The Effects of Water Depth on the Growth of Two Emergent Plants in an In-Situ Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
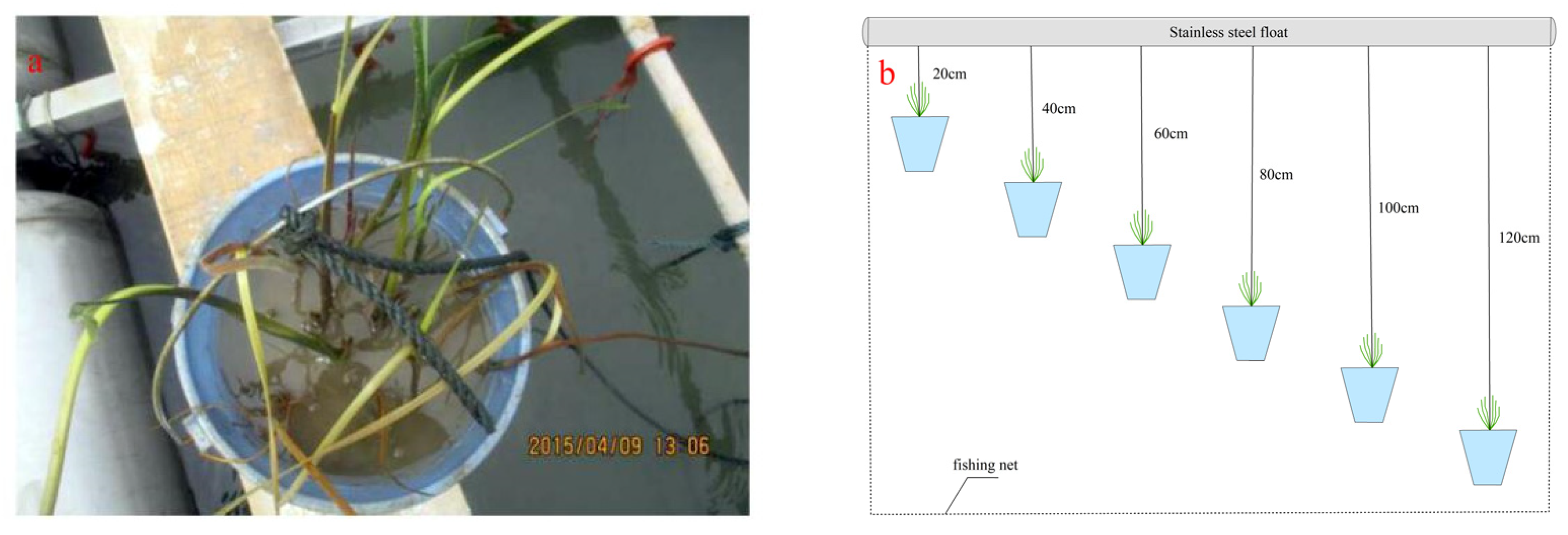
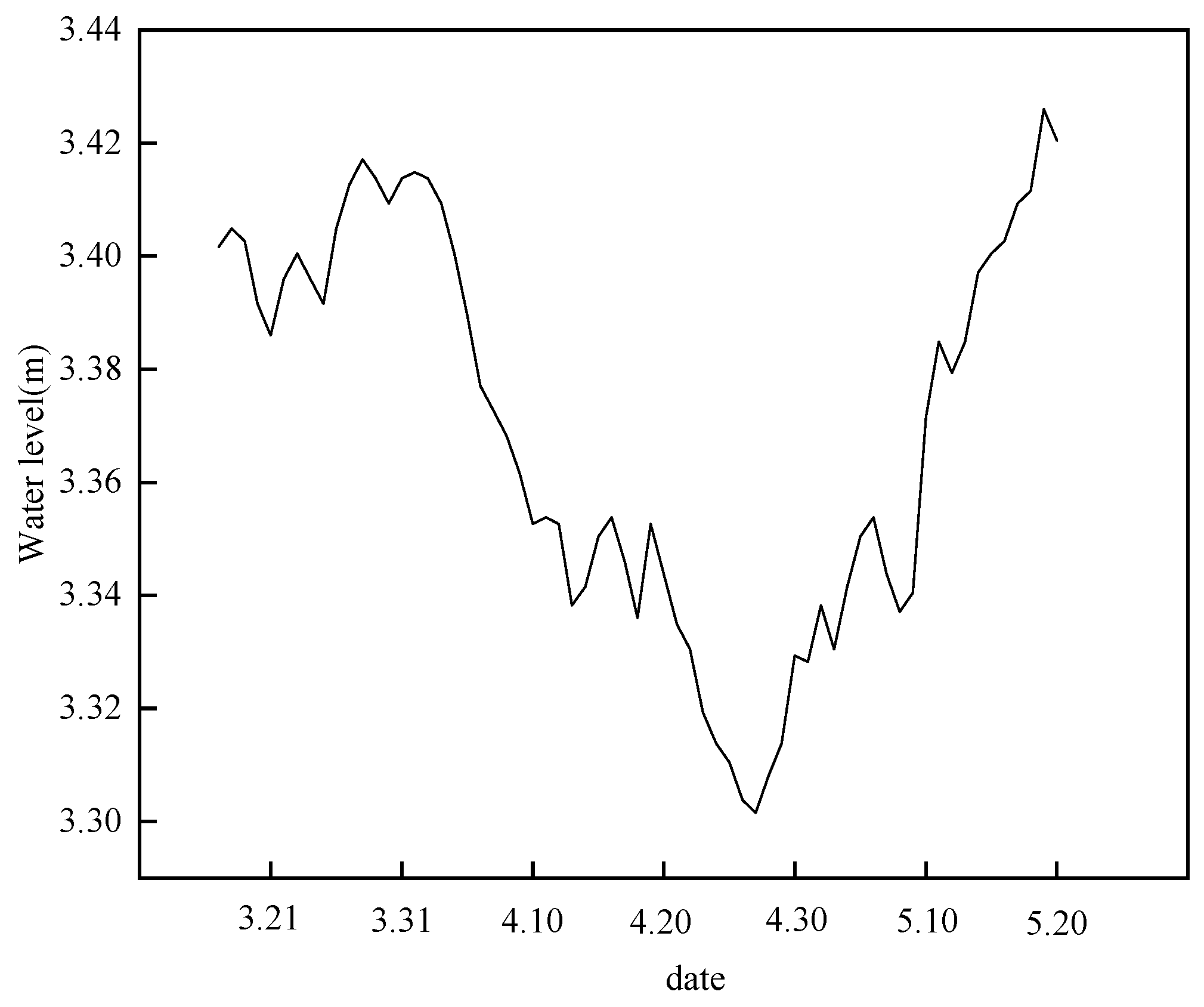
2.2. Experiment Design
2.3. Measurement Indicators
2.3.1. Morphological Indicators
2.3.2. Chlorophyll Fluorescence Parameters
2.4. Statistical Analysis
3. Results and Analysis
3.1. The Effect of Water Depth on the Growth of the Emergent Plants
3.1.1. Effects of Different Water Depths on Germination of the Emergent Plants
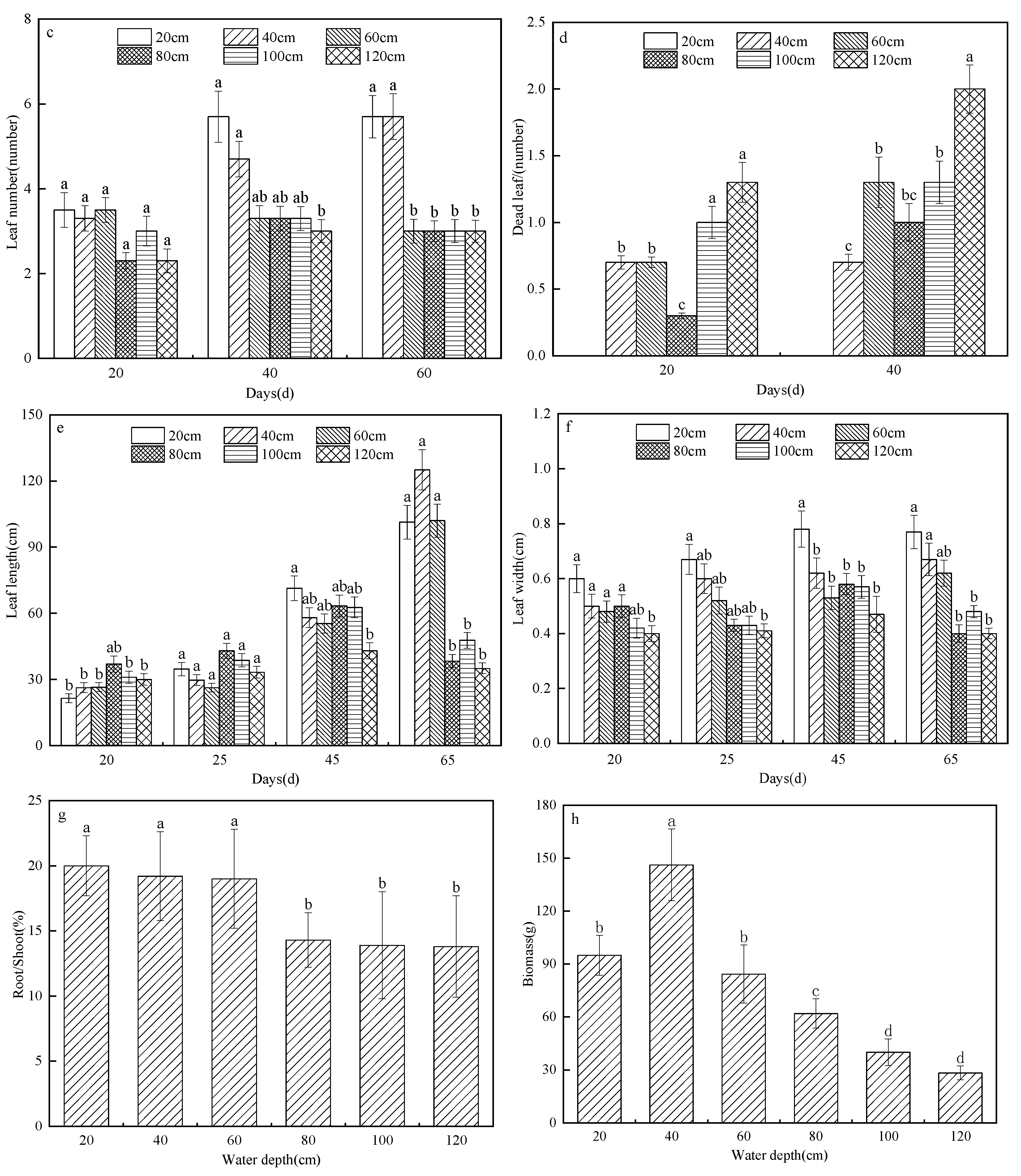
3.1.2. Effects of Different Water Depths on the Morphological Characteristics of the Emergent Plants
