Do Changes in Prey Community in the Environment Affect the Feeding Selectivity of Silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedures
2.2. Water Physiochemical Measurements
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Data Analysis
2.4.1. Environmental Parameters
2.4.2. Alpha-Diversity Analysis
2.4.3. Beta-Diversity Analysis
2.4.4. Relationship between Water Taxonomic Composition and Environmental Factors
2.4.5. Feeding Selectivity
3. Results
3.1. Temporal Variations in Environmental Factors
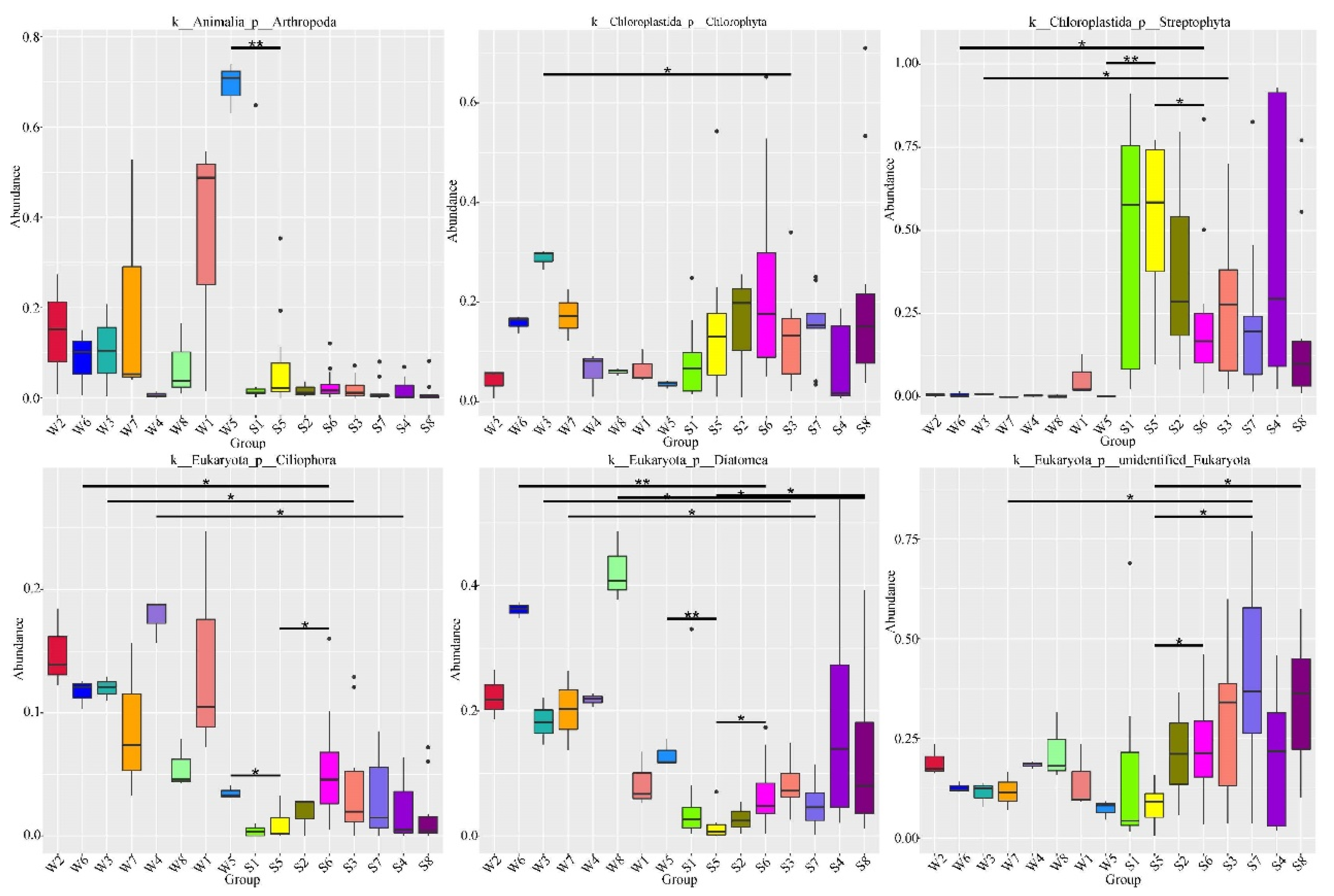
3.2. Spatio-Temporal Variations in the Diversity and Taxonomic Composition of Water and Fish Gut Samples
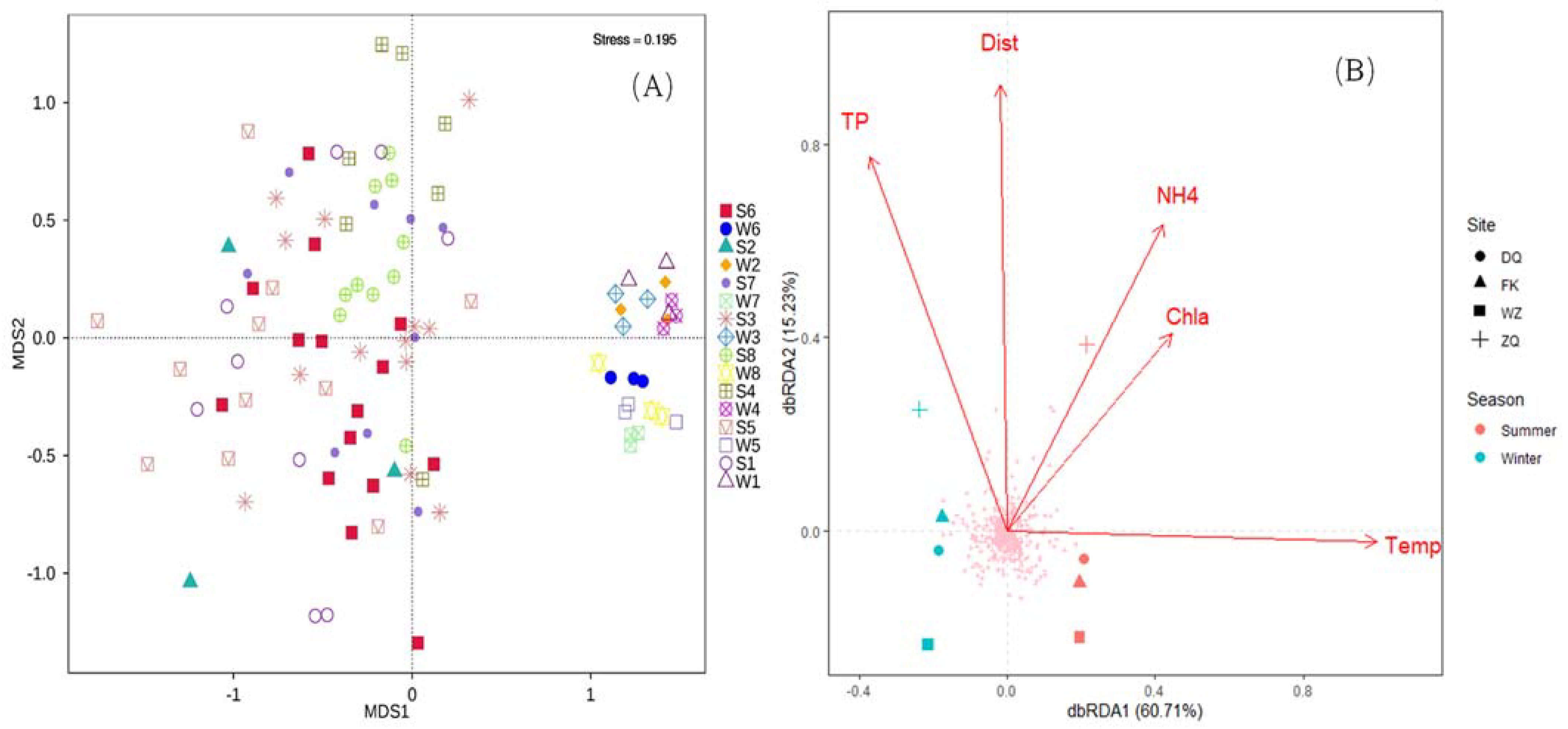
3.3. Environmental Factors Influencing the Taxonomic Composition of Water
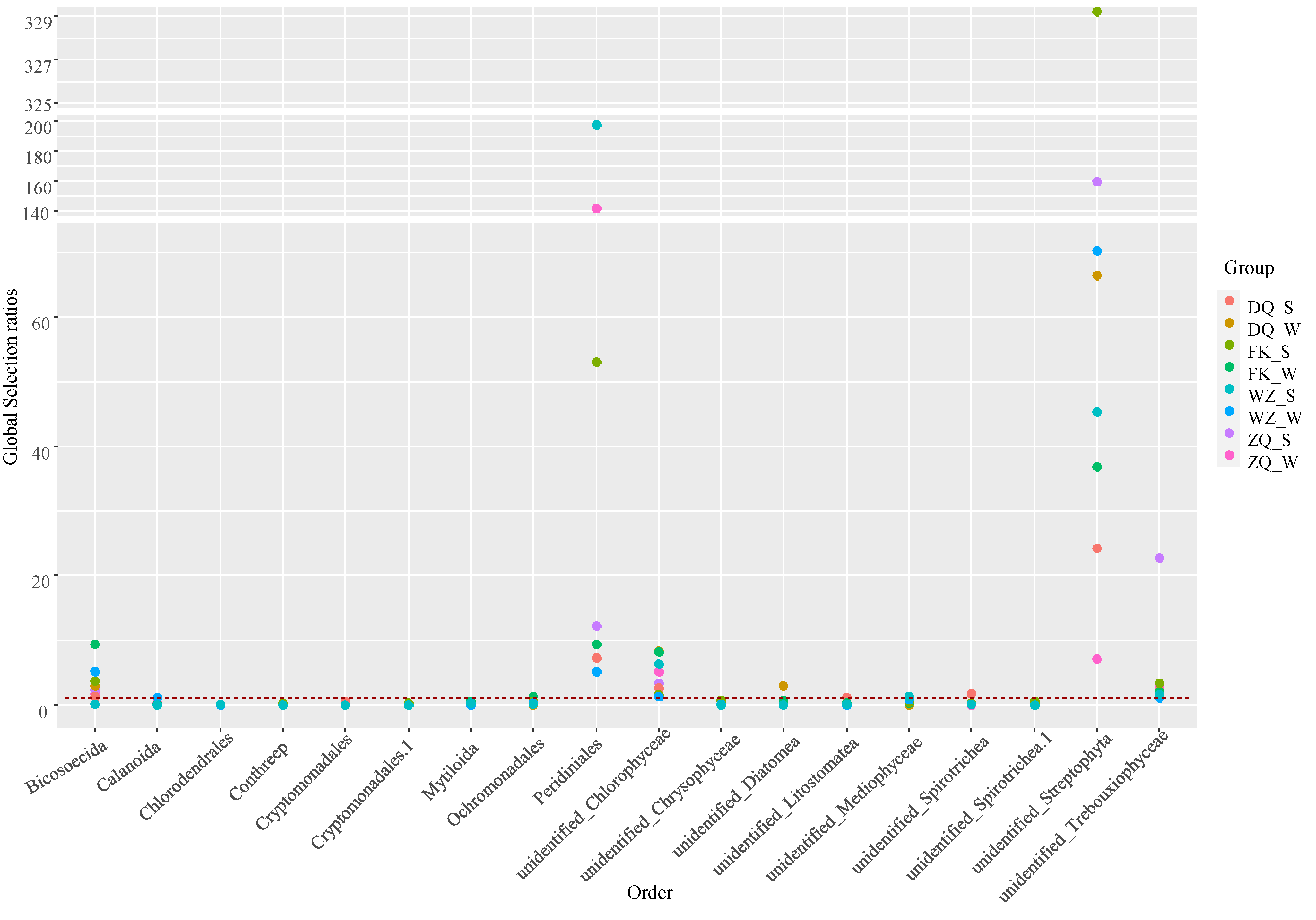
3.4. Dietary Selectivity of Fish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Yang, J.; Zhu, S.; Li, Y.; Chen, W.; Hu, Z. Insight into the combinatorial transcriptional regulation on α-amylase gene in animal groups with different dietary nutrient content. Genomics 2020, 112, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Kreiling, A.-K.; O’Gorman, E.J.; Pálsson, S.; Benhaïm, D.; Leblanc, C.A.; Ólafsson, J.S.; Kristjánsson, B.K. Seasonal variation in the invertebrate community and diet of a top fish predator in a thermally stable spring. Hydrobiologia 2021, 848, 531–545. [Google Scholar]
- Behrens, M.D.; Lafferty, K.D. Temperature and diet effects on omnivorous fish performance: Implications for the latitudinal diversity gradient in herbivorous fishes. Can. J. Fish. Aquat. Sci. 2007, 64, 867–873. [Google Scholar] [CrossRef]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Zhu, S.; Li, J.; Li, S.; Li, X. Individual dietary specialization reduces intraspecific competition, rather than feeding activity, in black amur bream (Megalobrama terminalis). Sci. Rep. 2020, 10, 17961. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, J.-P.; Su, L.-H.; Fan, J.-J.; Chang, H.-Y.; Wang, T.-T.; Wang, L.; Lin, H.