Heterologous Grafting Improves Cold Tolerance of Eggplant
Abstract
:1. Introduction
2. Material and Methods
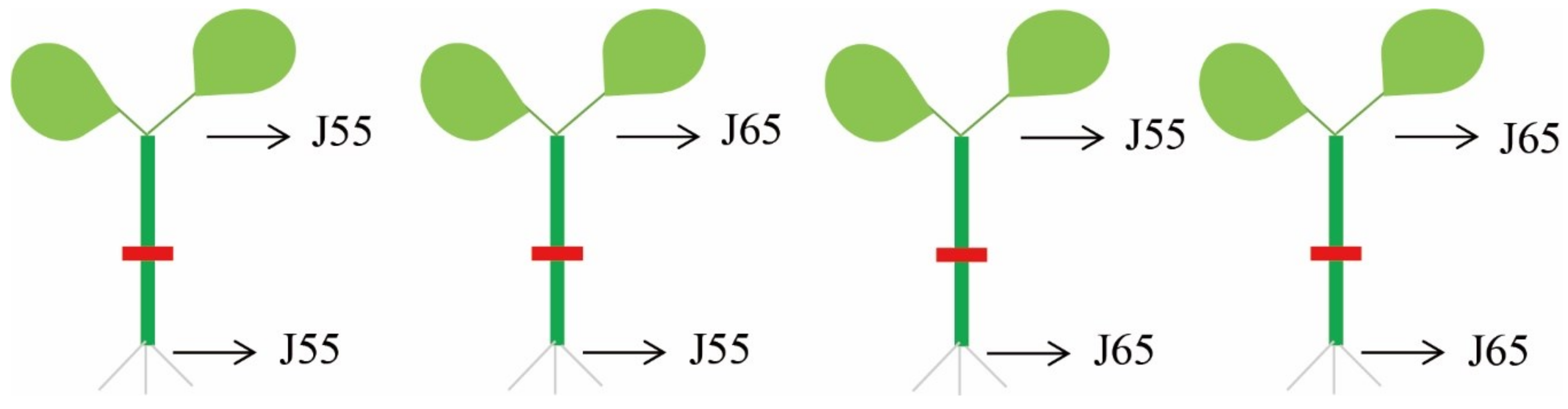
2.1. Plant Materials and Cultivation
2.2. Chilling Treatment
2.3. Chilling Injury Index Statistical
2.4. Measurement of the Maximum Photochemical Efficiency of PSⅡ
2.5. RNA-Seq and Transcriptome Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
3. Results
3.1. Morphological and Physiological Responses to Cold Stress
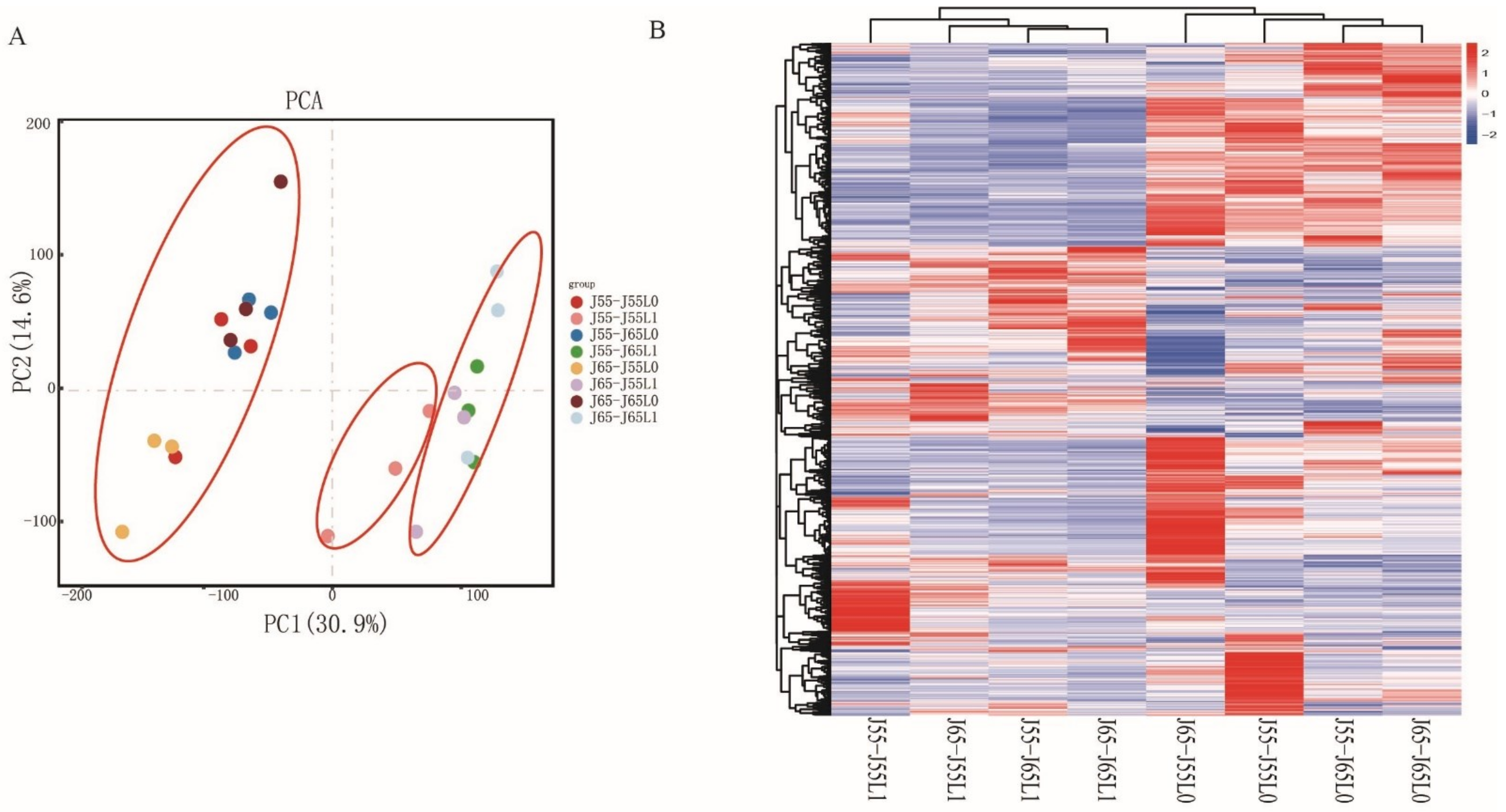
3.2. Transcriptome Sequencing, Assembly, and Mapping
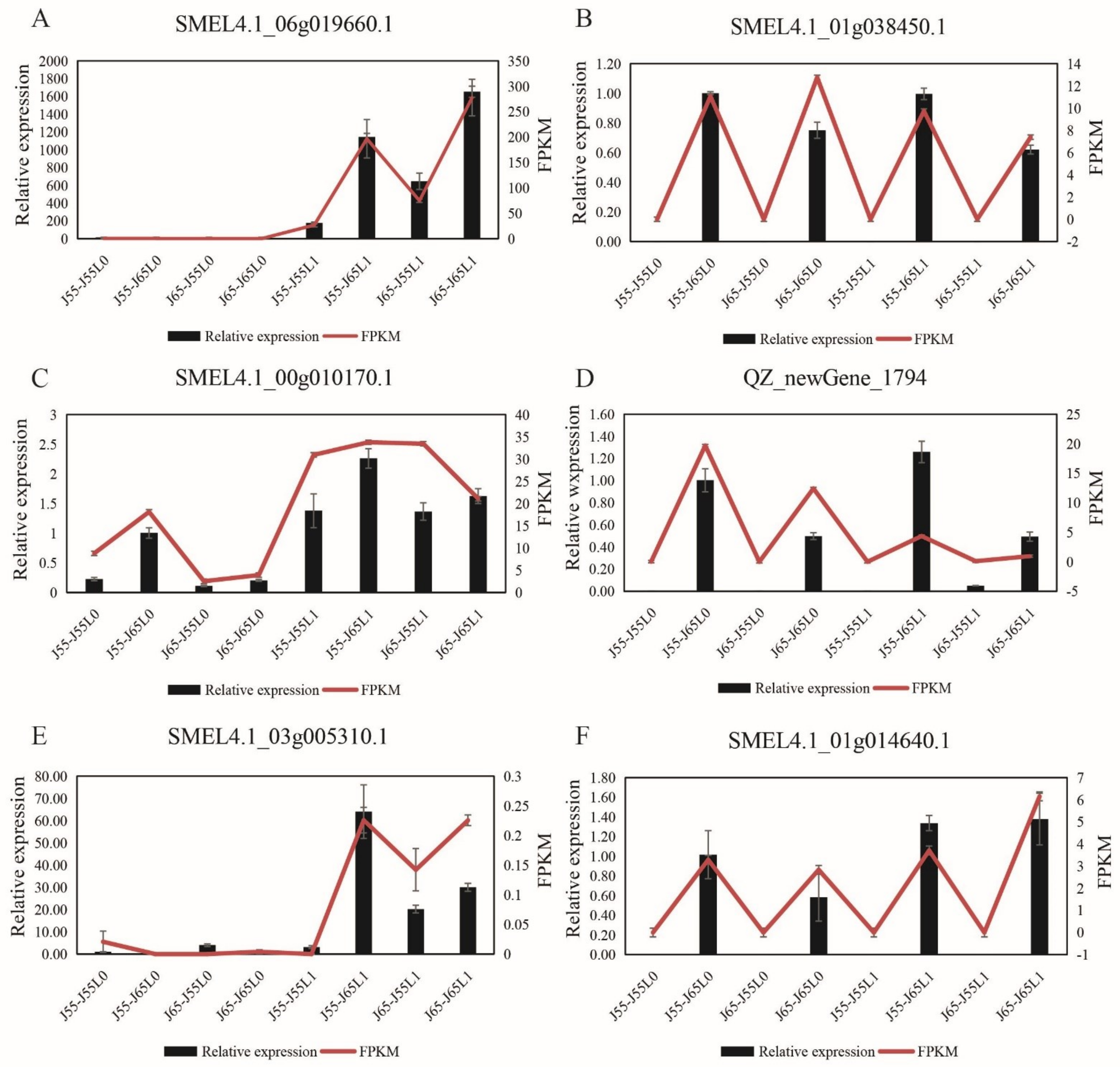
3.3. Validation of Transcriptome Data
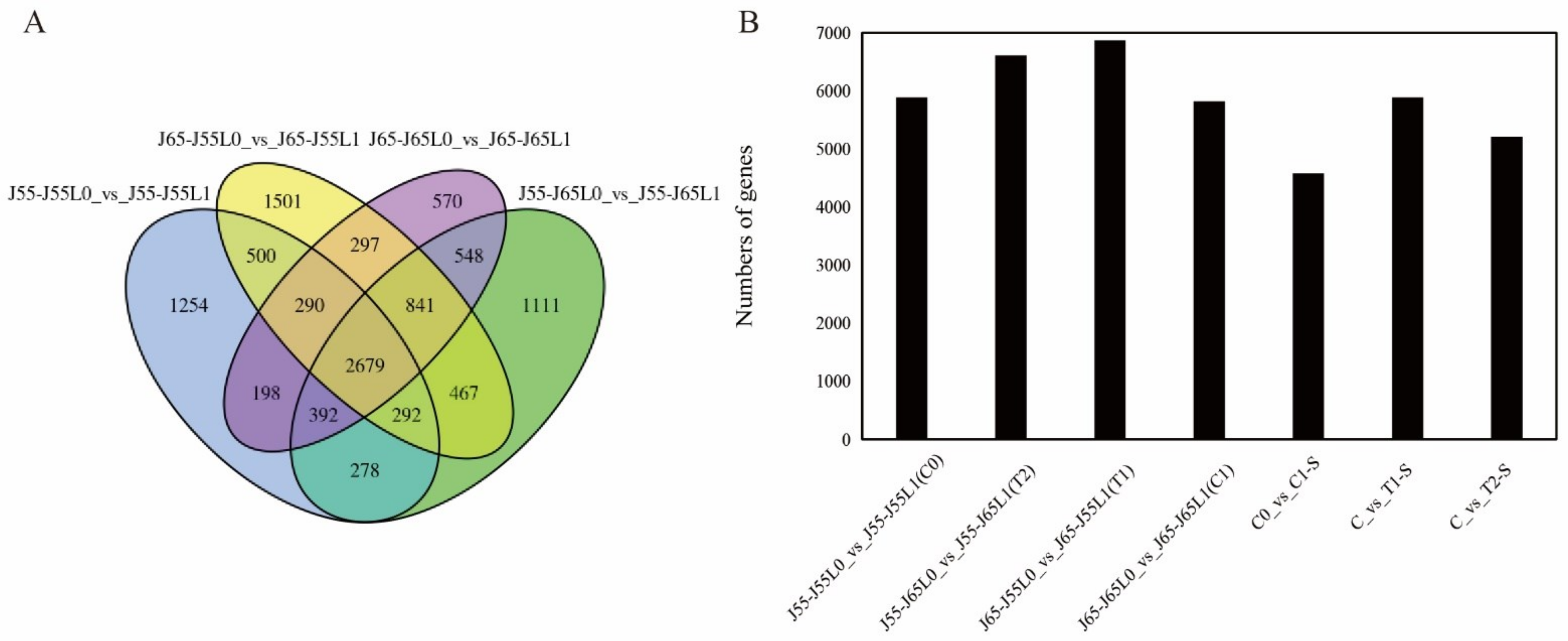
3.4. Identification of DEGs between the Grafting Combinations
3.5. GO Enrichment Analysis of the Identified DEGs
3.6. KEGG Pathway Enrichment Analysis of Different Expression Genes
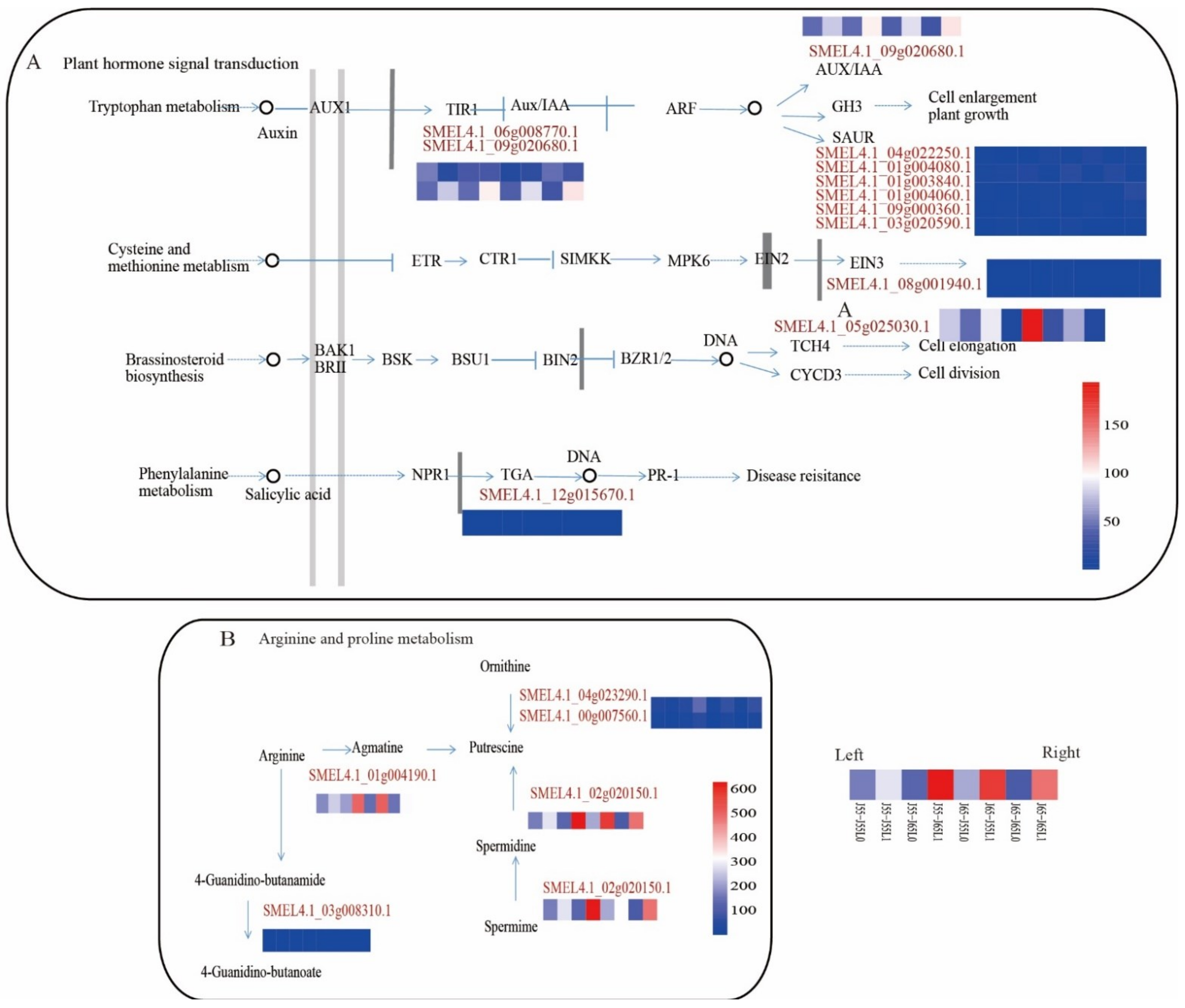
3.7. Analysis of DEGs in Plant Hormone Signal Transduction, Arginine and Proline Metabolism
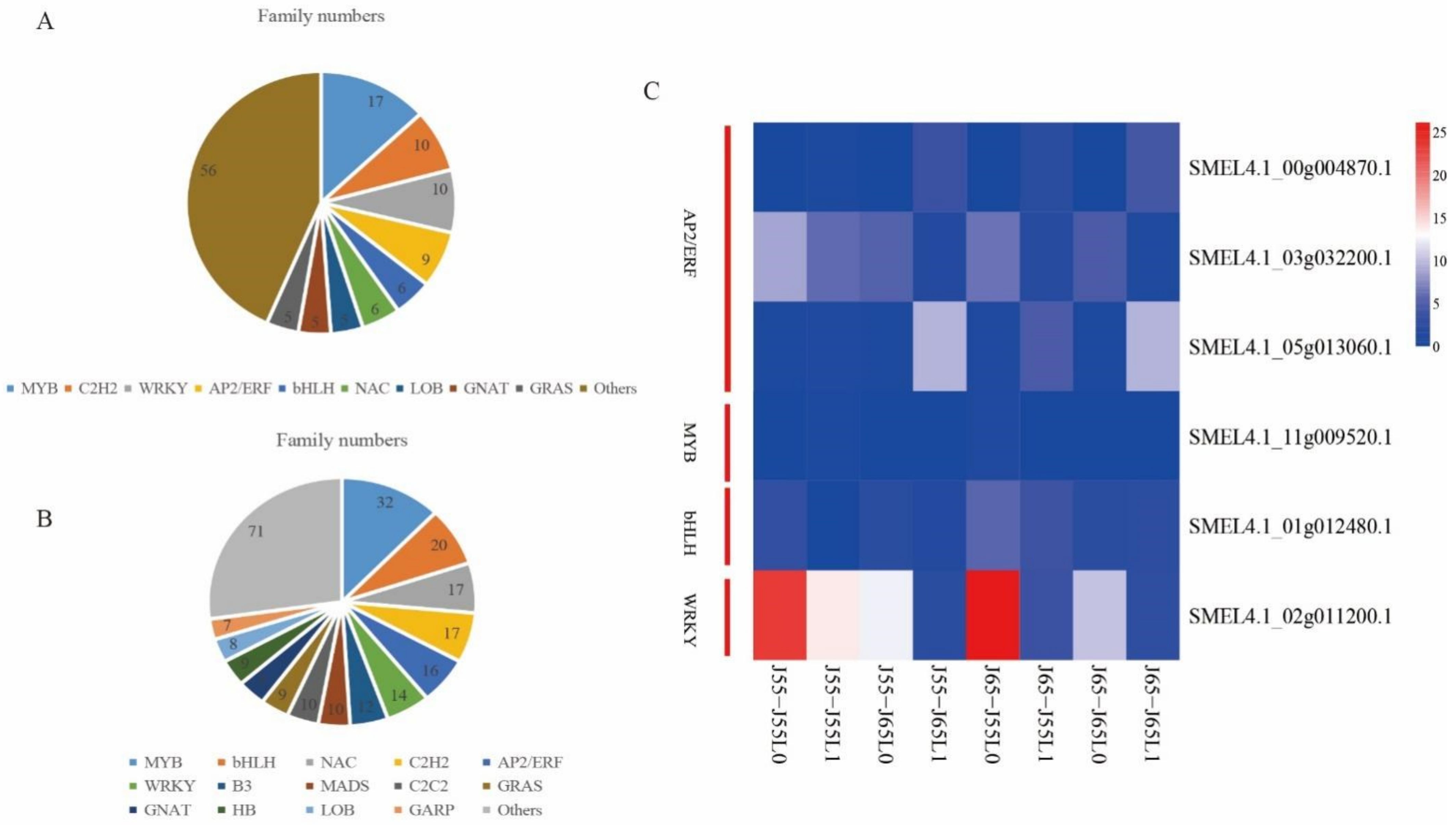
3.8. Identification for Transcription Factors
4. Discussion
4.1. Grafting Can Increase the Eggplant’s Cold Tolerance
4.2. Plant Hormone Signal Transduction Is an Important Pathway Involved in Promoting the Eggplant Cold Tolerance
4.3. Putrescine Biosynthesis Improve the Cold Tolerance of Grafted Eggplants