3.2. Effects of Different Water Depths on the Photosynthetic Fluorescence Characteristics of the Emergent Plants
4. Discussion
4.1. Effects of Different Water Depths on the Survival of the Emergent Plants
4.2. Effects of Different Water Depths on the Morphological Characteristics of the Emergent Plants
4.3. Effects of Different Water Depths on the Biomass of the Emergent Plants
4.4. Effects of Different Water Depths on Chlorophyll Fluorescence Parameters of the Emergent Plants
5. Conclusions
- (1)
- The in-situ water depth experiment showed that water depth had no significant effect on the germination of T. orientalis or Z. caduciflora, and the germination rates of the two were 90% and 85%, respectively.
- (2)
- The water depth changed the morphological characteristics of T. orientalis and Z. caduciflora. Plant height, tiller number, leaf length, leaf width, leaf number, and the root-shoot ratio decreased with increasing water depth, whereas the number of dead leaves increased with increasing water depth.
- (3)
- The biomass of the emergent plants increased first and then decreased with increasing water depth, and the biomass was largest when the water depth was 40 cm.
- (4)
- The maximum Fv/Fm of the emergent leaves was higher in the 20–60 cm water depth range. The ETRmax values of the treatment groups were significantly different. The ETRmax of the two emergent plants was highest when water depth was 20 cm.