-J.; Yang, Y. Fish feeding groups, food selectivity, and diet shifts associated with environmental factors and prey availability along a large subtropical river, China. Aquat. Sci. 2019, 81, 31. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Li, Y.; Zhu, S.; Huang, Y.; Wu, Z.; Liu, Q.; Li, X. Small-subunit ribosomal DNA sequencing analysis of dietary shifts during gonad maturation in wild black Amur bream (Megalobrama terminalis) in the lower reaches of the Pearl River. Fish. Sci. 2017, 83, 955–965. [Google Scholar]
- Barros, A.; Hobbs, J.A.; Willmes, M.; Parker, C.M.; Bisson, M.; Fangue, N.A.; Rypel, A.L.; Lewis, L.S. Spatial heterogeneity in prey availability, feeding success, and dietary selectivity for the threatened longfin smelt. Estuaries. Coast. 2022, 45, 1766–1779. [Google Scholar]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef]
- Deagle, B.E.; Thomas, A.C.; Shaffer, A.K.; Trites, A.W.; Jarman, S.N. Quantifying sequence proportions in a DNA-based diet study using Ion Torrent amplicon sequencing: Which counts count? Mol. Ecol. Resour. 2013, 13, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Gopalakrishnan, S.; Lynggaard, C.; Nielsen, M.; Gilbert, M.T.P. Promises and pitfalls of using high-throughput sequencing for diet analysis. Mol. Ecol. Resour. 2019, 19, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. P. Roy Soc. B-Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B.; List, F.B.C.A.; Bolchacova, E. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Procl. Nat. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef] [PubMed]
- Moon-van der Staay, S.Y.; De Wachter, R.; Vaulot, D. Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 2001, 409, 607–610. [Google Scholar] [CrossRef]
- Tanabe, A.S.; Nagai, S.; Hida, K.; Yasuike, M.; Fujiwara, A.; Nakamura, Y.; Takano, Y.; Katakura, S. Comparative study of the validity of three regions of the 18S-rRNA gene for massively parallel sequencing-based monitoring of the planktonic eukaryote community. Mol. Ecol. Resour. 2016, 16, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Decelle, J.; Romac, S.; Sasaki, E.; Not, F.; Mahe, F. Intracellular diversity of the V4 and V9 regions of the 18S rRNA in marine protists (radiolarians) assessed by high-throughput sequencing. PLoS ONE 2014, 9, e104297. [Google Scholar]
