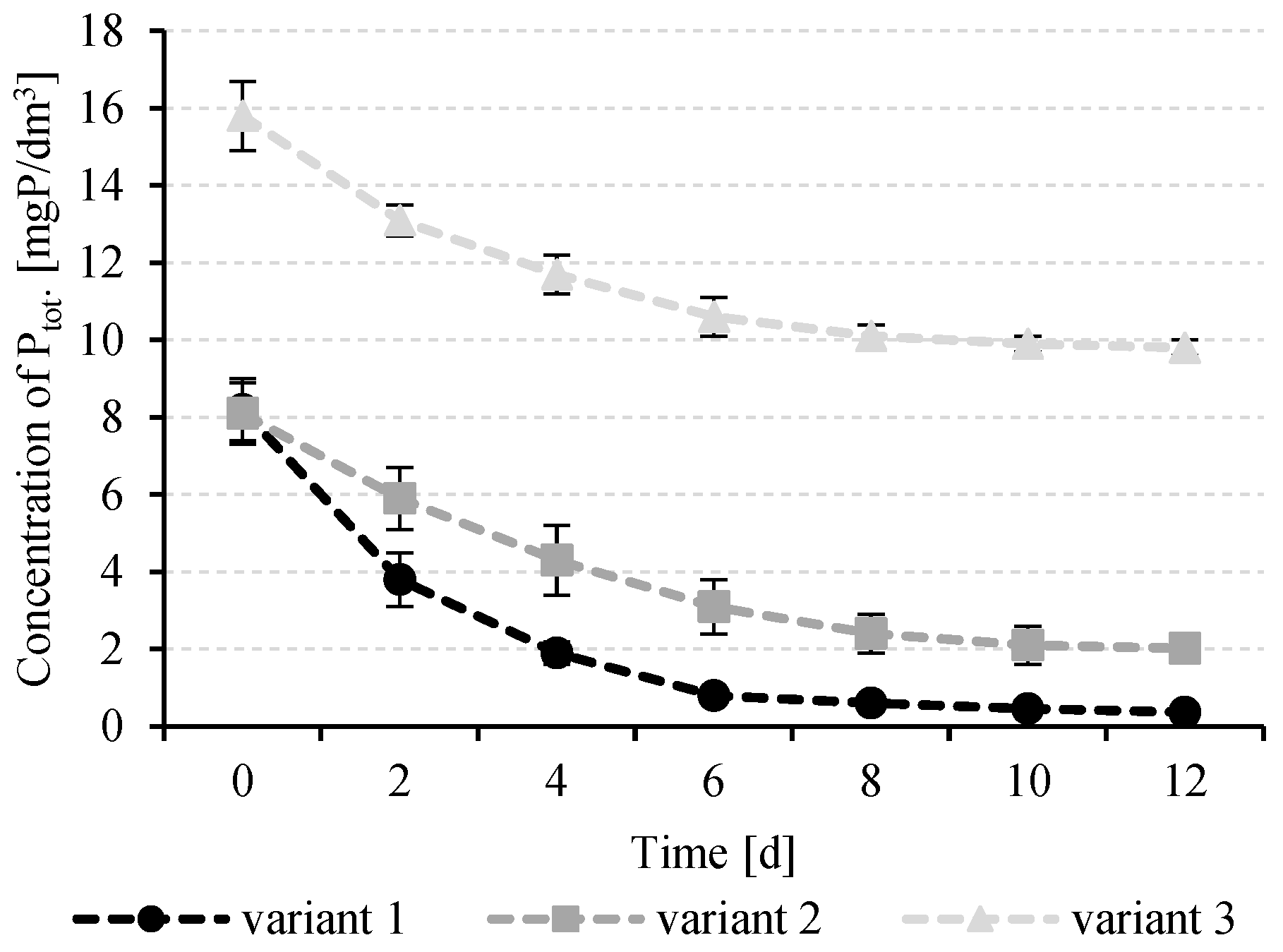
3.3. Removal of Biogenes by Tetraselmis sp.
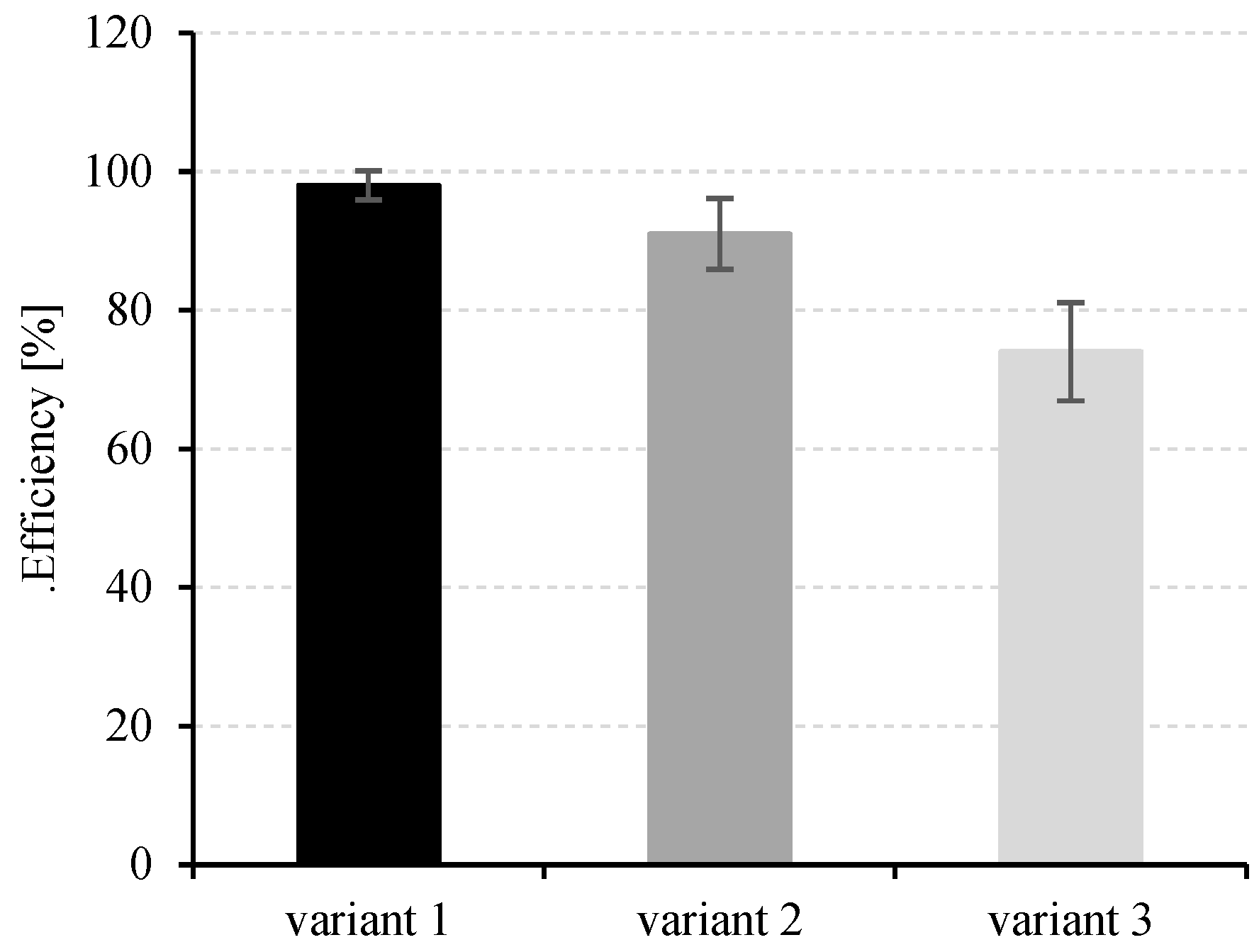
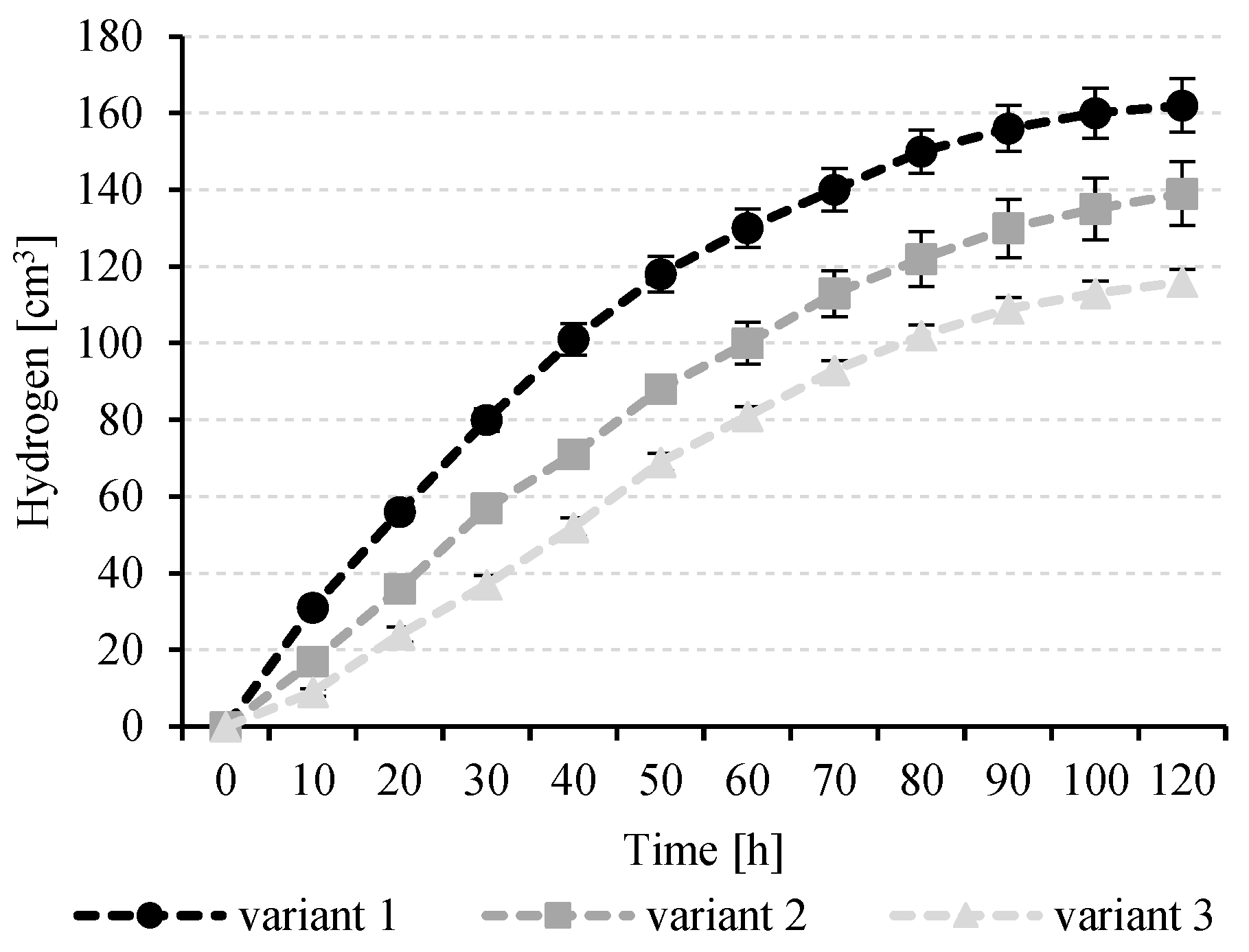
3.4. Hydrogen Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karolinczak, B.; Miłaszewski, R.; Dąbrowski, W. Cost Optimization of Wastewater and Septage Treatment Process. Energies 2020, 13, 6406. [Google Scholar] [CrossRef]
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Achieving Energy Neutrality in Wastewater Treatment Plants through Energy Savings and Enhancing Renewable Energy Production. Rev. Environ. Sci. Bio/Technol. 2018, 17, 655–689. [Google Scholar] [CrossRef]
- Calicioglu, O.; Femeena, P.V.; Mutel, C.L.; Sills, D.L.; Richard, T.L.; Brennan, R.A. Techno-Economic Analysis and Life Cycle Assessment of an Integrated Wastewater-Derived Duckweed Biorefinery. ACS Sustain. Chem. Eng. 2021, 9, 9395–9408. [Google Scholar] [CrossRef]
- Zhi, R.; Yang, A.; Zhang, G.; Zhu, Y.; Meng, F.; Li, X. Effects of Light-Dark Cycles on Photosynthetic Bacteria Wastewater Treatment and Valuable Substances Production. Bioresour. Technol. 2019, 274, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Sikosana, M.L.; Sikhwivhilu, K.; Moutloali, R.; Madyira, D.M. Municipal Wastewater Treatment Technologies: A Review. Procedia Manuf. 2019, 35, 1018–1024. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.M.Y.; Ong, H.C. Progress and Challenges of Contaminate Removal from Wastewater Using Microalgae Biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef]
- Spiller, M.; Moretti, M.; De Paepe, J.; Vlaeminck, S.E. Environmental and Economic Sustainability of the Nitrogen Recovery Paradigm: Evidence from a Structured Literature Review. Resour. Conserv. Recycl. 2022, 184, 106406. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New Frontiers from Removal to Recycling of Nitrogen and Phosphorus from Wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Maryjoseph, S.; Ketheesan, B. Microalgae Based Wastewater Treatment for the Removal of Emerging Contaminants: A Review of Challenges and Opportunities. Case Stud. Chem. Environ. Eng. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Yap, J.K.; Sankaran, R.; Chew, K.W.; Halimatul Munawaroh, H.S.; Ho, S.H.; Rajesh Banu, J.; Show, P.L. Advancement of Green Technologies: A Comprehensive Review on the Potential Application of Microalgae Biomass. Chemosphere 2021, 281, 130886. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae Culture Quality Indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J.; Dudek, M.; Świca, I.; Rudnicka, A. The Cultivation of Lipid-Rich Microalgae Biomass as Anaerobic Digestate Valorization Technology—A Pilot-Scale Study. Processes 2020, 8, 517. [Google Scholar] [CrossRef]
- Raper, E.; Stephenson, T.; Anderson, D.R.; Fisher, R.; Soares, A. Industrial Wastewater Treatment through Bioaugmentation. Process Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef]
- Gondi, R.; Kavitha, S.; Yukesh Kannah, R.; Parthiba Karthikeyan, O.; Kumar, G.; Kumar Tyagi, V.; Rajesh Banu, J. Algal-Based System for Removal of Emerging Pollutants from Wastewater: A Review. Bioresour. Technol. 2022, 344, 126245. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Gunasegavan, R.D.N.; Mustar, S.; Lee, J.C.; Noh, M.F.M. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef]
- Dudek, M.; Dębowski, M.; Nowicka, A.; Kazimierowicz, J.; Zieliński, M. The Effect of Autotrophic Cultivation of Platymonas Subcordiformis in Waters from the Natural Aquatic Reservoir on Hydrogen Yield. Resources 2022, 11, 31. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Thakur, S.; Mishra, T.; Negi, P.; Mishra, S.; Hesham, A.E.-L.; Rastegari, A.A.; Yadav, N.; Yadav, A.N. Bioprospecting of Microbes for Biohydrogen Production: Current Status and Future Challenges. Bioprocess. Biomol. Prod. 2019, 443–471. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen Production. Sol. Hydrog. Prod. Process. Syst. Technol. 2019, 45–83. [Google Scholar] [CrossRef]
- Ghosh, A.; Sangtani, R.; Samadhiya, K.; Kiran, B. Maximizing Intrinsic Value of Microalgae Using Multi-Parameter Study: Conjoint Effect of Organic Carbon, Nitrate, and Phosphate Supplementation. Clean Technol. Environ. Policy 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Kim, G.; Mujtaba, G.; Lee, K.; Kim, G.; Mujtaba, G.; Lee, K. Effects of Nitrogen Sources on Cell Growth and