- (5)
- Pioneer species of emergent plants have strong morphological plasticity to water depth changes but have a particular tolerance range. The suitable water depth range for T. orientalis and Z. caduciflora seedlings is 20–60 cm, and a higher water depth for a long time is not conducive to growth.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.L.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Zhu, G.W.; Shi, K.; Zhou, Y.Q. Profound Changes in the Physical Environment of Lake Taihu from 25 Years of Long-Term Observations: Implications for Algal Bloom Outbreaks and Aquatic Macrophyte Loss. Water Resour. Res. 2018, 54, 4319–4331. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, J.; Elser, J.J.; Gardner, W.S.; Deng, J.M.; Brookes, J.D. Water depth underpins the relative roles and fates of nitrogen and phosphorus in lakes. Environ. Sci. Technol. 2020, 54, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.G.; Gu, X.H.; Cao, Y.; Luo, J.H.; Zeng, Q.F.; Chen, H.H.; Jeppesen, E. Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. Sci. Total Environ. 2021, 756, 143642. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Hu, Z.B.; Ye, C.; Wei, W.W.; Li, C.H.; Chen, H.S. Nitrogen and phosphorus content and correlation analysis in water quality and aquatic plants in the estuary of Taihu Lake. Environ. Eng. 2019, 37, 74–80+102. (In Chinese) [Google Scholar]
- Xu, J.L.; Liu, J.; Hu, J.Q.; Wang, H.X.; Sheng, L.X.; Dong, X.L. Nitrogen and phosphorus removal in simulated wastewater by two aquatic plants. Environ. Sci. Pollut. Res. 2021, 28, 63237–63249. [Google Scholar] [CrossRef]
- Wang, W.; Cui, J.; Li, J.F.; Du, J.M.; Chang, Y.J.; Cui, J.W.; Liu, X.J.; Fan, X.Y.; Yao, D.R. Removal effects of different emergent-aquatic-plant groups on Cu, Zn, and Cd compound pollution from simulated swine wastewater. J. Environ. Manag. 2021, 296, 113251. [Google Scholar] [CrossRef]
- Lu, D.F.; Wei, L.W.; Shen, Q.W.; Xiong, H.J.; Zheng, Y.S. Effect of aquatic plant combination on the enrichment of heavy metals in polluted water. China. Landsc. Arc. 2020, 36, 105–110. (In Chinese) [Google Scholar]
- Wu, X.F.; He, Y.; Huang, Z.P.; Zhang, D.D. Study on the purification of domestic sewage with different treatment gradients in Zibai-Rice Constructed Wetland. J. Anhui Agric. Univ. 2021, 48, 834–841. [Google Scholar]
- Ndlela, L.L.; Oberholster, P.J.; van Wyk, J.H.; Cheng, P.H. An over-view of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae. 2016, 60, 11–26. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L.; Miller, T.R. Variable cyanobacterial toxin and metabolite profiles across six eutrophic lakes of differing physiochemical characteristics. Toxins 2017, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lv, X.G.; Jiang, M.; Zhang, W.G.; Wu, H.T. Effects of light and water depth on seed germination of Phragmites australis in the wetlands of Songnen Plain. Chin. J. Plant Ecol. 2015, 39, 616–620. (In Chinese) [Google Scholar]
- Zhang, Q.; Qiu, S.Y.; Zhu, Y.; Cui, X.H.; He, Q.; Li, B. Propagule types and environmental stresses matter in saltmarsh plant restoration. Ecol. Eng. 2020, 143, 105693. [Google Scholar] [CrossRef]
- Grabas, G.P.; Fiorino, G.E.; Reinert, A. Vegetation species richness is associated with daily water-level fluctuations in Lake Ontario coastal wetlands. J. Great Lakes Res. 2019, 45, 805–810. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.L.; Liu, H.F.; Liang, L.Q.; Cai, Y.P.; Wang, X.; Li, C.H. Vegetation dynamics under water-level fluctuations: Implications for wetland restoration. J. Hydrol. 2020, 581, 124418. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H.L. Stomatal limitations to photosynthesis and their critical water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Nakayama, K.; Komai, K.; Tada, K.; Lina, H.C.; Yajima, H.; Yano, S.; Hipsey, M.R.; Tsai, J.W. Modeling dissolved inorganic carbon considering submerged aquatic vegetation. Ecol. Model. 2020, 431, 109188. [Google Scholar] [CrossRef]