- Choi, J.; Park, J.S. Comparative analyses of the V4 and V9 regions of 18S rDNA for the extant eukaryotic community using the Illumina platform. Sci. Rep. 2020, 10, 6519. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, T.; Bass, D.; Nebel, M.; Christen, R.; Jones, M.D.M.; Breiner, H.-W.; Richards, T.A. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 2010, 19, 21–31. [Google Scholar] [CrossRef]
- Hu, S.K.; Liu, Z.; Lie, A.A.Y.; Countway, P.D.; Kim, D.Y.; Jones, A.C.; Gast, R.J.; Cary, S.C.; Sherr, E.B.; Sherr, B.F.; et al. Estimating protistan diversity using high-throughput sequencing. J. Eukaryot. Microbiol. 2015, 62, 688–693. [Google Scholar] [CrossRef]
- Luddington, I.A.; Kaczmarska, I.; Lovejoy, C. Distance and character-based evaluation of the V4 region of the 18S rRNA gene for the identification of diatoms (Bacillariophyceae). PLoS ONE 2012, 7, e45664. [Google Scholar] [CrossRef]
- Kuo, J.; Chen, C.-Y.; Han, C.-C.; Ju, Y.-M.; Tew, K.S. Analyses of diet preference of larval orange-spotted grouper (Epinephelus coioides) grown under inorganic fertilization method using next-generation sequencing. Aquaculture 2021, 531, 735916. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Zhao, Y.; Yang, H. Sea cucumber (Apostichopus japonicus) eukaryotic food source composition determined by 18s rDNA barcoding. Mar. Biol. 2016, 163, 153. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Li, X.; Liu, Q.; Li, J.; Li, Y.; Yang, J.; Zhu, S. Fish community structure and environmental effects of West River. South China Fish. Sci. 2020, 16, 42–52. [Google Scholar]
- Xie, P.; Liu, J. Practical success of biomanipulation using filter-feeding fish to control cyanobacteria clooms: A synthesis of decades of research and application in a subtropical hypereutrophic Lake. Sci. World J. 2001, 1, 337–356. [Google Scholar] [CrossRef]
- Shen, R.; Gu, X.; Chen, H.; Mao, Z.; Zeng, Q.; Jeppesen, E. Silver carp (Hypophthalmichthys molitrix) stocking promotes phytoplankton growth by suppression of zooplankton rather than through nutrient recycling: An outdoor mesocosm study. Freshw. Biol. 2021, 66, 1074–1088. [Google Scholar] [CrossRef]
