The Deformation and Shear Vortex Width of Flexible Vegetation Roots in an Artificial Floating Bed Channel
Abstract
:1. Introduction
2. Study Methods
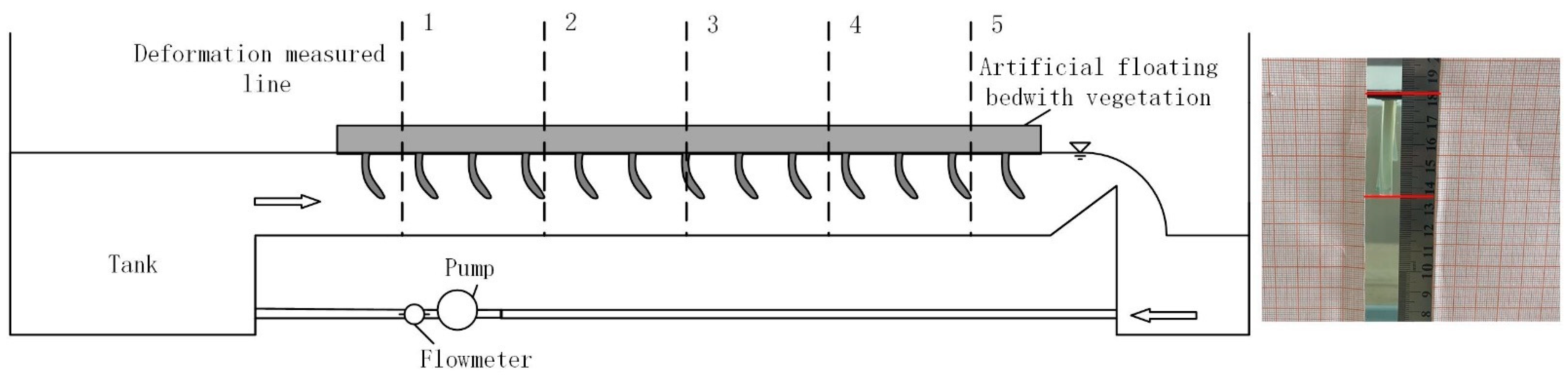
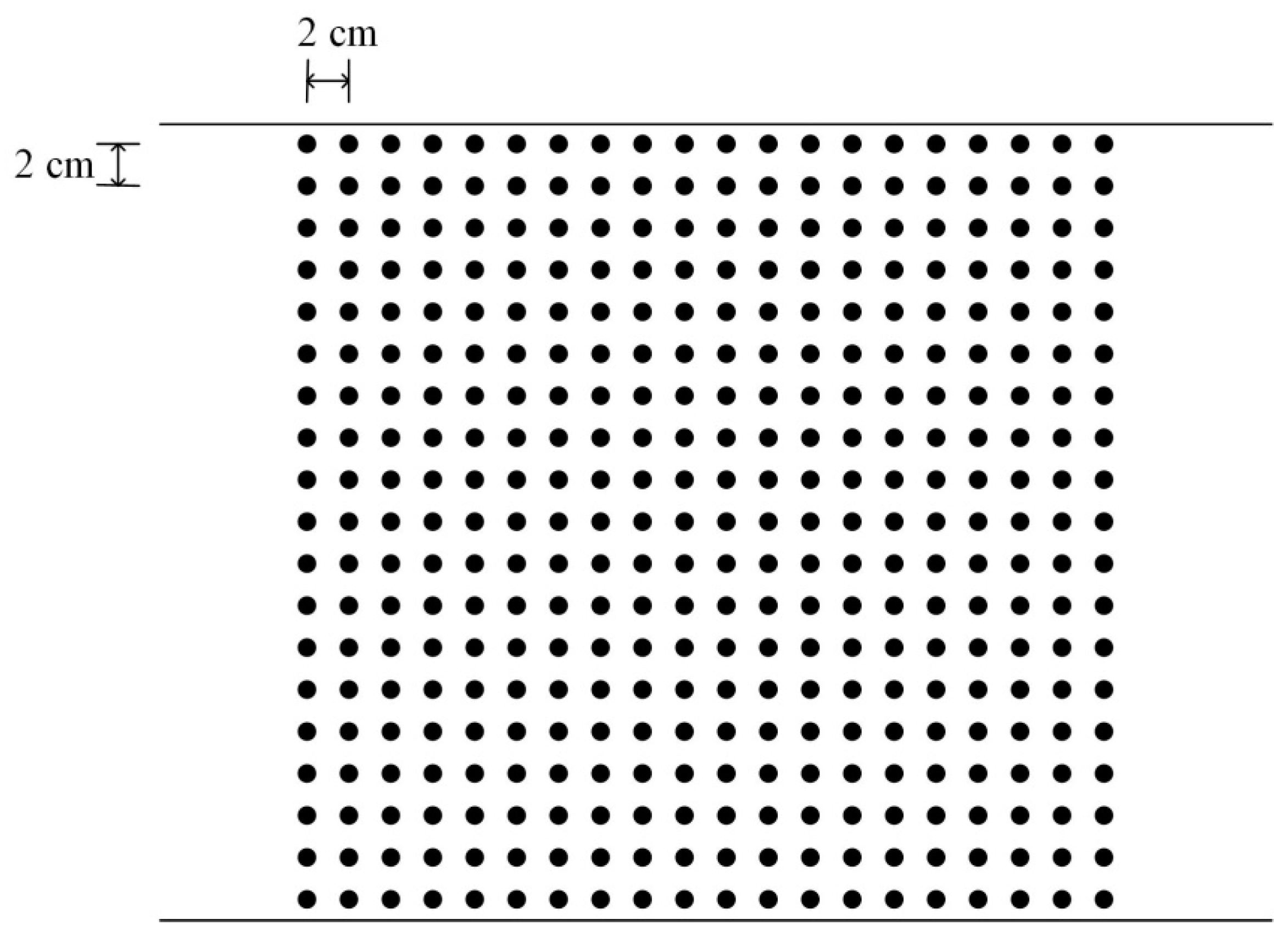
2.1. Experimental Study
2.2. The Effective Blade Length
