Predicting the Potential Distribution Area of the Platanus orientalis L. in Turkey Today and in the Future
Abstract
:1. Introduction
2. Materials
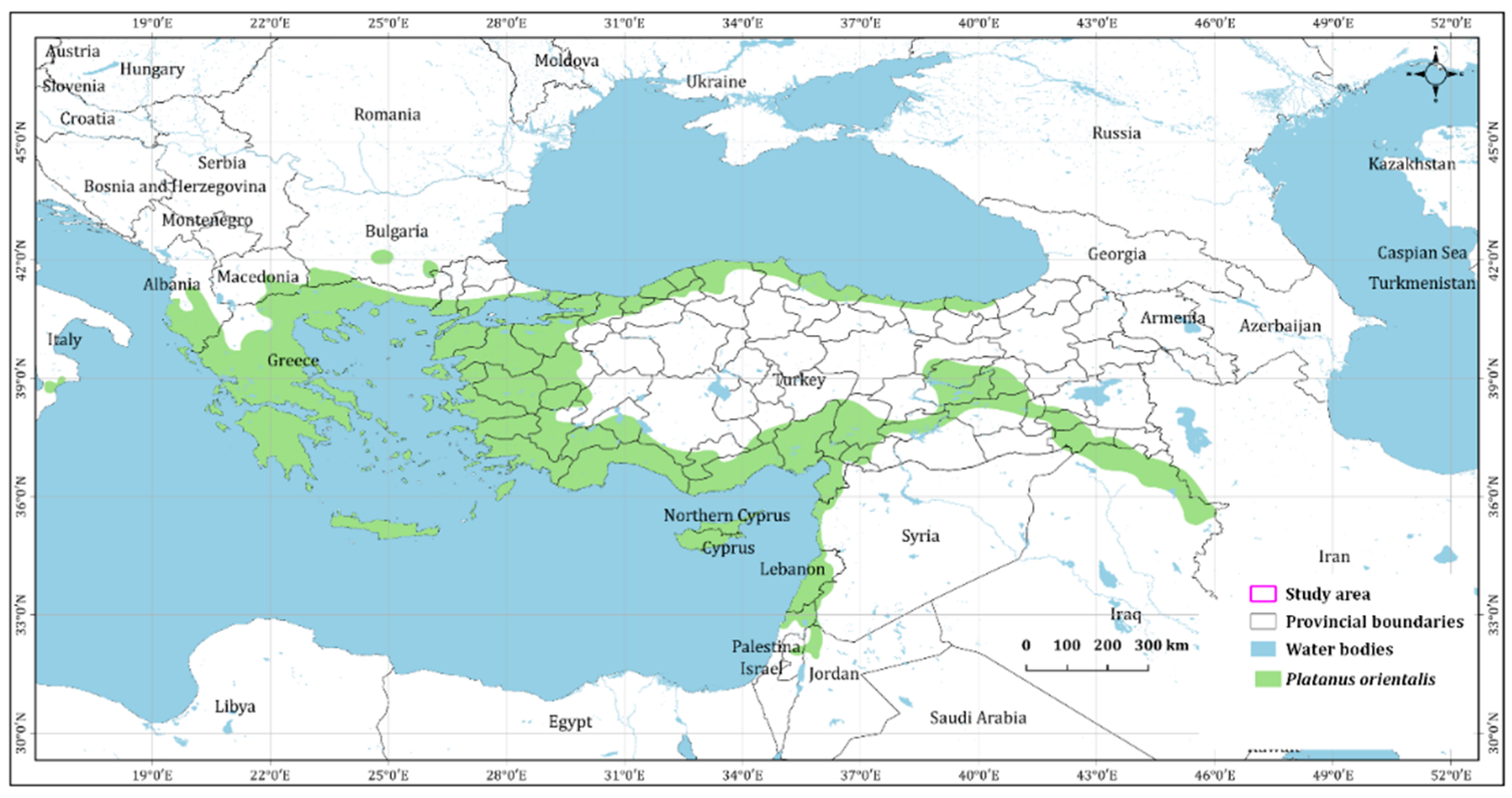
2.1. Platanus orientalis L. and Occurrence Data
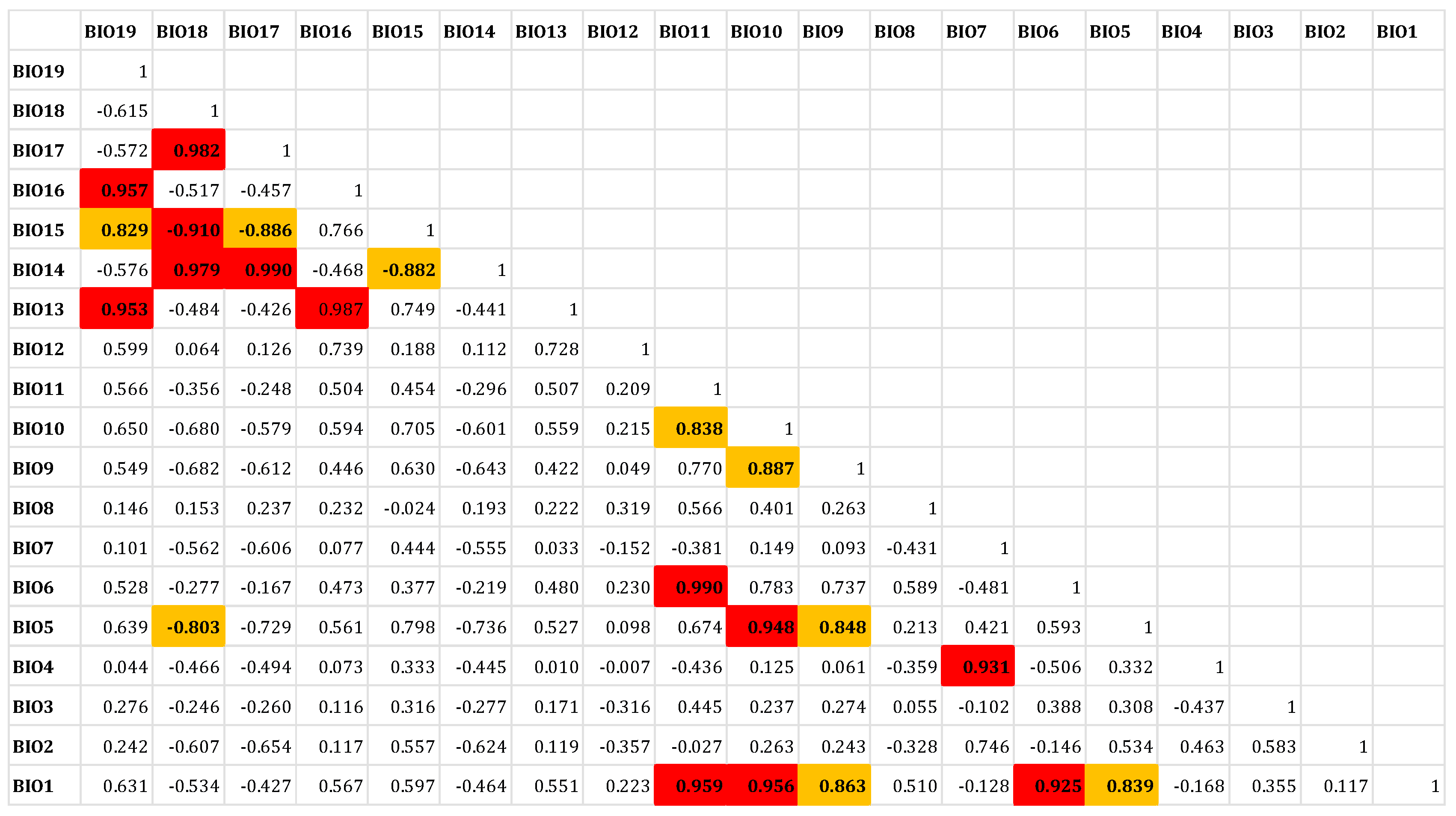
2.2. Environmental Variables
3. Method