- Radke, R.J.; Kahl, U. Effects of a filter-feeding fish [silver carp, Hypophthalmichthys molitrix (Val.)] on phyto- and zooplankton in a mesotrophic reservoir: Results from an enclosure experiment. Freshw. Biol. 2002, 47, 2337–2344. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Zhong, P.; Zhang, X.; Ning, J.; Larsen, S.E.; Chen, D.; Gao, Y.; He, H.; Jeppesen, E. Successful restoration of a tropical shallow eutrophic lake: Strong bottom-up but weak top-down effects recorded. Water Res. 2018, 146, 88–97. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, J.A.; Anderson, A.M.; Casper, A.F. Multi-trophic response to invasive silver carp (Hypophthalmichthys molitrix) in a large floodplain river. Freshw. Biol. 2018, 63, 597–611. [Google Scholar] [CrossRef]
- Domaizon, I.; Dévaux, J. Impact of moderate silver carp biomass gradient on zooplankton communities in a eutrophic reservoir. Consequences for the use of silver carp in biomanipulation. C. R. Acad. Sci. Ser.-III–Sci. Vie 1999, 322, 621–628. [Google Scholar] [CrossRef]
- Peng, G.; Zhou, X.; Xie, B.; Huang, C.; Uddin, M.M.; Chen, X.; Huang, L. Ecosystem stability and water quality improvement in a eutrophic shallow lake via long-term integrated biomanipulation in Southeast China. Ecol. Eng. 2021, 159, 106119. [Google Scholar] [CrossRef]
- Editorial Committee of Encyclopedia of Rivers and Lakes in China. Section of Zhujiang River basin. In Encyclopedia of Rivers and Lakes in China; China Water & Power Press: Beijing, China, 2013; pp. 1–6. [Google Scholar]
- Cheng, F.; Li, W.; Castello, L.; Murphy, B.R.; Xie, S. Potential effects of dam cascade on fish: Lessons from the Yangtze River. Rev. Fish Biol. Fish. 2015, 25, 569–585. [Google Scholar] [CrossRef]
- Liu, Q.; Lai, Z.; Wang, C.; Ni, J.; Gao, Y. Seasonal variation significantly affected bacterioplankton and eukaryoplankton community composition in Xijiang River, China. Environ. Monit. Assess. 2022, 194, 55. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Li, X.; Huang, Y.; Liu, Y. Resource status and spatial distribution characteristics of four major Chinese carps in the Pearl River. Acta Hydrobiol. Sin. 2017, 41, 1–9. [Google Scholar]