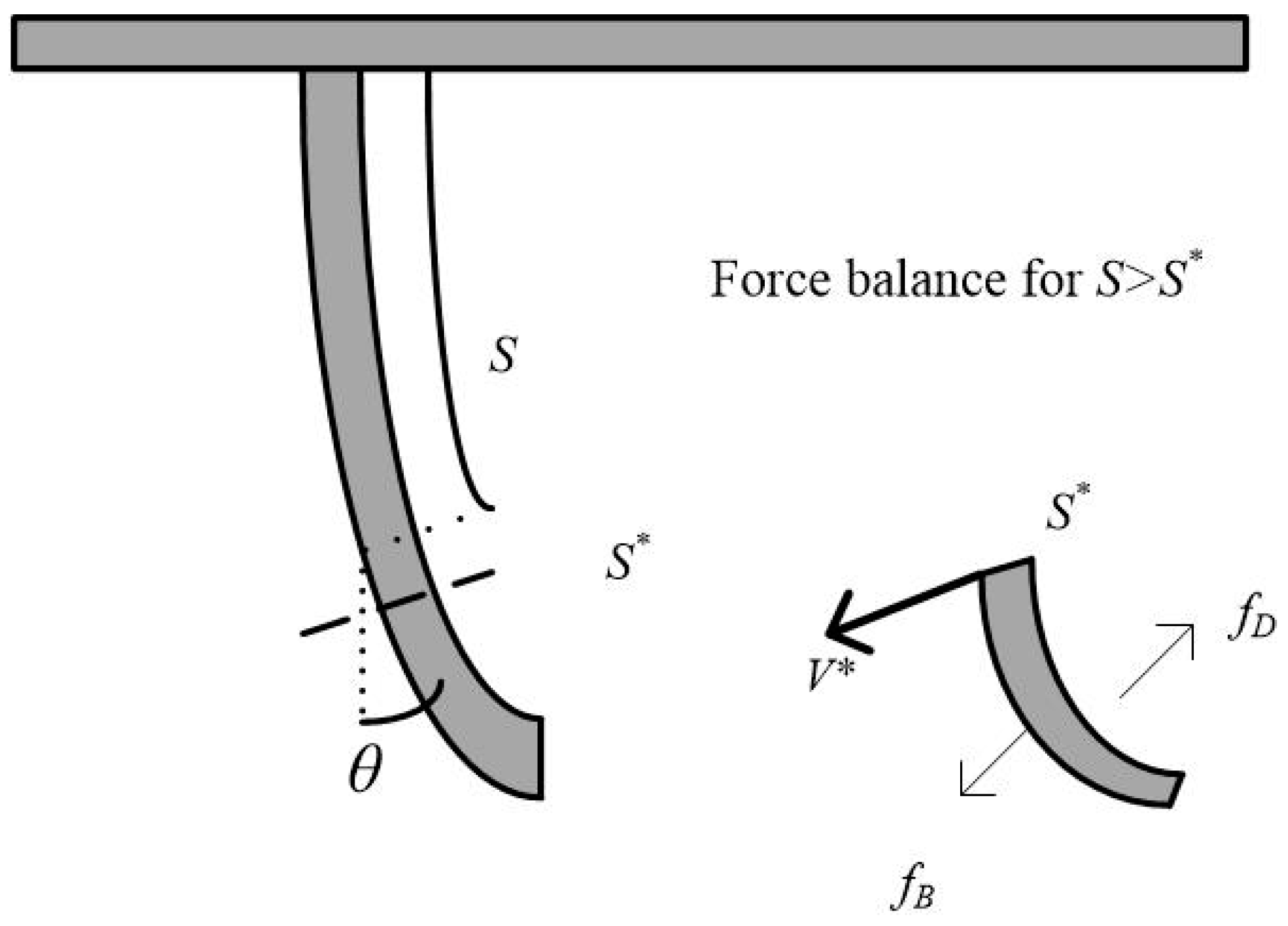
2.3. The Formula of
2.4. Error Analysis
3. Results
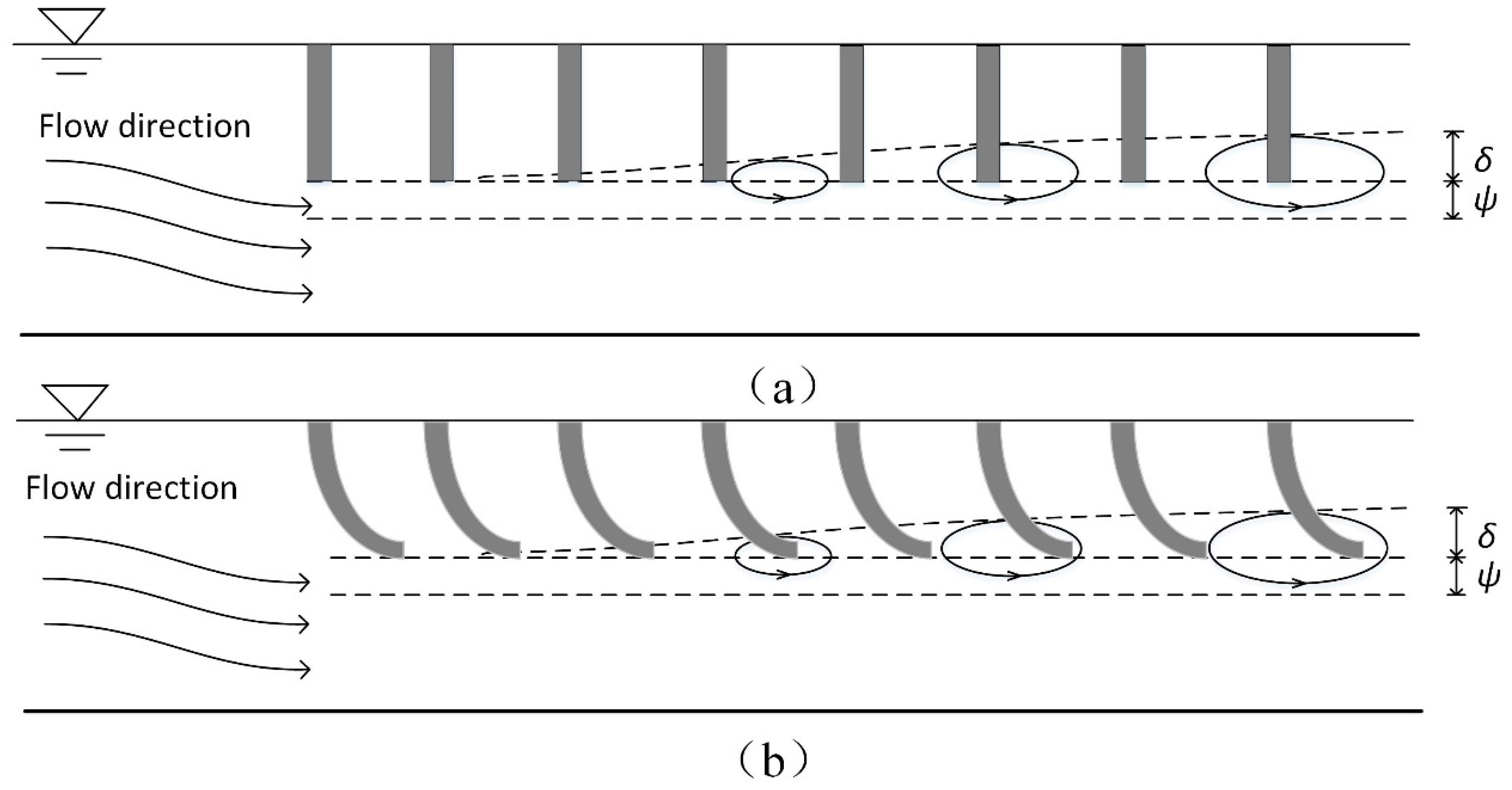
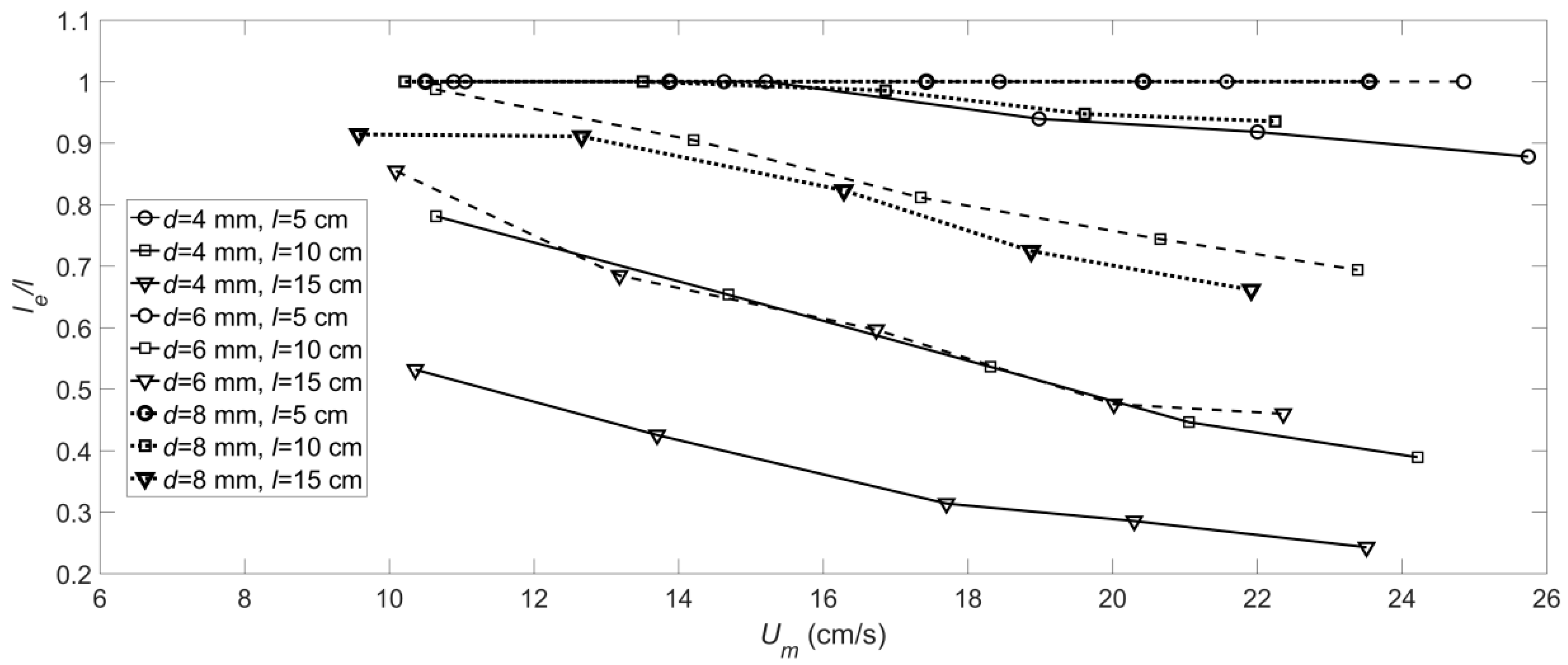
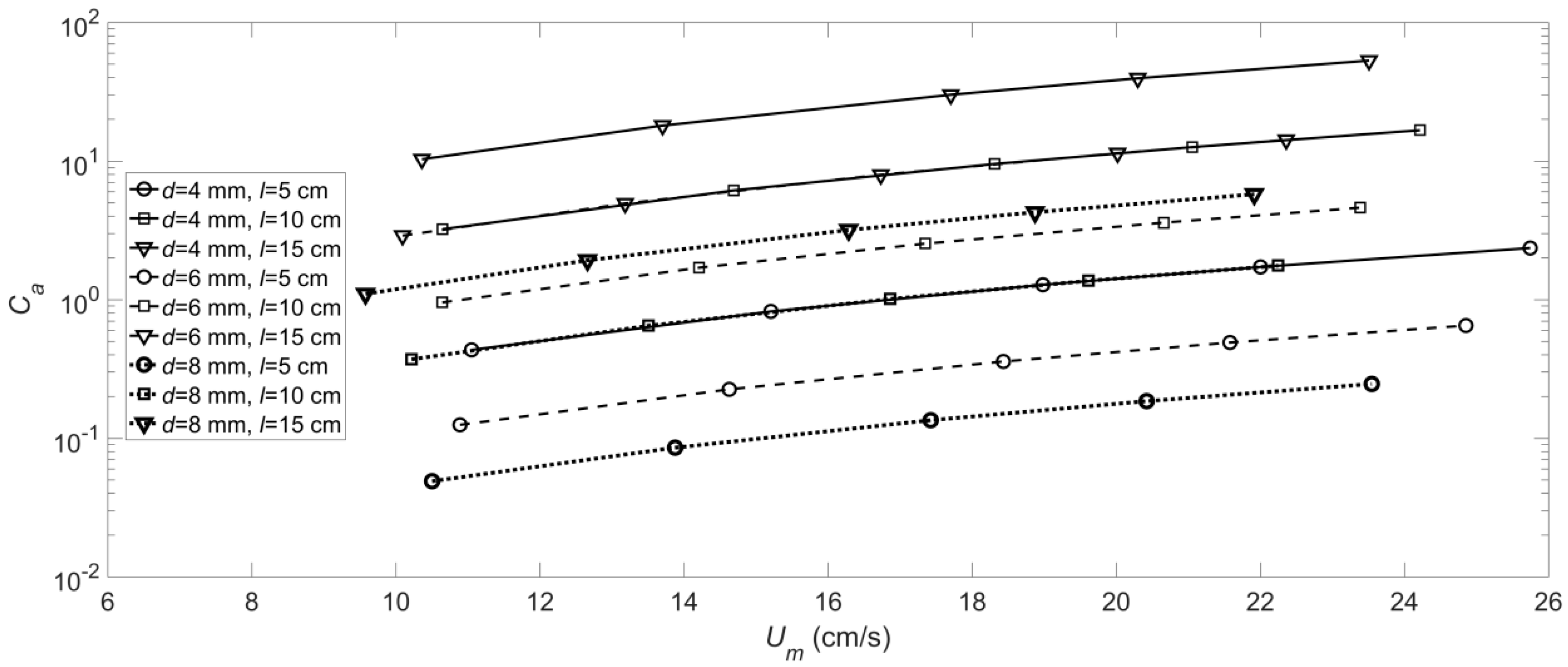
3.1. The Deformation of the Flexible Vegetation