4.4. MYB, AP2/ERF Transcription Factor Family Involved in the Cold Tolerance Improving of Grafted Eggplant Combination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gao, H.; Kang, L.; Liu, Q.; Cheng, N.; Wang, B.; Cao, W. Effect of 24-epibrassinolide treatment on the metabolism of eggplant fruits in relation to development of pulp browning under chilling stress. J. Food Sci. Technol. 2014, 52, 3394–3401. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, D.; Li, S.; Liu, Y.; Chen, H. SmBICs Inhibit Anthocyanin Biosynthesis in Eggplant (Solanum melongena L.). Plant Cell Physiol. 2021, 6, 1001–1011. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, L.; Yu, Z.; Wang, L.; Cheng, Y. Cold-responsive miRNAs and their target genes in the wild eggplant species Solanum aculeatissimum. BMC Genom. 2017, 18, 1. [Google Scholar]
- Shi, J.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q. Effect of low-temperature conditioning combined with methyl jasmonate treatment on the chilling resistance of eggplant (Solanum melongena L.) fruit. J. Food Sci. Technol. 2019, 56, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Pan, Y.; Li, J.; Chen, X.; Pan, Y.; Wang, Y.; Tian, S.; Zhang, X. Heterologous expression of Arabidopsis C-repeat binding factor 3 (AtCBF3) and cold-regulated 15A (AtCOR15A) enhanced chilling tolerance in transgenic eggplant (Solanum melongena L.). Plant Cell Rep. 2014, 33, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Niu, C.; Nie, X.; Li, Y.; Wei, M. Transcriptome and Physiological Analysis of Rootstock Types and Silicon Affecting Cold Tolerance of Cucumber Seedlings. Plants 2022, 11, 445. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Musa, I.; Rafii, M.Y.; Ahmad, K.; Ramlee, S.I.; Hatta, M.A.; Oladosu, Y.; Muhammad, I.I.; Chukwu, S.C.; Mat Sulaiman, N.N.; Fatai Arolu, A.; et al. Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks. Plants 2020, 9, 1583. [Google Scholar] [CrossRef]
- Gisbert-Mullor, R.; Ceccanti, C.; Gara Padilla, Y.; López-Galarza, S.; Calatayud, Á.; Conte, G.; Guidi, L. Effect of Grafting on the Production, Physico-Chemical Characteristics and Nutritional Quality of Fruit from Pepper Landraces. Antioxidants 2020, 9, 501. [Google Scholar] [CrossRef]
- Darré, M.; Valerga, L.; Zaro, M.J.; Lemoine, M.L.; Concellón, A.; Vicente, A.R. Eggplant grafting on a cold-tolerant rootstock reduces fruit chilling susceptibility and improves antioxidant stability during storage. J Sci Food Agric. 2021, 102, 3350–3358. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Zhang, R.; Liu, M.; Wang, C.; Liu, Z.; Xiang, C.; Lu, X.; Zhang, X.; Li, X.; et al. Rootstock-scion exchanging mRNAs participate in the pathways of amino acids and fatty acid metabolism in cucumber under early chilling stress. Hortic Res. 2022, 9, uhac031, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, Y.; Zhang, X.; Zhang, Y.; Bi, H.; Ai, X. Salicylic