- Lan, Z.; Huang, H.; Chen, Y.; Liu, J.; Chen, J.; Li, L.; Chen, J. Testing mechanisms underlying responses of plant functional traits to flooding duration gradient in a lakeshore meadow. J. Freshw. Ecol. 2019, 34, 481–495. [Google Scholar] [CrossRef]
- Chen, X.S.; Li, Y.F.; Cai, Y.H.; Xie, Y.H.; Deng, Z.M.; Li, F.; Hou, Z.Y. Differential strategies to tolerate flooding in Polygonum hydropiper plants originating from low-and high-elevation habitats. Front. Plant Sci. 2020, 9, 1970. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Chen, Y.; Leng, F.; Jin, B.; Fang, C.; Chen, J. The effects of flooding regimes and soil nutrients on lakeshore plant diversity in a pristine lake and a human managed lake in subtropical China. J. Freshw. Ecol. 2019, 34, 757–769. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, L.; Cao, H.B.; Tang, C.D.; Wang, X.Y.; Tian, B.; Shen, J. A dynamic biomass model of emergent aquatic vegetation under different water levels and salinity. Ecol. Model. 2021, 440, 109398. [Google Scholar] [CrossRef]
- Jin, B.F.; Guo, Y.H. Primary studies on the reproductive characteristics of Potomogeton maackianus. Acta. Hydrobiol. Sin. 2001, 25, 439–448. [Google Scholar]
- Wang, H.Y.; Chen, J.K.; Zhou, J. Influence of water level gradient on plant growth, repreduction and biomass allocation of wetland plant species. Chin. J. Plant Ecol. 1999, 23, 269–274. [Google Scholar]
- Blanch, S.J.; Ganf, G.C.; Walker, K.F. Growth and resource allocation in response to flooding in the emergent sedge Bolboschoenus medianus. Aquat. Bot. 1999, 63, 145–160. [Google Scholar] [CrossRef]
- Armstrong, J.; Afreen-Zobayed, F.; Blyth, S.; Armstrong, W. Phragmites australis: Effects of shoot submergence on seedling growth, and survival and radial oxygen loss from roots. Aquat. Bot. 1999, 64, 275–289. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Nõges, T.; Nõges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.B.; Liu, Y.; Zhang, G.S.; Zhang, Q.J.; Duan, H.L. Response of wetland plant functional traits to hydrological processes: A review. Chin. J. Ecol. 2018, 37, 952–959. (In Chinese) [Google Scholar]
- Wang, H.L.; Zhang, X.K.; Wan, A. Morphological responses of Zizania latifolia seedlings to short-term inundation at different periods. J. Lake Sci. 2018, 30, 192–198. (In Chinese) [Google Scholar]
- Zhang, D.; Qi, Q.; Wang, X.; Tong, S.; Lv, X.; An, Y.; Zhu, X. Physiological responses of Carex schmidtii Meinsh to alternating flooding-drought conditions in the Momoge wetland, northeast China. Aquat. Bot. 2019, 153, 33–39. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Chen, Z.L.; Li, L.; Shao, Y. Response of dominant plant species to periodic flooding in the riparian zone of the Three Gorges Reservoir (TGR), China. Sci. Total Environ. 2020, 747, 141101. [Google Scholar] [CrossRef]
- Jia, W.T.; Ma, M.H.; Chen, J.L.; Wu, S.J. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Xie, H.M.; Wei, W.W.; Li, Y.K. Effect of different water depths on morphological and physiological traits of emergent plants. Res. Environ. Sci. 2021, 34, 2706–2713. (In Chinese) [Google Scholar]
- Yuan, G.X.; Wu, A.P.; Chu, Z.S. Effects of water depth on the growth of four emergent macrophytes. Acta Sci. Circumstantiae. 2011, 31, 2690–2697. (In Chinese) [Google Scholar]