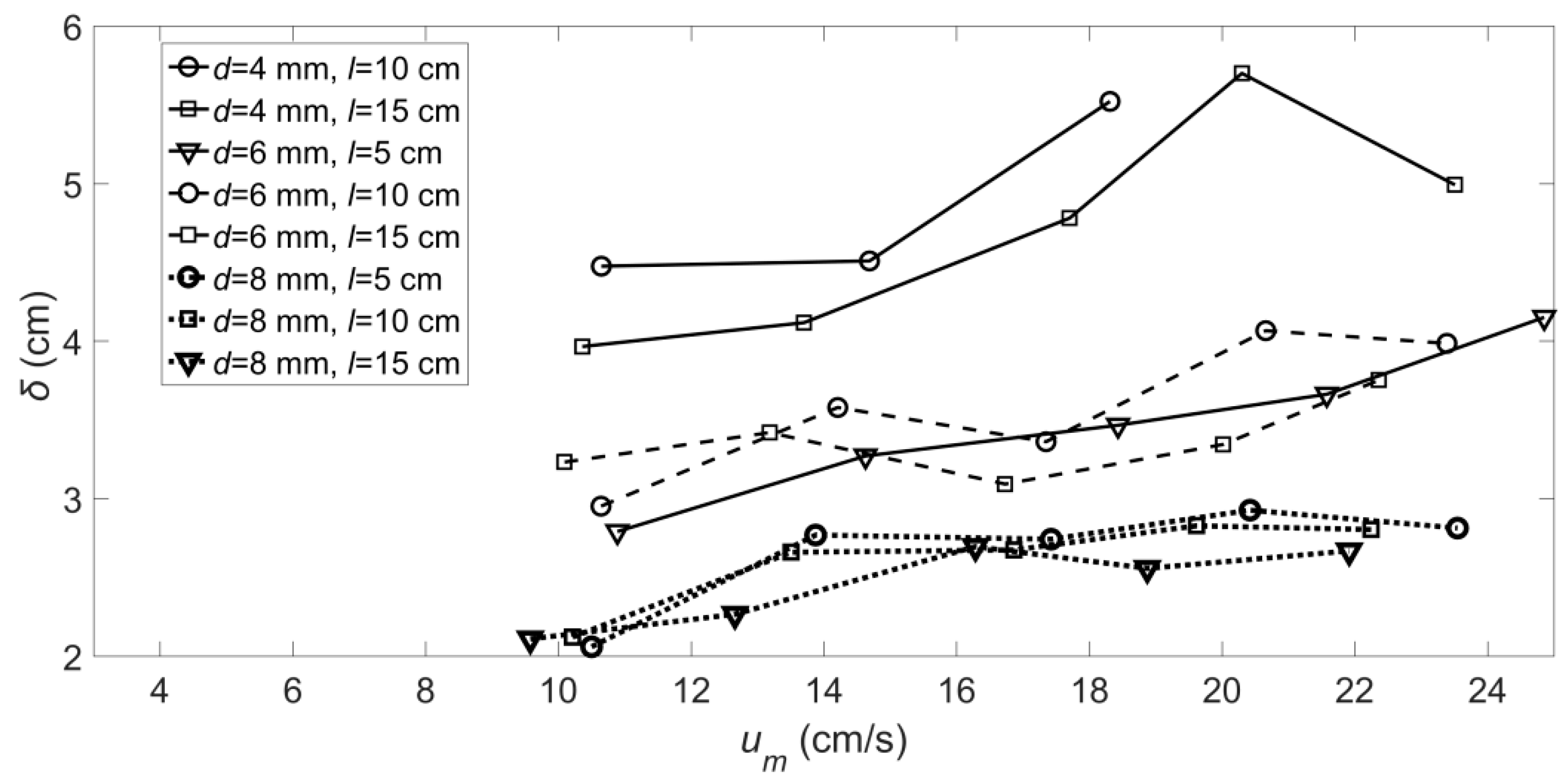
3.2. The Vortex Width of the Flexible Vegetated Channel
4. Discussion
5. Conclusions
- (1)
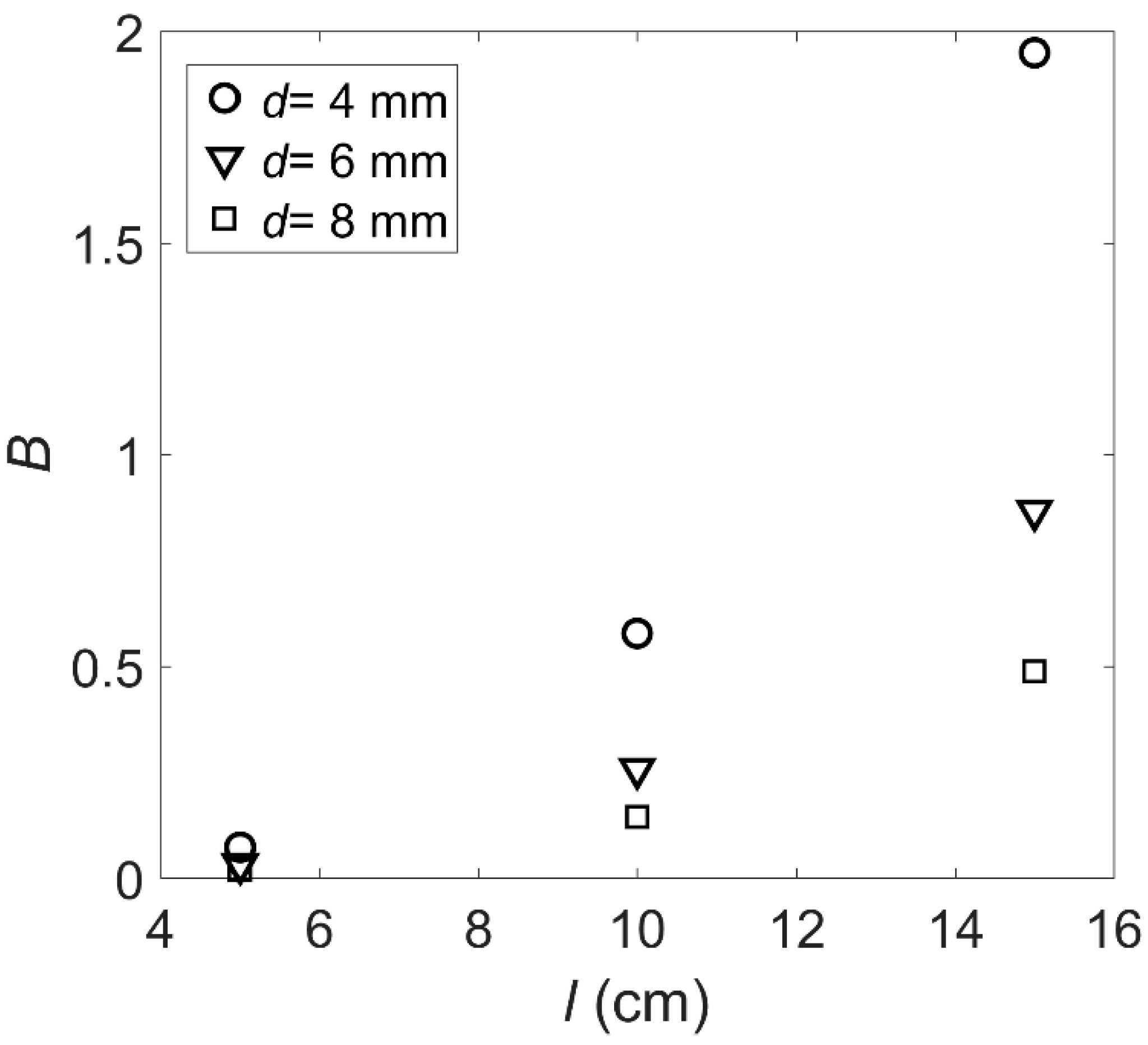
- The and values of the roots of the artificial floating island’s flexible vegetation under hydrodynamic conditions were obtained. The value of gradually increases with the increase of velocity, and the smaller the diameter is, the greater the value of the root with a longer length. The value of will increase with the increase of root length and will decrease with the increase of root diameter.