Acid Is Involved in Rootstock–Scion Communication in Improving the Chilling Tolerance of Grafted Cucumber. Front. Plant Sci. 2021, 12, 693344. [Google Scholar] [CrossRef] [PubMed]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting From a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Front. Plant Sci. 2021, 11, 621999. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, Q.; Chen, P.; Chen, G.; Zhou, R. Effects of Rootstocks on Cryotolerance and Overwintering Survivorship of Genic Male Sterile Lines in Upland Cotton (Gossypium hirsutum L.). PLoS ONE 2013, 8, e63534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ai, X.; Zheng, N.; Jiang, F.; Li, Q. Effects of graft on lipid peroxidation and antioxidative enzyme activities of Capsicum annum seedlings under low temperature and weak light intensity. Ying Yong Sheng Tai Xue Bao 2010, 21, 1289–1294. [Google Scholar]
- Zhou, Y.; Zhou, J.; Huang, L.; Ding, X.; Shi, K.; Yu, J. Grafting of Cucumis sativus onto Cucurbita ficifolia leads to improved plant growth, increased light utilization and reduced accumulation of reactive oxygen species in chilled plants. J. Plant Res. 2009, 122, 529–540. [Google Scholar] [CrossRef]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion-rootstock interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, X.; Zhang, A.; Zha, D. Screening and Transcriptome Analysis of Different Materials with Low Temperature Tolerance in Eggplant (Solanum melongena). Mol. Plant Breed. 2021, 19, 4779–4787. [Google Scholar]