- Liang, B.; Gao, F.L.; Guo, H.Y.; Ma, C.C. Influence of different submergence gradients on growth and some physiological characteristics of Phragmites communis seedings. J. Tianjin Norm. Univ. Nat. Sci. Ed. 2015, 35, 62–66. (In Chinese) [Google Scholar]
- He, S.W.; Li, Y.; Zhao, H.G.; Pan, J.Z. Preliminary study on the optical properties of Lake Gehu. J. Lake Sci. 2014, 26, 707–712. (In Chinese) [Google Scholar]
- He, S.W.; Li, Y.; Pan, J.Z.; Gao, Y.; Wu, X.D. OpticaI propenies after multi-treatments in northern part of Lake Gehu, Jiangsu Province in summer. J. Lake Sci. 2015, 27, 311–318. (In Chinese) [Google Scholar]
- Zhao, F.; Zhang, W.; Liu, Y.; Wang, L. Responses of growth and photosynthetic fluorescent characteristics in Ottelia acuminata to a water-depth gradient. J. Freshwater Ecol. 2018, 33, 285–297. [Google Scholar] [CrossRef]
- Laine, A.; Byrne, K.A.; Kiely, G.; Tuittila, E.S. Patterns in vegetation and CO2 dynamics along a water level gradient in a lowland blanket bog. Ecosystems 2007, 10, 890–905. [Google Scholar] [CrossRef]
- Luo, W.; Xie, Y. Growth and morphological responses to water level and nutrient supply in three emergent macrophyte species. Hydrobiologia 2009, 624, 151–160. [Google Scholar] [CrossRef]
- Squires, L.; Valk, A.G.V. Water-depth tolerances of the dominant emergent macrophytes of the Delta Marsh, Manitoba. Can. J. Bot. 1992, 70, 1860–1867. [Google Scholar] [CrossRef]
- Vretare, V.; Weisner, S.E.B. Influence of pressurized ventilation on performance of an emergent macrophyte (Phragmites australis). J. Ecol. 2000, 88, 978–987. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, Y.; Su, Z.; Zhou, D. Physiological Responses of Typical Wetland Plants Following Flooding Process-From an Eco-Hydrological Model Perspective. Front Ecol. Evol. 2022, 10, 721244. [Google Scholar] [CrossRef]
- Li, Y.; Bian, H.; Ren, B.; Xie, Y.; Ding, X.; Yao, X.; Zhou, Q. Morphological responses of two plant species from different elevations in the Dongting Lake wetlands, China, to variation in water levels. Nord. J. Bot. 2019, 37, e01987. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.G.; Chen, F.; Meng, Y.J.; Chandrasekaran, U.; Luo, X.F.; Yang, W.Y.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Armstrong, W.; Beckett, P.M.; Colmer, T.D.; Setter, T.L.; Greenway, H. Tolerance of roots to low oxygen: ‘Anoxic’cores, the phytoglobin-nitric oxide cycle, and energy or oxygen sensing. J. Plant Physiol. 2019, 239, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Qi, X.; Qiang, X.; Chen, X. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar]
- Sharma, P.; Asaeda, T.; Fujino, T. Effect of water depth on the rhizome dynamics of Typha angustifolia. Wetl. Ecol. Manag. 2008, 16, 43–49. [Google Scholar] [CrossRef]
- Zhou, L.F.; Kang, S.Y.; Zhang, J. Effects of different water depths on growth states of typha orientalis presl, water quality and sediment physical and chemical properties. J. Jilin Univ. Earth Sci. Ed. 2021, 51, 231–239. (In Chinese) [Google Scholar]
- Tang, S.W.; Cao, Y.; Xu, L.M.; Luo, S.S.; Ma, Y.S. Simulation Experiment on Adaptation of Typha orientalis to Flooded Habitat. Wetl. Sci. 2019, 17, 582–592. (In Chinese) [Google Scholar]
- Yu, Y.X.; Li, Y.; Wang, H.J.; Wu, X.D.; Zhang, M.; Wang, H.Z.; Hamilton, D.P.; Jeppesen, E. Submersed macrophyte restoration with artificial light-emitting diodes: A mesocosm experiment. Ecotoxicol. Environ. Saf. 2021, 228, 113044. [Google Scholar] [CrossRef]