- (2)
- According to the stress analysis, the simulation formula of flexible root deformation is given. The simulated deformation is in good agreement with the actual deformation, which can effectively provide support for the application-design of artificial floating islands.
- (3)
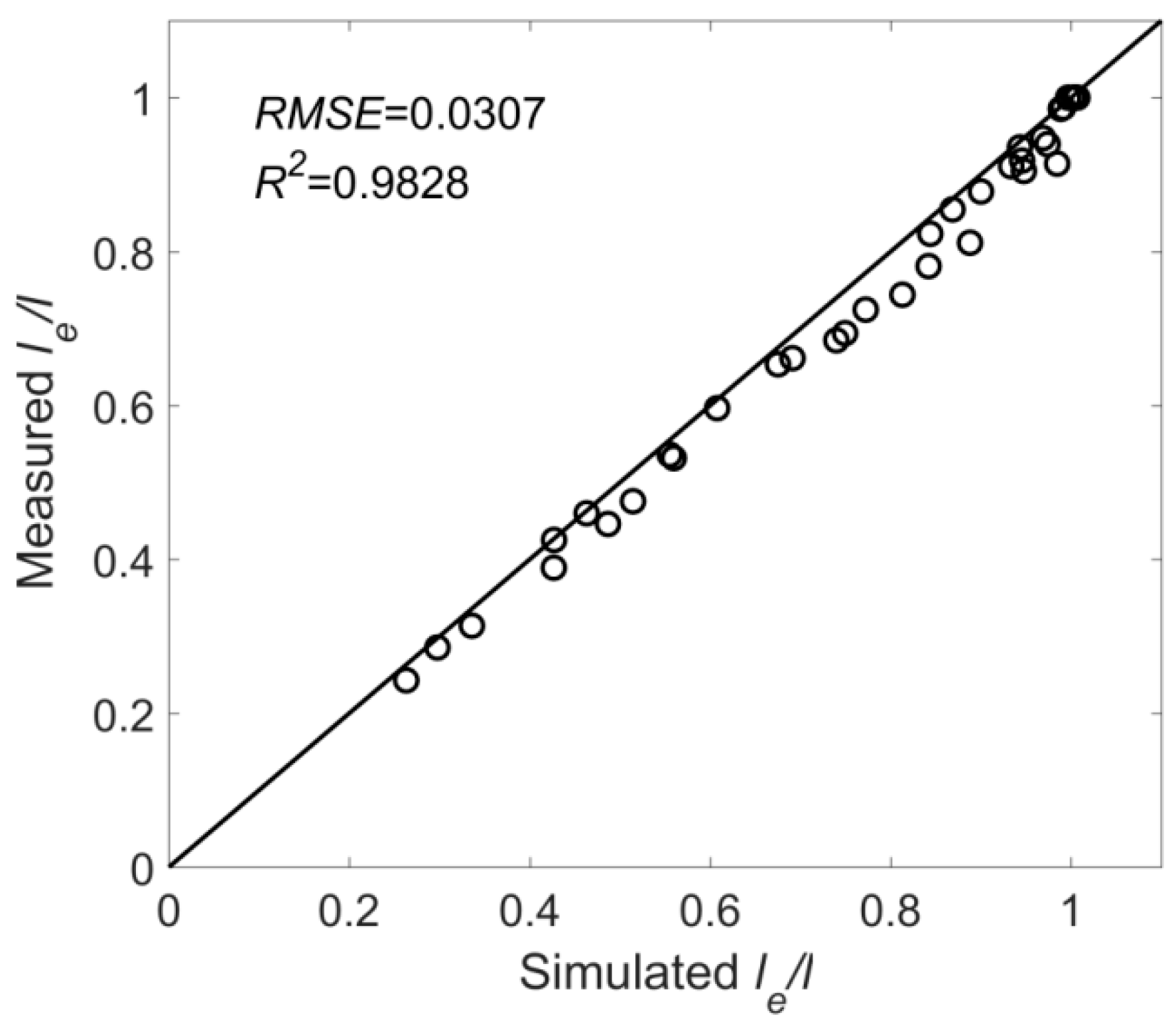
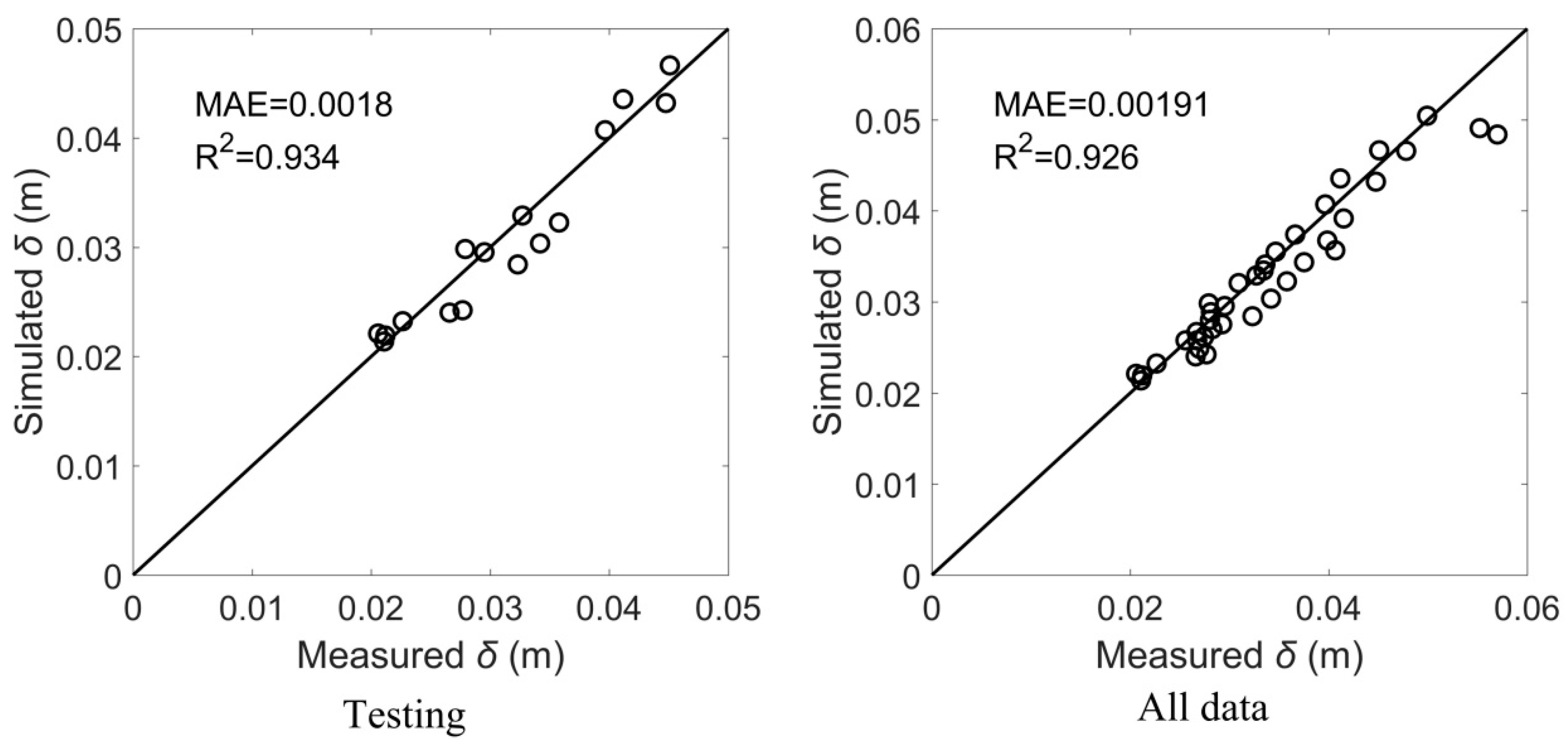
- Based on the theoretical analysis and machine learning, a formula for the shear vortex width of flexible vegetation is established, . The formula simulation has a high precision; R2 was 0.926 and MAE reached 0.00191.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.G.; Xue, W.Y.; Huai, W.X. Effect of vegetation on flow structure and dispersion in strongly curved channels. J. Hydrodyn. Ser. B 2015, 27, 286–291. [Google Scholar] [CrossRef]
- Huai, W.; Hu, Y.; Zeng, Y.; Han, J. Velocity distribution for open channel flows with suspended vegetation. Adv. Water Resour. 2012, 49, 56–61. [Google Scholar] [CrossRef]
- Aberle, J.; Järvelä, J. Flow resistance of emergent rigid and flexible floodplain vegetation. J. Hydraul. Res. 2013, 51, 33–45. [Google Scholar] [CrossRef]
- Follett, E.; Chamecki, M.; Nepf, H. Evaluation of a random displacement model for predicting particle escape from canopies using a simple eddy diffusivity model. Agric. For. Meteorol. 2016, 224, 40–48. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ku, C.R.; Lu, H.L. Effects of aquatic ecological indicators of sustainable green energy landscape facilities. Ecol. Eng. 2014, 71, 144–153. [Google Scholar] [CrossRef]
- Yeh, N.; Yeh, P.; Chang, Y.H. Artificial floating islands for environmental improvement. Renew. Sustain. Energy Rev. 2015, 47, 616–622. [Google Scholar] [CrossRef]
- Huett, D.O.; Morris, S.G.; Smith, G.; Hunt, N. Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res. 2005, 39, 3259–3272. [Google Scholar] [CrossRef]
- Guo, P.; Zhu, Y.; Song, X.; Ding, B.; Zou, G.; Fu, Z.; Lv, Q. Remediation of eutrophic water by terrestrial plant in enclosure: The purification effect and dynamics processes of Lolium multi florum for removing NH~3-N. J.-Zhejiang Univ.-Sci. Ed. 2007, 34, 76. [Google Scholar]
- Song, H.; Li, X.; Wang, X.; Lu, X. Enhancing nitrogen removal performance of vegetated floating-bed by adding Hyriopsiscumingii Lea and an artificial medium. Fresenius Environ. Bull. 2011, 20, 2435–2441. [Google Scholar]
- Sooknah, R.D.; Wilkie, A.C. Nutrient removal by floating aquatic macrophytes cultured in anaerobically digested flushed dairy manure wastewater. Ecol. Eng. 2004, 22, 27–42. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Jin, H. Nitrogen removal from polluted river by enhanced floating bed grown canna. Ecol. Eng. 2009, 35, 135–140. [Google Scholar] [CrossRef]
- Garbett, P. An investigation into the application of floating reed bed and barley straw techniques for the remediation of eutrophic waters. Water Environ. J. 2005, 19, 174–180. [Google Scholar] [CrossRef]
- Miyazaki, A.; Kubota, F.; Agata, W.; Yamamoto, Y.; Song, X. Plant production and water purification efficiency by rice and umbrella plants grown in a floating culture system under various water environmental conditions. J. Fac. Agric. Kyushu Univ. 2000, 45, 29–38. [Google Scholar] [CrossRef]
- Nakai, S.; Zou, G.; Song, X.; Pan, Q.; Zhou, S.; Hosomi, M. Release of anti-cyanobacterial allelochemicals from aquatic and terrestrial plants applicable for artificial floating islands. J. Water Environ. Technol. 2008, 6, 55–63. [Google Scholar] [CrossRef]
- Stewart, F.M.; Muholland, T.; Cunningham, A.B.; Kania, B.G.; Osterlund, M.T. Floating islands as an alternative to constructed wetlands for treatment of excess nutrients from agricultural and municipal wastes–results of laboratory-scale tests. Land Contam. Reclam. 2008, 16, 25–33. [Google Scholar] [CrossRef]