- Zhang, M.; Zhang, H.; Tan, J.; Huang, S.; Chen, X.; Jiang, D.; Xiao, X. Transcriptome Analysis of Eggplant Root in Response to Root-Knot Nematode Infection. Pathogens 2021, 10, 470. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, F.; Gao, Y.; Wang, D.; Wang, K. Parallel Bud Mutation Sequencing Reveals that Fruit Sugar and Acid Metabolism Potentially Influence Stress in Malus. Int. J. Mol. Sci. 2019, 20, 5988. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.; Zhang, J. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 Regulates microRNA-Mediated Cold Stress Response in Arabidopsis Roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Jain, M. Genome-wide survey and comprehensive expression profiling of Aux/IAA gene family in chickpea and soybean. Front. Plant Sci. 2015, 6, 918. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, J.P. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009, 276, 3148–3162. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; He, H.; Han, T.; Di, P.; Sechet, J.; Fang, L.; Liang, Y.; Scheller, H.V.; Mortimer, J.C.; et al. Xyloglucan endotransglucosylase-hydrolase30 negatively affects salt tolerance in Arabidopsis. J. Exp. Bot. 2019, 70, 5495–5506. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Jiang, Z.; Liu, L.; Pu, J.; Zhang, W.; Wang, S.; Xu, F. The Xyloglucan Endotransglucosylase/Hydrolase Gene XTH22/TCH4 Regulates Plant Growth by Disrupting the Cell Wall Homeostasis in Arabidopsis under Boron Deficiency. Int. J. Mol. Sci. 2022, 23, 1250. [Google Scholar] [CrossRef]
- Takahashi, D.; Johnson, K.L.; Hao, P.; Tuong, T.; Erban, A.; Sampathkumar, A.; Bacic, A.; Livingston, D.P.; Kopka, J.; Kuroha, T.; et al. Cell wall modification by the xyloglucan endotransglucosylase/hydrolaseXTH19 influences freezing tolerance after cold and sub-zero acclimation. Plant Cell Environ. 2021, 44, 915–930. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepperCaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenicArabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef]
- Kou, S.; Chen, L.; Tu, W.; Scossa, F.; Wang, Y.; Liu, J.; Fernie, A.R.; Song, B.; Xie, C. The arginine decarboxylase gene ADC1, associated to the putrescine pathway, plays an important role in potato cold-acclimated freezing tolerance as revealed by transcriptome and metabolome analyses. Plant J. 2018, 96, 1283–1298. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Shi, J.; Zhang, Y.; Liu, T.; Qi, H. Abscisic acid and putrescine synergistically regulate the cold tolerance of melon seedlings. Plant Physiol. Biochem. 2021, 166, 1054–1064. [Google Scholar] [CrossRef]