- Coops, H.; Beklioglu, M.; Crisman, T.L. The role of water-level fluctuations in shallow lake ecosystems—Workshop conclusions. Hydrobiologia 2003, 506, 23–27. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.J.; Yu, Q.; Wang, H.Z.; Liang, X.M.; Liu, M.; Jeppesen, E. Effects of Artificial LED Light on the Growth of Three Submerged Macrophyte Species during the Low-Growth Winter Season: Implications for Macrophyte Restoration in Small Eutrophic Lakes. Water 2019, 11, 1512. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.J.; Li, Y.; Xu, C.; Yu, Q.; Liu, M.; Zhang, M.; Wang, H.Z.; Hamilton, D.P.; Jeppesen, E. Can artificial light promote submerged macrophyte growth in summer? Aquat. Ecol. 2021, 10, 89–98. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef]
- Vander Lee, G.H.; Kraak, M.H.S.; Verdonschot, R.C.F.; Verdonschot, J.V.F.M. Oxygen drives benthic-pelagic decomposition pathways in shallow wetlands. Sci. Rep. 2017, 7, 15051. [Google Scholar] [CrossRef] [PubMed]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; Willey: London, UK, 1979. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Tilman, D. Plant Strategies and the Structure and Dynamics of Plant Communities; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Garssen, A.G.; Baattrup-Pedersen, A.; Voesenek, L.A.C.J.; Verhoeven, J.T.; Soons, M.B. Riparian plant community responses to increased flooding: A meta-analysis. Glob. Chang. Biol. 2015, 21, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Perata, P.; Voesenek, L. Flooding and low oxygen responses in plants. Funct. Plant Biol. 2017, 44, iii–vi. [Google Scholar] [CrossRef]
- Cyril, A.; Sophie, B.; Adam, C.; Guillaume, T. Metabolomics analysis of postphotosynthetic effects of gaseous O2 on primary metabolism in illuminated leaves. Funct. Plant Biol. 2017, 44, 929–940. [Google Scholar]
- Wu, X.D.; Wang, G.X.; Li, Z.G.; Xia, J.; Wei, H.N.; Xu, K.; Zhou, F. Effects of drought stress on growth and chlorophyll fluorescence parameters of Typha orientalis. J. Ecol. Rural Environ. 2012, 28, 103–107. (In Chinese) [Google Scholar]
- Wei, G.W.; Chen, Y.H.; Sun, X.S.; Matsubara, S.Z.; Luo, F.L.; Yu, F.H. Elevation-dependent selection for plasticity in leaf and root traits of Polygonum hydropiper in response to flooding. Environ. Exp. Bot. 2021, 182, 104331. [Google Scholar] [CrossRef]
- Zhao, Y.X. Response Mechanism of Erhai Purple Willow and Calamus to Waterlevel Rise; Hunan Agricultural University: Changsha, China, 2019. (In Chinese) [Google Scholar]
- Kang, S.Y.; Zhou, L.F. Growth Status of Typha angustifolia at Different Water Depths and Their Influence Factors in Shifosi Reservoir in 2018. Wetl. Sci. 2019, 17, 359–364. (In Chinese) [Google Scholar]
- Bai, J.S. Effects of Habitat Change on Sprouting and Growth of Dominant Plants in Herbaceous Marsh Wetland. Master’s Thesis, Northeast Institute of Geography and Agroecology Chinese Academy of Science, Changchun, China, 2021. (In Chinese). [Google Scholar]
- Li, Q.; Wang, G.X. Influence of silts on growth and development of Hydrilla verticillata’s seeding in silt waters. Acta Ecol. Sin. 2010, 30, 995–1002. (In Chinese) [Google Scholar]
- Gafny, S.; Gasith, A. Spatially and temporally sporadic appearance of macrophytes in the littoral zone of Lake Kinneret, Israel: Taking advantage of a window of opportunity. Aquat. Bot. 1999, 62, 249–267. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, X.K.; Wan, A.; Wang, H.L.; Xie, J. Effects of water depth and substrate type on rhizome bud sprouting and growth in Zizania latifolia. Wetl. Ecol. Manag. 2018, 26, 277–284. [Google Scholar] [CrossRef]