- Wen, L.; Recknagel, F. In situ removal of dissolved phosphorus in irrigation drainage water by planted floats: Preliminary results from growth chamber experiment. Agric. Ecosyst. Environ. 2002, 90, 9–15. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, C. (2008, May). Purification of stream flowing into Dian Lake by ecological floating-bed system. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 2932–2935. [Google Scholar]
- Ai, Y.D.; Liu, M.Y.; Huai, W.X. Numerical investigation of flow with floating vegetation island. J. Hydrodyn. 2020, 32, 31–43. [Google Scholar] [CrossRef]
- Zong, L.; Nepf, H. Flow and deposition in and around a finite patch of vegetation. Geomorphology 2010, 116, 363–372. [Google Scholar] [CrossRef]
- Järvelä, J. Determination of flow resistance caused by non-submerged woody vegetation. Int. J. River Basin Manag. 2004, 2, 61–70. [Google Scholar] [CrossRef]
- Lu, J.; Dai, H.C. Numerical modeling of pollution transport in flexible vegetation. Appl. Math. Model. 2018, 64, 93–105. [Google Scholar] [CrossRef]
- Vogel, S. Drag and reconfiguration of broad leaves in high winds. J. Exp. Bot. 1989, 40, 941–948. [Google Scholar] [CrossRef]
- Huai, W.X.; Zhang, J.; Katul, G.G.; Cheng, Y.G.; Tang, X.; Wang, W.J. The structure of turbulent flow through submerged flexible vegetation. J. Hydrodyn. 2019, 31, 274–292. [Google Scholar] [CrossRef]
- Huai, W.X.; Li, S.; Katul, G.G.; Liu, M.Y.; Yang, Z.H. Flow dynamics and sediment transport in vegetated rivers: A review. J. Hydrodyn. 2021, 33, 400–420. [Google Scholar] [CrossRef]
- Bai, Y.; Duan, Y. The vertical distribution of suspended sediment and phosphorus in a channel with ice cover. Environ. Sci. Pollut. Res. 2021, 28, 37953–37962. [Google Scholar] [CrossRef]
- Nepf, H.; Ghisalberti, M.; White, B.; Murphy, E. Retention time and dispersion associated with submerged aquatic canopies. Water Resour. Res. 2007, 43. [Google Scholar] [CrossRef]
- Liu, C.; Shan, Y.; Lei, J.; Nepf, H. Floating treatment islands in series along a channel: The impact of island spacing on the velocity field and estimated mass removal. Adv. Water Resour. 2019, 129, 222–231. [Google Scholar] [CrossRef]
- Tymiński, T.; Kałuża, T. Investigation of mechanical properties and flow resistance of flexible riverbank vegetation. Pol. J. Environ. Stud. 2012, 21, 201–207. [Google Scholar]
- Łoboda, A.M.; Karpiński, M.; Bialik, R.J. On the relationship between aquatic plant stem characteristics and drag force: Is a modeling application possible? Water 2018, 10, 540. [Google Scholar] [CrossRef]
- Bai, Y.; Xuan, W. Drag force coefficient of the flexible vegetation root in an artificial floating bed channel. Ecol. Eng. 2022, 179, 106619. [Google Scholar] [CrossRef]
- Luhar, M.; Coutu, S.; Infantes, E.; Fox, S.; Nepf, H. Wave-induced velocities inside a model seagrass bed. J. Geophys. Res. Ocean. 2010, 115. [Google Scholar] [CrossRef]
- Chapman, J.A.; Wilson, B.N.; Gulliver, J.S. Drag force parameters of rigid and flexible vegetal elements. Water Resour. Res. 2015, 51, 3292–3302. [Google Scholar] [CrossRef]
- Wu, W.M.; Ozeren, Y.; Wren, D.G.; Chen, Q.; Zhang, G.; Holland, M.; Chatagnier, J. Phase I Report for SERRI Project No. 80037: Investigation of surge and wave reduction by vegetation. Lab. Publ. 2011, 1, 315. [Google Scholar]
- Cavallaro, L.; Viviano, A.; Paratore, G.; Foti, E. Experiments on surface waves interacting with flexible aquatic vegetation. Ocean. Sci. J. 2018, 53, 461–474. [Google Scholar] [CrossRef]
- Möller, I.; Kudella, M.; Rupprecht, F.; Spencer, T.; Paul, M.; Van Wesenbeeck, B.K.; Wolters, G.; Jensen, K.; Bouma, T.J.; Miranda-Lange, M.; et al. Wave attenuation over coastal salt marshes under storm surge conditions. Nat. Geosci. 2014, 7, 727–731. [Google Scholar] [CrossRef] [Green Version]
- Augustin, L.N. Laboratory Experiments and Numerical Modeling of Wave Attenuation through Artificial Vegetation. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 2010. [Google Scholar]
- Anderson, M.E.; Smith, J.M. Wave attenuation by flexible, idealized salt marsh vegetation. Coast. Eng. 2014, 83, 82–92. [Google Scholar] [CrossRef]