- The State Environment Protection Administration. Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Barbour, A.B.; Montgomery, M.L.; Adamson, A.A.; Díaz-Ferguson, E.; Silliman, B.R. Mangrove use by the invasive lionfish Pterois volitans. Mar. Ecol. Prog. Ser. 2010, 401, 291–294. [Google Scholar] [CrossRef]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. ISME J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2013. Available online: https://mirrors.tuna.tsinghua.edu.cn/CRAN/ (accessed on 1 July 2021).
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Modell. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- Duncan, E.M.; Arrowsmith, J.A.; Bain, C.E.; Bowdery, H.; Broderick, A.C.; Chalmers, T.; Fuller, W.J.; Galloway, T.S.; Lee, J.H.; Lindeque, P.K.; et al. Diet-related selectivity of macroplastic ingestion in green turtles (Chelonia mydas) in the eastern Mediterranean. Sci. Rep. 2019, 9, 11581. [Google Scholar] [CrossRef]
- Wang, C.; Lai, Z.; Li, X.; Gao, Y.; Li, Y.; Yu, Y. Annual variation pattern of phytoplankton community at the downstream of Xijiang River. Acta Ecol. Sin. 2013, 33, 4398–4408. [Google Scholar] [CrossRef][Green Version]
- Bahnwart, M.; HÜbener, T.; Schubert, H. Downstream changes in phytoplankton composition and biomass in a lowland river-lake system (Warnow River, Germany). Hydrobiologia 1999, 391, 99–111. [Google Scholar] [CrossRef]
- Tragin, M.; Zingone, A.; Vaulot, D. Comparison of coastal phytoplankton composition estimated from the V4 and V9 regions of the 18S rRNA gene with a focus on photosynthetic groups and especially Chlorophyta. Environ. Microbiol. 2018, 20, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Bagley, M.; Elk, M.; Pilgrim, E.; Martinson, J.; Santo Domingo, J. Spatial and temporal dynamics of a freshwater eukaryotic plankton community revealed via 18S rRNA gene metabarcoding. Hydrobiologia 2018, 818, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, B.B.; Flinn, M.B. Diet of invasive silver carp (Hypophthalmichthys molitrix) in a mainstem reservoir ecosystem. J. Ky. Acad. Sci. 2019, 79, 3–11. [Google Scholar]