- Palpurina, S.; Chytrý, M.; Hölzel, N.; Tichý, L.; Wagner, V.; Horsák, M.; Axmanová, I.; Hájek, M.; Hájková, P.; Freitag, M.; et al. The type of nutrient limitation affects the plant species richness-productivity relationship: Evidence from dry grasslands across Eurasia. J. Ecol. 2019, 107, 1038–1050. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Shay, J.M. The effects of water level on the growth and reproduction of scirpus maritimus var. paludosus. Can. J. Bot. 1981, 59, 118–121. [Google Scholar] [CrossRef]
- Grace, J.B. Effects of water depth on Typha latifolia and Typha domingensis. Am. J. Bot. 1989, 76, 762–768. [Google Scholar] [CrossRef]
- Cui, H.X.; Pu, Y.H.; Xiong, B.H. The Growth and Reproduction Response of Potomogeton Malaianus Miq. To Water Depth Gradient. Acta Hydrobiol. Sin. 1999, 23, 270–272. (In Chinese) [Google Scholar]
- Clevering, O.A.; Hundscheid, M.P.J. Plastic and non-plastic variation in growth of newly established clones of Scirpus (Bolboschoenus) maritimus L. grown at different water depths. Aquat. Bot. 1998, 62, 1–17. [Google Scholar] [CrossRef]
- Han, Z.; Wang, S.Y.; Liu, X.B.; Peng, W.Q.; Ge, G.; Huang, A.P. Ecological thresholds for the dominated wetland plants of Poyang Lake along the gradient of flooding duration. J. Hydraul. Eng. 2019, 50, 252–262. (In Chinese) [Google Scholar]
- Yan, H.; Liu, R.Q.; Liu, Z.N.; Wang, X.; Luo, W.P.; Sheng, L.X. Growth and physiological responses to water depths in Carex schmidtii Meinsh. PLoS ONE 2015, 10, e0128176. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tan, J.L. Recent advances in the application of chlorophyll a fluorescence from photosystem II. Photochem. Photobiol. 2015, 91, 1–14. [Google Scholar] [CrossRef]
- Wang, W.L.; Wang, G.X.; Li, Q. Influence of water turbidity on growth of the seedlings of Potamogeton cripus. J. Ecol. 2006, 26, 3586–3593. (In Chinese) [Google Scholar]
- Cheng, X.R.; Shu, J.; Liu, J.; Wang, W.; Yu, M.K. Growth, photosynthesis and fluorescence characteristics of Begonia fimbristipula and Gynura divaricata under different light conditions. Acta Bot. Boreali-Occident. Sin. 2014, 34, 1426–1431. (In Chinese) [Google Scholar]
- Zhang, S.R. A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chin. Bull. Bot. 1999, 16, 444–448. (In Chinese) [Google Scholar]
- Dawson, S.; Dennison, W. Effects of ultraviolet and photosynthetically active radiation on five seagrass species. Mar Biol. 1996, 125, 629–638. [Google Scholar] [CrossRef]
- Gao, G.Q.; Lv, S.H.; Lv, N.Z. Light-response of PSⅡ fluorescence parameters on Vallisneria natans and Potamogeton malaianus to various water depths in Poyang Lake. Guihaia 2018, 38, 1626–1634. (In Chinese) [Google Scholar]
- Han, B.P.; Han, Z.G.; Fu, X. Algal Photosynthesis: Mechanisms and Models; Science Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]






| Water (mg/L) | Sediment (mg/g) | |||||
|---|---|---|---|---|---|---|
| TP | TN | NH3-N | CODMn | Chl-a | TP | TN |
| 0.089 | 2.97 | 0.21 | 3 | 0.03 | 0.54 | 2.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Wu, X.; Gao, Z.; Ge, X.; Xiong, J.; Tan, L.; Wei, H. The Effects of Water Depth on the Growth of Two Emergent Plants in an In-Situ Experiment. Sustainability 2022, 14, 11309. https://doi.org/10.3390/su141811309
Lin X, Wu X, Gao Z, Ge X, Xiong J, Tan L, Wei H. The Effects of Water Depth on the Growth of Two Emergent Plants in an In-Situ Experiment. Sustainability. 2022; 14(18):11309. https://doi.org/10.3390/su141811309
Chicago/Turabian StyleLin, Xiaowen, Xiaodong Wu, Zhenni Gao, Xuguang Ge, Jiale Xiong, Lingxiao Tan, and Hongxu Wei. 2022. "The Effects of Water Depth on the Growth of Two Emergent Plants in an In-Situ Experiment" Sustainability 14, no. 18: 11309. https://doi.org/10.3390/su141811309