- Losada, I.J.; Maza, M.; Lara, J.L. A new formulation for vegetation-induced damping under combined waves and currents. Coast. Eng. 2016, 107, 1–13. [Google Scholar] [CrossRef]
- Koftis, T.; Prinos, P.; Stratigaki, V. Wave damping over artificial Posidonia oceanica meadow: A large-scale experimental study. Coast. Eng. 2013, 73, 71–83. [Google Scholar] [CrossRef]
- Chastel, T.; Botten, K.; Durand, N.; Goutal, N. Bulk drag coefficient of a subaquatic vegetation subjected to irregular waves: Influence of Reynolds and Keulegan-Carpenter numbers. La Houille Blanche 2020, 106, 34–42. [Google Scholar] [CrossRef]
- Luhar, M.; Nepf, H.M. Flow-induced reconfiguration of buoyant and flexible aquatic vegetation. Limnol. Oceanogr. 2011, 56, 2003–2017. [Google Scholar] [CrossRef]
- Abdelrhman, M.A. Modeling coupling between eelgrass Zostera marina and water flow. Mar. Ecol. Prog. Ser. 2007, 338, 81–96. [Google Scholar] [CrossRef]
- Gosselin, F.; De Langre, E.; Machado-Almeida, B.A. Drag reduction of flexible plates by reconfiguration. J. Fluid Mech. 2010, 650, 319–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Cheng, J.; Wang, W.J.; Zeng, L.; Wang, B. Drag coefficient of emergent flexible vegetation in steady nonuniform flow. Water Resour. Res. 2020, 56, e2020WR027613. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, Y.H.; Zha, W. Velocity distribution and turbulence structure of open channel flow with floating-leaved vegetation. J. Hydrol. 2020, 590, 125298. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.; Liu, Y. Predicting the bulk drag coefficient of flexible vegetation in wave flows based on a genetic programming algorithm. Ocean. Eng. 2021, 223, 108694. [Google Scholar] [CrossRef]
- Liu, M.Y.; Huai, W.X.; Yang, Z.H.; Zeng, Y.H. A genetic programming-based model for drag coefficient of emergent vegetation in open channel flows. Adv. Water Resour. 2020, 140, 103582. [Google Scholar] [CrossRef]
- Huai, W.; Li, C. Longitudinal dispersion in open channel flow with suspended canopies. Water Sci. Technol. 2016, 74, 722–728. [Google Scholar] [CrossRef]
- Plew, D.R. Depth-averaged drag coefficient for modeling flow through suspended canopies. J. Hydraul. Eng. 2011, 137, 234–247. [Google Scholar] [CrossRef]
- Bradley, K.; Houser, C. Relative velocity of seagrass blades: Implications for wave attenuation in low-energy environments. J. Geophys. Res. Earth Surf. 2009, 114. [Google Scholar] [CrossRef]
- Alben, S.; Shelley, M.; Zhang, J. Drag reduction through self-similar bending of a flexible body. Nature 2002, 420, 479–481. [Google Scholar] [CrossRef]
- Li, R.M.; Shen, H.W. Effect of tall vegetations on flow and sediment. J. Hydraul. Div. 1973, 99, 793–814. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, J.; Liu, Y.; Wang, Z. Study of the Impact of Vegetation Direction and Slope on Drag Coefficient. Iran. J. Sci. Technol. Trans. Civ. Eng. 2018, 42, 381–390. [Google Scholar] [CrossRef]
- Tanino, Y.; Nepf, H.M. Laboratory investigation of mean drag in a random array of rigid, emergent cylinders. J. Hydraul. Eng. 2008, 134, 34–41. [Google Scholar] [CrossRef]
- Caroppi, G.; Västilä, K.; Gualtieri, P.; Järvelä, J.; Giugni, M.; Rowiński, P.M. Comparison of flexible and rigid vegetation induced shear layers in partly vegetated channels. Water Resour. Res. 2021, 57, e2020WR028243. [Google Scholar] [CrossRef]
- White, B.L.; Nepf, H.M. A vortex-based model of velocity and shear stress in a partially vegetated shallow channel. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Shan, Y.Q.; Liu, C.; Luo, M.K.; Yang, K.J. A simple method for estimating bed shear stress in smooth and vegetated compound channels. J. Hydrodyn. 2016, 28, 497–505. [Google Scholar] [CrossRef]
- Yang, J.Q.; Kerger, F.; Nepf, H.M. Estimation of the bed shear stress in vegetated and bare channels with smooth beds. Water Resour. Res. 2015, 51, 3647–3663. [Google Scholar] [CrossRef]
- Liu, J.L.; Liu, J.K.; Anderson, J.T.; Zhang, R.; Zhang, Z.M. Potential of aquatic macrophytes and artificial floating island for removing contaminants. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2016, 150, 702–709. [Google Scholar] [CrossRef]