- Ochs, C.A.; Pongruktham, O.; Killgore, K.J.; Hoover, J.J. Phytoplankton prey selection by Hypophthalmichthys molitrix val. (silver carp) in a lower Mississippi river backwater lake. Southeast. Nat. 2019, 18, 113–129. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Lin, Q.; Han, B. Effects of silver carp (Hypophthalmichthys molitrix) and nutrients on the plankton community of a deep, tropical reservoir: An enclosure experiment. Freshw. Biol. 2013, 58, 100–113. [Google Scholar] [CrossRef]
- Wang, L.; Liu, P.; Sun, J.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Comparison and combination of selective grazing on natural seston by benthic bivalves (Hyriopsis cumingii) and pelagic fish (Hypophthalmichthys molitrix). Environ. Sci. Pollut. Res. 2018, 25, 33423–33431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, P.; Huang, X. A review of nontraditional biomanipulation. Sci. World J. 2008, 8, 362037. [Google Scholar] [CrossRef]
- Ma, H.; Cui, F.; Liu, Z.; Fan, Z.; He, W.; Yin, P. Effect of filter-feeding fish silver carp on phytoplankton species and size distribution in surface water: A field study in water works. J. Environ. Sci. 2010, 22, 161–167. [Google Scholar] [CrossRef]
- Vörös, L.; Oldal, I.; Présing, M.; V-Balogh, K. Size-selective filtration and taxon-specific digestion of plankton algae by silver carp (Hypophthalmichthys molitrix Val.). In Shallow Lakes’ 95; Springer: Dordrecht, The Netherlands, 1997; pp. 223–228. [Google Scholar]
- Gning, N.; Vidy, G.; Thiaw, O.T. Feeding ecology and ontogenic diet shifts of juvenile fish species in an inverse estuary: The Sine-Saloum, Senegal. Estuar. Coast. Shelf Sci. 2008, 76, 395–403. [Google Scholar] [CrossRef]
- Latli, A.; Michel, L.N.; Lepoint, G.; Kestemont, P. River habitat homogenisation enhances trophic competition and promotes individual specialisation among young of the year fish. Freshw. Biol. 2019, 64, 520–531. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, F.; Murphy, B.R.; Xie, S. Downstream effects of the Three Gorges Dam on larval dispersal, spatial distribution, and growth of the four major Chinese carps call for reprioritizing conservation measures. Can. J. Fish. Aquat. Sci. 2018, 75, 141–151. [Google Scholar] [CrossRef]