Biochemical Composition of Marine Chlorophyte Tetraselmis sp. for Lipid Production. Algae 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban Wastewater Treatment by Tetraselmis Sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen Availability Influences Phosphorus Removal in Microalgae-Based Wastewater Treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, F.; Ge, F.; Tao, N.; Zhou, Q.; Wong, M. Mechanisms of Ammonium Assimilation by Chlorella Vulgaris F1068: Isotope Fractionation and Proteomic Approaches. Bioresour. Technol. 2015, 190, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Deng, M.; Yu, X.; Zhang, W. Two-Stage Photo-Biological Production of Hydrogen by Marine Green Alga Platymonas Subcordiformis. Biochem. Eng. J. 2004, 19, 69–73. [Google Scholar] [CrossRef]
- Karolinczak, B.; Abrowski, W.D.; Zyłka, R.; Dymaczewski, Z.; Zuorro, A. Evaluation of Dairy Wastewater Treatment Systems Using Carbon Footprint Analysis. Energies 2021, 14, 5366. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Karolinczak, B.; Gajewska, M.; Wojciechowska, E. Application of Subsurface Vertical Flow Constructed Wetlands to Reject Water Treatment in Dairy Wastewater Treatment Plant. Environ. Technol. 2016, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Dȩbowski, M.; Zieliński, M.; Krzemieniewski, M.; Rokicka, M.; Kupczyk, K. Effectiveness of Dairy Wastewater Treatment in a Bioreactor Based on the Integrated Technology of Activated Sludge and Hydrophyte System. Environ. Technol. 2014, 35, 1350–1357. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of Decentralized Wastewater Treatment Technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Peng, Y.Y.; He, S.; Wu, F. Biochemical Processes Mediated by Iron-Based Materials in Water Treatement: Enhancing Nitrogen and Phosphorus Removal in Low C/N Ratio Wastewater. Sci. Total Environ. 2021, 775, 145137. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus Recovery from Municipal Wastewater Treatment: Critical Review of Challenges and Opportunities for Developing Countries. J. Environ. Manage. 2019, 248, 109268. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kundu, P.; Adhikari (Nee Pramanik), S. Two Stage Treatability and Biokinetic Study of Dairy Wastewater Using Bacterial Consortium and Microalgae. Biocatal. Agric. Biotechnol. 2022, 43, 102387. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of Microalgae in Adjusted Wastewater to Enhance Biofuel Production and Reduce Environmental Impact: Pyrolysis Performances and Life Cycle Assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Geng, Y.; Cui, D.; Yang, L.; Xiong, Z.; Pavlostathis, S.G.; Shao, P.; Zhang, Y.; Luo, X.; Luo, S. Resourceful Treatment of Harsh High-Nitrogen Rare Earth Element Tailings (REEs) Wastewater by Carbonate Activated Chlorococcum Sp. Microalgae. J. Hazard. Mater. 2022, 423, 127000. [Google Scholar] [CrossRef]
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Aravind Kumar, J. Treatment of Heavy Metals Containing Wastewater Using Biodegradable Adsorbents: A Review of Mechanism and Future Trends. Chemosphere 2022, 295, 133724. [Google Scholar] [CrossRef]
- Ferreira, C.S.G.; Nunes, B.A.; de Henriques-Almeida, J.M.M.; Guilhermino, L. Acute Toxicity of Oxytetracycline and Florfenicol to the Microalgae Tetraselmis Chuii and to the Crustacean Artemia Parthenogenetica. Ecotoxicol. Environ. Saf. 2007, 67, 452–458. [Google Scholar] [CrossRef]
- Naorbe, M.C.; Serrano, A.E. Effects of Heavy Metals on Cell Density, Size, Specific Growth Rate and Chlorophyll a of Tetraselmis Tetrathele under Controlled Laboratory Conditions. Aquac. Aquarium, Conserv. Legis. 2018, 11, 589–597. [Google Scholar]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and Characterization of Microalgae with Potential for Nutrient Removal from Municipal Wastewater and Simultaneous Lipid Production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current Perspective on Wastewater Treatment Using Photobioreactor for Tetraselmis Sp.: An Emerging and Foreseeable Sustainable Approach. Environ. Sci. Pollut. Res. 2021 2021, 1, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.C.; Agustini, C.B.; Trierweiler, L.F.; Gutterres, M. Influence of Period Light on Cultivation of Microalgae Consortium for the Treatment of Tannery Wastewaters from Leather Finishing Stage. J. Clean. Prod. 2020, 263, 121618. [Google Scholar] [CrossRef]