- Cuevas, J.C.; López-Cobollo, R.; Alcázar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine Is Involved in Arabidopsis Freezing Tolerance and Cold Acclimation by Regulating Abscisic Acid Levels in Response to Low Temperature. Plant Physiol. 2008, 148, 1094–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Wang, F.; Rao, J. Effect of Putrescine Treatment on Chilling Injury, Fatty Acid Composition and Antioxidant System in Kiwifruit. PLoS ONE 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo citrange rootstock (Citrus sinensis Osb. ×Poncirus trifoliata L. Raf.) enhances natural chilling stress tolerance of common clementine (Citrus clementina Hort. ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Gao, M.; Lu, J.; Huang, Y.; Bie, Z. Spatial–Temporal Response of Reactive Oxygen Species and Salicylic Acid Suggest Their Interaction in Pumpkin Rootstock-Induced Chilling Tolerance in Watermelon Plants. Antioxidants 2021, 10, 2024. [Google Scholar] [CrossRef]
- An, J.; Li, R.; Qu, F.; You, C.; Wang, X.; Hao, Y. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018, 96, 562–577. [Google Scholar] [CrossRef]
- Dong, J.; Cao, L.; Zhang, X.; Zhang, W.; Yang, T.; Zhang, J.; Che, D. An R2R3-MYB Transcription Factor RmMYB108 Responds to Chilling Stress of Rosa multiflora and Conferred Cold Tolerance of Arabidopsis. Front. Plant Sci. 2021, 12, 696919. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Ngatia, J.N.; Wang, Y.; Khoso, M.A.; Farooq, U.; Chen, S. AP2/ERF, an important cold stress-related transcription factor family in plants: A review. Physiol. Mol. Biol. Plants 2021, 27, 1953–1968. [Google Scholar] [CrossRef]