- Huang, K.; Qin, J. Research on designed lowest navigable water level at downstream of the third and fourth ship locks of Changzhou hydro-complex. HongShui River 2012, 31, 31–35. [Google Scholar]
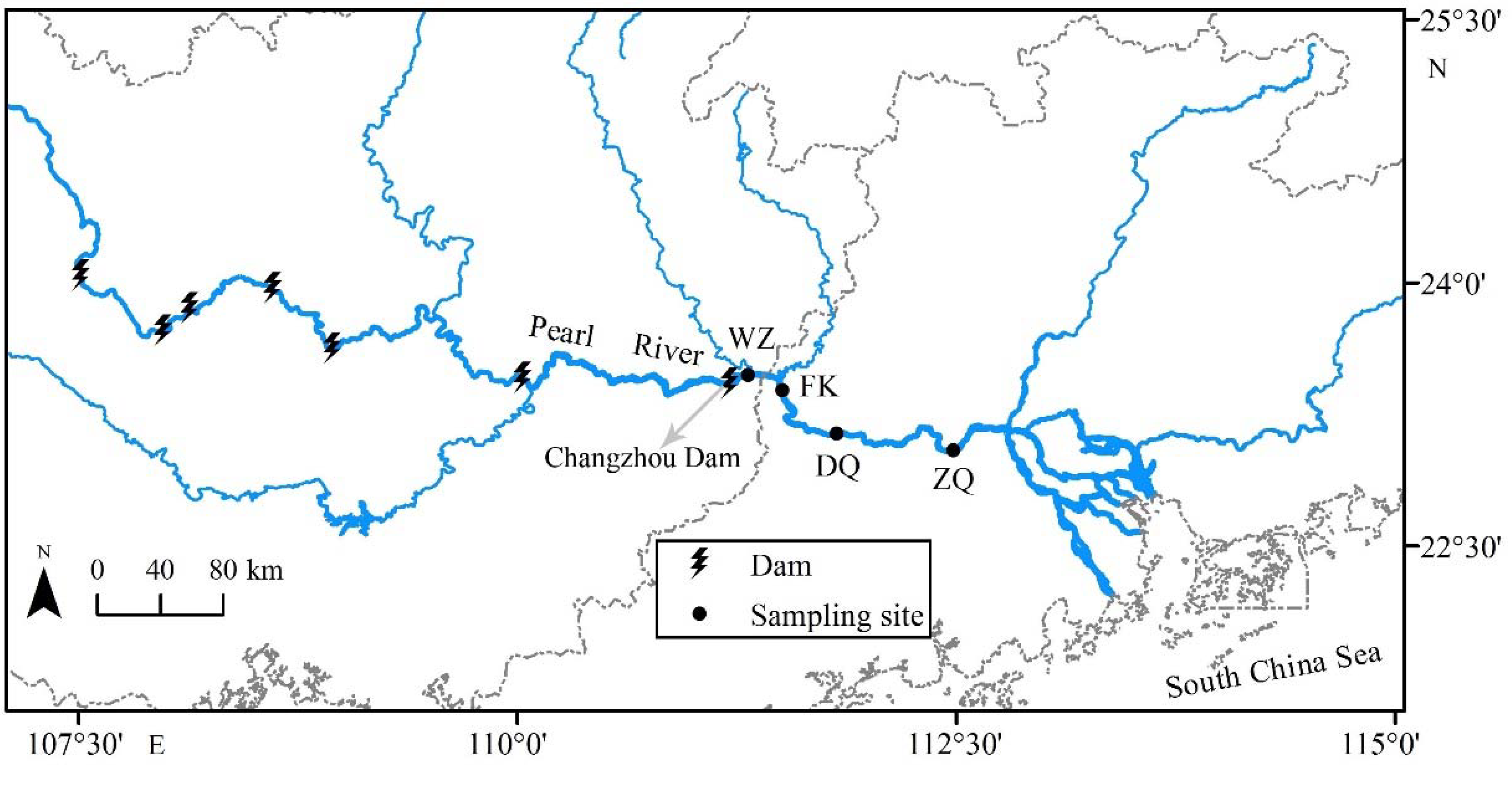

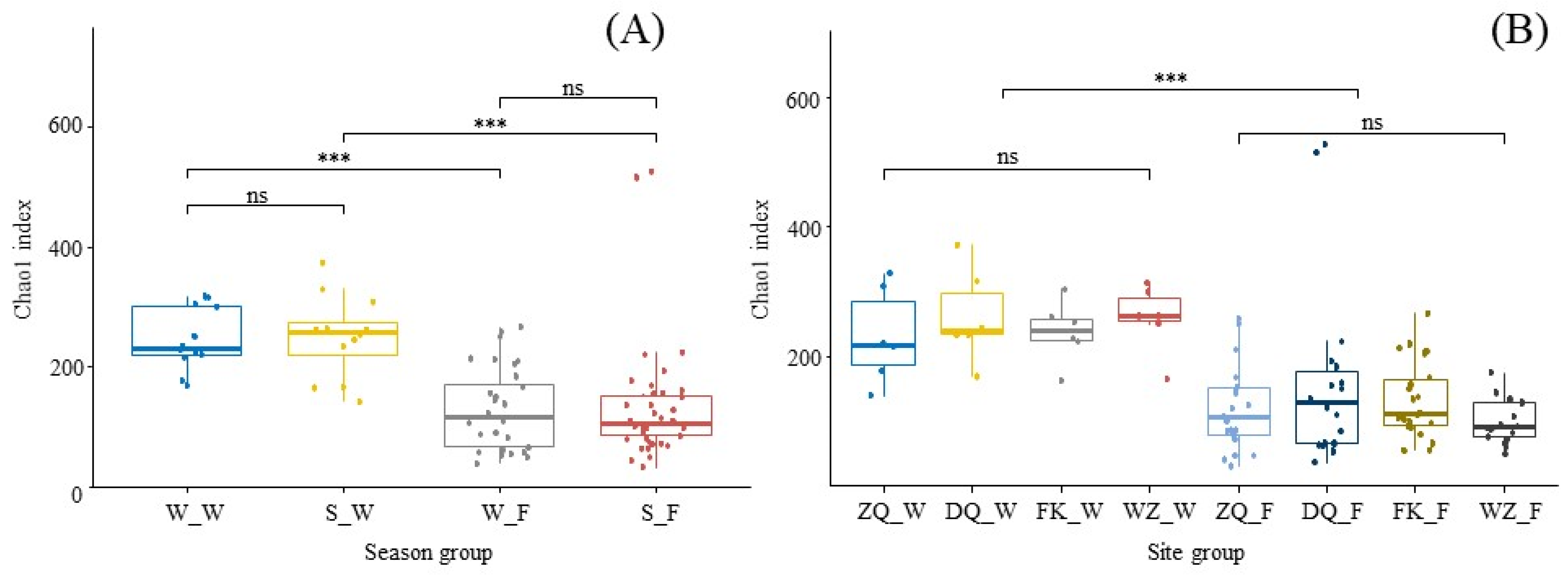

| Season | Site | Latitude (N) | Longitude (E) | Sample | N | SL/mm (Mean ± SD) | M/g (Mean ± SD) | Group |
|---|---|---|---|---|---|---|---|---|
| Winter | ZQ | 23°2′37″ | 112°29′8″ | Fish | 9 | 367.2 ± 69.6 | 1046.0 ± 755.2 | S1 |
| Water | 3 | - | - | W1 | ||||
| DQ | 23°8′20″ | 111°49′11″ | Fish | 3 | 484.0 ± 52.4 | 2368.5 ± 961.2 | S2 | |
| Water | 3 | - | - | W2 | ||||
| FK | 23°23′3” | 111°30′42″ | Fish | 13 | 459.3 ± 40.9 | 1946.4 ± 589.6 | S3 | |
| Water | 3 | - | - | W3 | ||||
| WZ | 23°28′18″ | 111°18′55″ | Fish | 7 | 420.6 ± 97.5 | 1651.2 ± 773.6 | S4 | |
| Water | 3 | - | - | W4 | ||||
| Summer | ZQ | 23°2′37″ | 112°29′8″ | Fish | 11 | 345.0 ± 18.1 | 810.0 ± 153.5 | S5 |
| Water | 3 | - | - | W5 | ||||
| DQ | 23°8′20″ | 111°49′11″ | Fish | 15 | 429.9 ± 53.5 | 1738.9 ± 754.2 | S6 | |
| Water | 3 | - | - | W6 | ||||
| FK | 23°23′3″ | 111°30′42″ | Fish | 9 | 406.7 ± 44.4 | 1379.0 ± 446.4 | S7 | |
| Water | 3 | - | - | W7 | ||||
| WZ | 23°28′18″ | 111°18′55″ | Fish | 10 | 462.7 ± 42.9 | 1903.5 ± 452.8 | S8 | |
| Water | 3 | - | - | W8 |
| Group | Based on Bray–Curtis Index | Based on Jaccard Index | ||||||
|---|---|---|---|---|---|---|---|---|
| MRPP | PERMANOVA | MRPP | PERMANOVA | |||||
| δ | p | F | p | δ | p | F | p | |
| Winter vs. summer | ||||||||
| Water | 0.505 | 0.001 | 11.541 | 0.001 | 0.668 | 0.001 | 7.007 | 0.001 |
| Fish samples from ZQ | 0.792 | 0.003 | 1.805 | 0.002 | 0.882 | 0.002 | 1.484 | 0.004 |
| Fish samples from DQ | 0.781 | 0.535 | 1.079 | 0.277 | 0.875 | 0.540 | 1.041 | 0.335 |
| Fish samples from FK | 0.761 | 0.031 | 1.51 | 0.035 | 0.861 | 0.035 | 1.322 | 0.02 |