- Xiang, Q.; Wei, X.; Yang, Z.; Xie, T.; Zhang, Y.; Li, D.; Pan, X.; Liu, X.; Zhang, X.; Yao, C. Acclimation to a Broad Range of Nitrate Strength on a Euryhaline Marine Microalga Tetraselmis Subcordiformis for Photosynthetic Nitrate Removal and High-Quality Biomass Production. Sci. Total Environ. 2021, 781, 146687. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.S. Effects of Water Culture Medium, Cultivation Systems and Growth Modes for Microalgae Cultivation: A Review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Tran, T.N.T.; Le, T.V.A.; Nguyen Phan, T.X.; Show, P.L.; Chia, S.R. Auto-Flocculation through Cultivation of Chlorella Vulgaris in Seafood Wastewater Discharge: Influence of Culture Conditions on Microalgae Growth and Nutrient Removal. J. Biosci. Bioeng. 2019, 127, 492–498. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, A.; Silva-Gálvez, A.L.; Aguilar-Juárez, Ó.; Senés-Guerrero, C.; Orozco-Nunnelly, D.A.; Carrillo-Nieves, D.; Gradilla-Hernández, M.S. Microalgae-Based Livestock Wastewater Treatment (MbWT) as a Circular Bioeconomy Approach: Enhancement of Biomass Productivity, Pollutant Removal and High-Value Compound Production. J. Environ. Manage. 2022, 308, 114612. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Qiu, S.; Li, M.; Yuan, W.; Ge, S. Granular Indigenous Microalgal-Bacterial Consortium for Wastewater Treatment: Establishment Strategy, Functional Microorganism, Nutrient Removal, and Influencing Factor. Bioresour. Technol. 2022, 353, 127130. [Google Scholar] [CrossRef]
- Huang, L.; Liu, J.; Li, Q.; Wang, C.; Wu, K.; Wang, C.; Zhao, X.; Yin, F.; Liang, C.; Zhang, W. A Review of Biogas Slurry Treatment Technology Based on Microalgae Cultivation. Curr. Opin. Environ. Sci. Health 2022, 25, 100315. [Google Scholar] [CrossRef]
- Hu, R.; Cao, Y.; Chen, X.; Zhan, J.; Luo, G.; Hao Ngo, H.; Zhang, S. Progress on Microalgae Biomass Production from Wastewater Phycoremediation: Metabolic Mechanism, Response Behavior, Improvement Strategy and Principle. Chem. Eng. J. 2022, 137187. [Google Scholar] [CrossRef]