- Xing, C.; Liu, Y.; Zhao, L.; Zhang, S.; Huang, X. A novel MYB transcription factor regulates ascorbic acid synthesis and affects cold tolerance. Plant Cell Environ. 2019, 42, 832–845. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X.; Liu, S.; Zhuang, Y. Identification of WRKY gene family and characterization of cold stress-responsiveWRKY genes in eggplant. PeerJ 2020, 8, e8777. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wu, S.; Li, Q.; Wang, X.; Li, X.; Liu, F.; Yang, J. Heterologous Grafting Improves Cold Tolerance of Eggplant. Sustainability 2022, 14, 11170. https://doi.org/10.3390/su141811170
Wang D, Wu S, Li Q, Wang X, Li X, Liu F, Yang J. Heterologous Grafting Improves Cold Tolerance of Eggplant. Sustainability. 2022; 14(18):11170. https://doi.org/10.3390/su141811170
Chicago/Turabian StyleWang, Duanhua, Shuanghua Wu, Qian Li, Xin Wang, Xuefeng Li, Feng Liu, and Jianguo Yang. 2022. "Heterologous Grafting Improves Cold Tolerance of Eggplant" Sustainability 14, no. 18: 11170. https://doi.org/10.3390/su141811170
APA StyleWang, D., Wu, S., Li, Q., Wang, X., Li, X., Liu, F., & Yang, J. (2022). Heterologous Grafting Improves Cold Tolerance of Eggplant. Sustainability, 14(18), 11170. https://doi.org/10.3390/su141811170