| Fish samples from WZ | 0.721 | 0.001 | 2.832 | 0.001 | 0.831 | 0.001 | 2.153 | 0.002 |
| Water vs. fish | ||||||||
| ZQ | 0.759 | 0.001 | 6.502 | 0.001 | 0.855 | 0.001 | 4.130 | 0.001 |
| DQ | 0.716 | 0.001 | 7.351 | 0.001 | 0.825 | 0.001 | 4.652 | 0.001 |
| FK | 0.728 | 0.001 | 6.646 | 0.001 | 0.835 | 0.001 | 4.266 | 0.001 |
| WZ | 0.715 | 0.001 | 6.473 | 0.001 | 0.825 | 0.001 | 4.173 | 0.001 |
| Four sites | ||||||||
| Water | 0.565 | 0.004 | 1.934 | 0.01 | 0.710 | 0.004 | 1.717 | 0.01 |
| Fish | 0.782 | 0.001 | 2.788 | 0.001 | 0.875 | 0.001 | 2.04 | 0.001 |
| Environmental Variables | Monte Carlo Permutation Test | ANOVA-like Permutation Test | ||||
|---|---|---|---|---|---|---|
| dbRDA1 | dbRDA2 | r2 | p | F | p | |
| Temp | 1.000 | 0.028 | 0.98 | 0.001 | 11.47 | 0.001 |
| TP | −0.475 | −0.880 | 0.66 | 0.001 | 2.38 | 0.021 |
| Chla | 0.773 | −0.634 | 0.33 | 0.013 | 1.68 | 0.066 |
| NH4+ | 0.592 | −0.806 | 0.52 | 0.002 | 1.98 | 0.038 |
| Dist | −0.030 | −1.000 | 0.74 | 0.001 | 1.40 | 0.156 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Liu, Q.; Zhu, S.; Li, Y.; Li, X.; Li, J. Do Changes in Prey Community in the Environment Affect the Feeding Selectivity of Silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China? Sustainability 2022, 14, 11175. https://doi.org/10.3390/su141811175
Xia Y, Liu Q, Zhu S, Li Y, Li X, Li J. Do Changes in Prey Community in the Environment Affect the Feeding Selectivity of Silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China? Sustainability. 2022; 14(18):11175. https://doi.org/10.3390/su141811175
Chicago/Turabian StyleXia, Yuguo, Qianfu Liu, Shuli Zhu, Yuefei Li, Xinhui Li, and Jie Li. 2022. "Do Changes in Prey Community in the Environment Affect the Feeding Selectivity of Silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China?" Sustainability 14, no. 18: 11175. https://doi.org/10.3390/su141811175
APA StyleXia, Y., Liu, Q., Zhu, S., Li, Y., Li, X., & Li, J. (2022). Do Changes in Prey Community in the Environment Affect the Feeding Selectivity of Silver Carp (Hypophthalmichthys molitrix) in the Pearl River, China? Sustainability, 14(18), 11175. https://doi.org/10.3390/su141811175