- Heo, S.W.; Ryu, B.G.; Nam, K.; Kim, W.; Yang, J.W. Simultaneous Treatment of Food-Waste Recycling Wastewater and Cultivation of Tetraselmis Suecica for Biodiesel Production. Bioprocess Biosyst. Eng. 2015, 38, 1393–1398. [Google Scholar] [CrossRef]
- Amit; Chandra, R.; Ghosh, U.K.; Nayak, J.K. Phycoremediation Potential of Marine Microalga Tetraselmis Indica on Secondary Treated Domestic Sewage for Nutrient Removal and Biodiesel Production. Environ. Sci. Pollut. Res. 2017, 24, 20868–20875. [Google Scholar] [CrossRef]
- Ran, C.; Zhang, F.; Sun, H.; Zhao, B. Effect of Culture Medium on Hydrogen Production by Sulfur-Deprived Marine Green Algae Platymonas Subcordiformis. Biotechnol. Bioprocess Eng. 2009 146 2010, 14, 835–841. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Kumar Ghodke, P.; Manna, S.; Chen, W.H. Emerging Technologies for Sustainable Production of Biohydrogen Production from Microalgae: A State-of-the-Art Review of Upstream and Downstream Processes. Bioresour. Technol. 2021, 342, 126057. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.J.; Kondo, A.; Chang, J.S. Recent Insights into Biohydrogen Production by Microalgae—From Biophotolysis to Dark Fermentation. Bioresour. Technol. 2017, 227, 373–387. [Google Scholar] [CrossRef]
- Dudek, M.; Nowicka, A.; Zieliński, M.; Kazimierowicz, J.; Dębowski, M. The Effect of Biomass Separation Method on the Efficiency of Hydrogen Production by Platymonas Subcordiformis. Int. J. Energy Environ. Eng. 2022, 2022, 1–11. [Google Scholar] [CrossRef]






| Indicator | Unit | Raw Wastewater | Pre-Treated Wastewater |
|---|---|---|---|
| BOD5 | mgO2/dm3 | 3010 ± 258 | 15.1 ± 3.2 |
| COD | mgO2/dm3 | 4280 ± 299 | 55.7 ± 9.8 |
| Ntot. | mg N/dm3 | 192 ± 9.6 | 23.6 ± 4.3 |
| Norg. | mg Norg./dm3 | 160 ± 12.1 | 2.2 ± 0.3 |
| N-NO3 | mg N-NO3/dm3 | 1.9 ± 0.3 | 20.7 ± 3.7 |
| N-NO2 | mg N-NO2/dm3 | 1.2 ± 0.5 | 1.7 ± 0.4 |
| N-NH4 | mg N-NH4/dm3 | 29 ± 6.2 | 1.9 ± 0.5 |
| Ptot. | mg P/dm3 | 48.2 ± 3.3 | 15.8 ± 2.4 |
| Porg. | mg Porg./dm3 | 33.7 ± 4.1 | 3.6 ± 1.1 |
| P-PO4 | mg P-PO4/dm3 | 15.6 ± 2.5 | 12.3 ± 2.9 |
| pH | - | 7.06 ± 0.14 | 7.06 ± 0.22 |
| Total suspended matter | mg/dm3 | 12.9 ± 1.6 | 4.2 ± 2.6 |
| Indicator | Unit | Variant 1 | Variant 2 | Variant 3 |
|---|---|---|---|---|
| BOD5 | mgO2/dm3 | 3.4 ± 0.3 | 7.9 ± 1.7 | 15.1 ± 3.2 |