- Gippel, C.J. Hydrological management of a lake with floating islands near Pirron Yallock, Victoria, Australia. J. Environ. Manag. 1993, 37, 219–238. [Google Scholar] [CrossRef]
- Yang, H. Changes in pollutant concentrations by artificial floating island installed in reservoir for irrigation. J. Korean Soc. Environ. Restor. Technol. 2006, 9, 23–32. [Google Scholar]

| Treatments | (cm) | Density (1/m2) | Discharge (L/s) | Slope | (cm) | (cm/s) | (mm) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 1600 | 8.17 | 0.0005 | 18.48 | 11.05 | 4 |
| 2 | 5 | 1600 | 11.88 | 0.0005 | 19.53 | 15.21 | 4 |
| 3 | 5 | 1600 | 15.68 | 0.0005 | 20.65 | 18.98 | 4 |
| 4 | 5 | 1600 | 18.60 | 0.0005 | 21.14 | 22.00 | 4 |
| 5 | 5 | 1600 | 22.91 | 0.0005 | 22.25 | 25.75 | 4 |
| 6 | 10 | 1600 | 8.22 | 0.0005 | 19.29 | 10.65 | 4 |
| 7 | 10 | 1600 | 11.99 | 0.0005 | 20.41 | 14.69 | 4 |
| 8 | 10 | 1600 | 15.82 | 0.0005 | 21.59 | 18.31 | 4 |
| 9 | 10 | 1600 | 18.62 | 0.0005 | 22.11 | 21.06 | 4 |
| 10 | 10 | 1600 | 22.51 | 0.0005 | 23.24 | 24.22 | 4 |
| 11 | 15 | 1600 | 8.38 | 0.0005 | 20.23 | 10.36 | 4 |
| 12 | 15 | 1600 | 11.77 | 0.0005 | 21.47 | 13.70 | 4 |
| 13 | 15 | 1600 | 15.98 | 0.0005 | 22.57 | 17.70 | 4 |
| 14 | 15 | 1600 | 18.73 | 0.0005 | 23.07 | 20.30 | 4 |
| 15 | 15 | 1600 | 22.82 | 0.0005 | 24.26 | 23.51 | 4 |
| 16 | 5 | 1600 | 8.30 | 0.0005 | 19.04 | 10.89 | 6 |
| 17 | 5 | 1600 | 11.77 | 0.0005 | 20.12 | 14.63 | 6 |
| 18 | 5 | 1600 | 15.63 | 0.0005 | 21.19 | 18.43 | 6 |
| 19 | 5 | 1600 | 18.74 | 0.0005 | 21.71 | 21.58 | 6 |
| 20 | 5 | 1600 | 22.66 | 0.0005 | 22.79 | 24.85 | 6 |
| 21 | 10 | 1600 | 8.48 | 0.0005 | 19.93 | 10.64 | 6 |
| 22 | 10 | 1600 | 11.96 | 0.0005 | 21.04 | 14.21 | 6 |
| 23 | 10 | 1600 | 15.39 | 0.0005 | 22.18 | 17.35 | 6 |
| 24 | 10 | 1600 | 18.80 | 0.0005 | 22.75 | 20.66 | 6 |
| 25 | 10 | 1600 | 22.33 | 0.0005 | 23.87 | 23.39 | 6 |
| 26 | 15 | 1600 | 8.39 | 0.0005 | 20.77 | 10.09 | 6 |
| 27 | 15 | 1600 | 11.61 | 0.0005 | 22.01 | 13.18 | 6 |
| 28 | 15 | 1600 | 15.44 | 0.0005 | 23.07 | 16.73 | 6 |
| 29 | 15 | 1600 | 19.00 | 0.0005 | 23.73 | 20.02 | 6 |
| 30 | 15 | 1600 | 22.24 | 0.0005 | 24.86 | 22.36 | 6 |
| 31 | 5 | 1600 | 8.38 | 0.0005 | 19.95 | 10.50 | 8 |
| 32 | 5 | 1600 | 11.70 | 0.0005 | 21.08 | 13.88 | 8 |
| 33 | 5 | 1600 | 15.48 | 0.0005 | 22.21 | 17.43 | 8 |
| 34 | 5 | 1600 | 18.57 | 0.0005 | 22.73 | 20.42 | 8 |
| 35 | 5 | 1600 | 22.52 | 0.0005 | 23.91 | 23.55 | 8 |
| 36 | 10 | 1600 | 8.49 | 0.0005 | 20.79 | 10.22 | 8 |
| 37 | 10 | 1600 | 11.87 | 0.0005 | 21.97 | 13.51 | 8 |
| 38 | 10 | 1600 | 15.58 | 0.0005 | 23.10 | 16.87 | 8 |
| 39 | 10 | 1600 | 18.68 | 0.0005 | 23.81 | 19.62 | 8 |
| 40 | 10 | 1600 | 22.18 | 0.0005 | 24.92 | 22.25 | 8 |
| 41 | 15 | 1600 | 8.30 | 0.0005 | 21.67 | 9.58 | 8 |
| 42 | 15 | 1600 | 11.57 | 0.0005 | 22.84 | 12.66 | 8 |
| 43 | 15 | 1600 | 15.74 | 0.0005 | 24.17 | 16.29 | 8 |
| 44 | 15 | 1600 | 18.60 | 0.0005 | 24.63 | 18.87 | 8 |
| 45 | 15 | 1600 | 22.72 | 0.0005 | 25.92 | 21.92 | 8 |
| Complexity | MAE | Solution |
|---|---|---|
| 1 | 0.00781 | |
| 4 | 0.00338 | |
| 6 | 0.00218 | |
| 8 | 0.00200 | |
| 10 | 0.00180 | |
| 12 | 0.001795 | |
| 15 | 0.00177 | |
| 17 | 0.00173 | |
| 19 | 0.00173 | |
| 25 | 0.00171 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Bai, Y.; Cao, X.; Li, E. The Deformation and Shear Vortex Width of Flexible Vegetation Roots in an Artificial Floating Bed Channel. Sustainability 2022, 14, 11661. https://doi.org/10.3390/su141811661
Qi Y, Bai Y, Cao X, Li E. The Deformation and Shear Vortex Width of Flexible Vegetation Roots in an Artificial Floating Bed Channel. Sustainability. 2022; 14(18):11661. https://doi.org/10.3390/su141811661
Chicago/Turabian StyleQi, Yiting, Yu Bai, Xin Cao, and Erpeng Li. 2022. "The Deformation and Shear Vortex Width of Flexible Vegetation Roots in an Artificial Floating Bed Channel" Sustainability 14, no. 18: 11661. https://doi.org/10.3390/su141811661