| COD | mgO2/dm3 | 11.7 ± 1.4 | 28.6 ± 3.1 | 55.7 ± 9.8 |
| Ntot. | mg N/dm3 | 22.8 ± 0.9 | 12.1 ± 1.4 | 23.6 ± 4.3 |
| Norg. | mg Norg./dm3 | 0.0 ± 0.0 | 1.2 ± 0.4 | 2.2 ± 0.3 |
| N-NO3 | mg N-NO3/dm3 | 20.4 ± 0.8 | 10.9 ± 1.6 | 20.7 ± 3.7 |
| N-NO2 | mg N-NO2/dm3 | 1.4 ± 0.4 | 0.9 ± 0.1 | 1.7 ± 0.4 |
| N-NH4 | mg N-NH4/dm3 | 0.0 ± 0.0 | 1.1 ± 0.3 | 1.9 ± 0.5 |
| Ptot. | mg P/dm3 | 8.2 ± 0.5 | 8.1 ± 1.3 | 15.8 ± 2.4 |
| Porg. | mg Porg./dm3 | 0.0 ± 0.0 | 1.7 ± 0.9 | 3.6 ± 1.1 |
| P-PO4 | mg P-PO4/dm3 | 8.2 ± 0.5 | 6.4 ± 2.0 | 12.3 ± 2.9 |
| pH | - | 7.1 ± 0.1 | 7.09 ± 0.1 | 7.06 ± 1.62 |
| Total suspended matter | mg/dm3 | 0.8 ± 0.2 | 2.3 ± 1.0 | 4.2 ± 2.6 |
| Variant | Biogas Composition [%] | H2 Production Per Biomass Concentration Unit | Rate of H2 Production (r) | Production Rate Constant (k) | ||
|---|---|---|---|---|---|---|
| H2 | CO2 | O2 | ||||
| [%] | [cm3/go.d.m.] | [cm3/h] | [1/d] | |||
| 1 | 57.2 ± 4.1 | 41.1 ± 3.4 | 1.7 ± 0.9 | 54 ± 2.1 | 1.73 ± 0.31 | 0.17 |
| 2 | 55.7 ± 5.6 | 42.3 ± 4.5 | 2.0 ± 1.1 | 69 ± 4.2 | 1.45 ± 0.37 | 0.13 |
| 3 | 55.4 ± 2.2 | 43.2 ± 1.8 | 1.4 ± 0.6 | 48 ± 1.9 | 1.22 ± 0.11 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, M.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Quattrocelli, P.; Nowicka, A. The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability 2022, 14, 12085. https://doi.org/10.3390/su141912085
Dudek M, Dębowski M, Kazimierowicz J, Zieliński M, Quattrocelli P, Nowicka A. The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability. 2022; 14(19):12085. https://doi.org/10.3390/su141912085
Chicago/Turabian StyleDudek, Magda, Marcin Dębowski, Joanna Kazimierowicz, Marcin Zieliński, Piera Quattrocelli, and Anna Nowicka. 2022. "The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System" Sustainability 14, no. 19: 12085. https://doi.org/10.3390/su141912085
APA StyleDudek, M., Dębowski, M., Kazimierowicz, J., Zieliński, M., Quattrocelli, P., & Nowicka, A. (2022). The Cultivation of Biohydrogen-Producing Tetraselmis subcordiformis Microalgae as the Third Stage of Dairy Wastewater Aerobic Treatment System. Sustainability, 14(19), 12085. https://doi.org/10.3390/su141912085