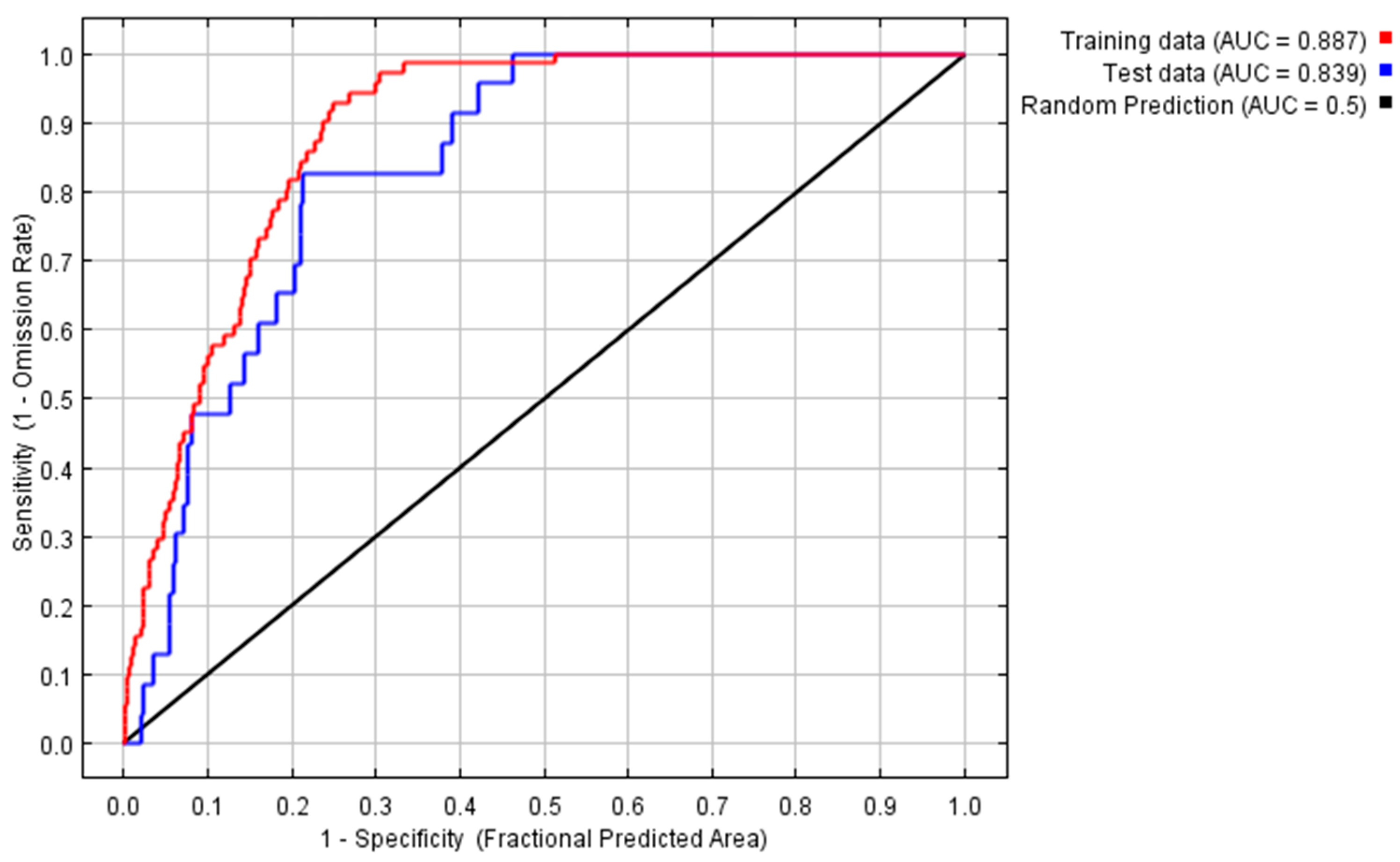
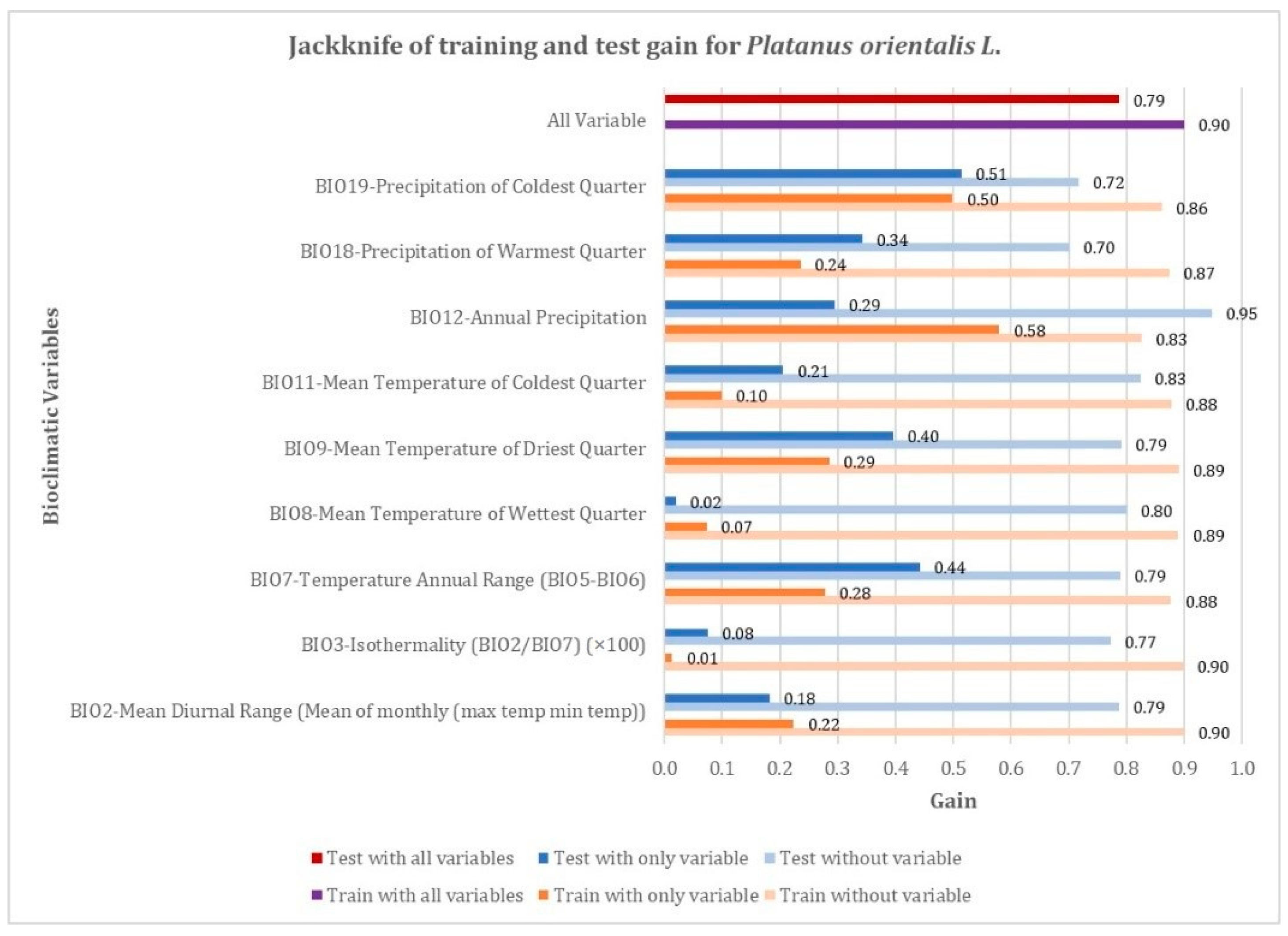
Statistical Analysis and Modelling Method
4. Results
4.1. Prediction of Platanus Orientalis Recent and Future Spatial Distribution
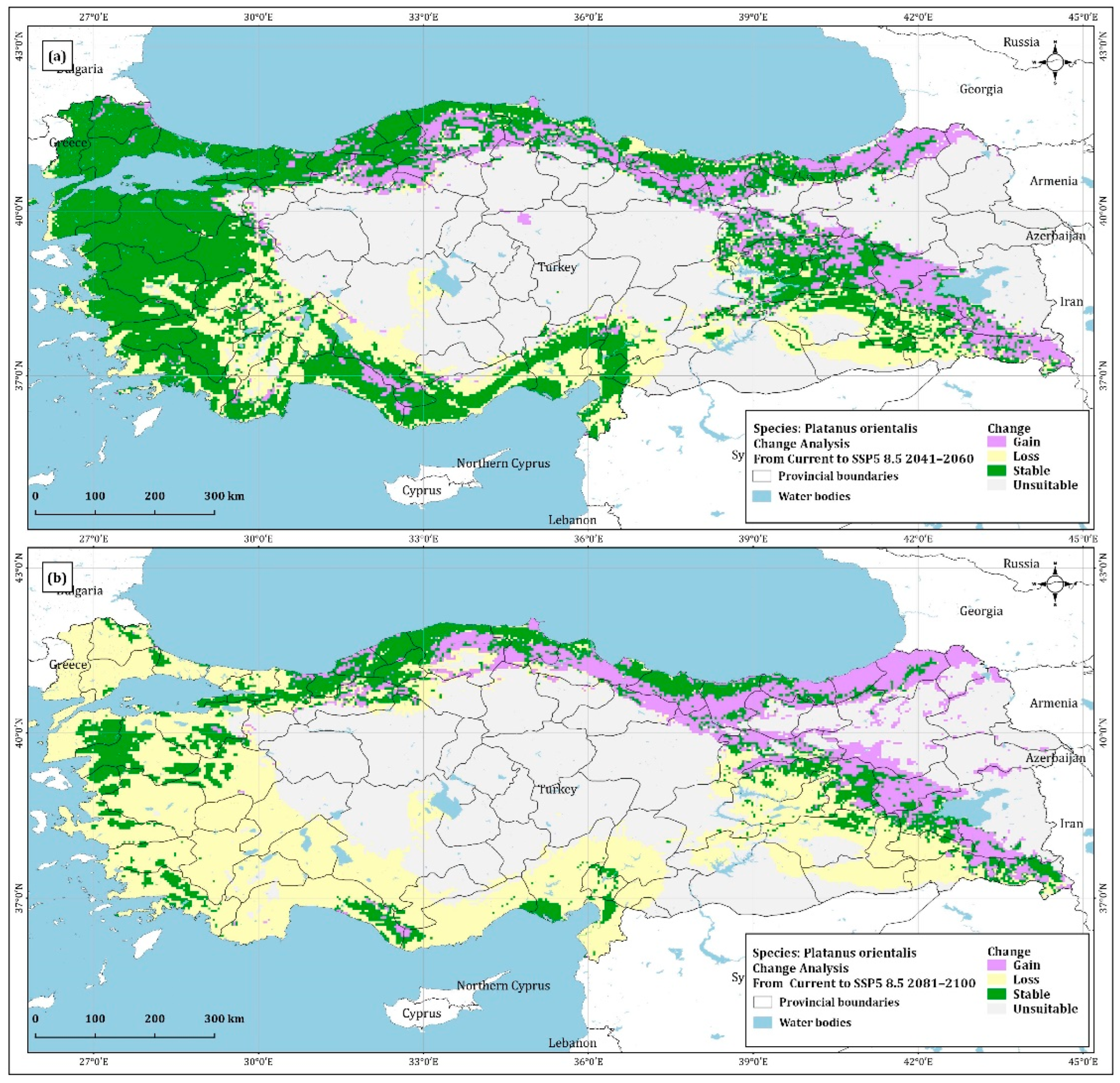
4.2. Change Analysis
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Secretariat, I.; Gullino, M.; Albajes, R.; Al-Jboory, I.; Angelotti, F.; Chakraborty, S.; Garrett, K.; Hurley, B.; Juroszek, P.; Makkouk, K. Scientific Review of the Impact of Climate Change on Plant Pests; FAO on behalf of the IPPC Secretariat: Rome, Italy, 2021; ISBN 92-5-134435-3. [Google Scholar]
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Riahi, K. IPCC, 2007: Climate Change 2007: Synthesis Report. 2008. Available online: https://pure.iiasa.ac.at/id/eprint/8667/ (accessed on 1 May 2022).
- Kumar, D.; Rawat, S.; Joshi, R. Predicting the current and future suitable habitat distribution of the medicinal tree Oroxylum Indicum (L.) Kurz in India. J. Appl. Res. Med. Aromat. Plants 2021, 23, 100309. [Google Scholar] [CrossRef]
- Gitay, H.; Suárez, A.; Watson, R.T.; Dokken, D.J. Climate Change and Biodiversity. 2002. Available online: https://www.osti.gov/etdeweb/biblio/20475293 (accessed on 1 May 2022).
- Peñuelas, J.; Sardans, J.; Estiarte, M.; Ogaya, R.; Carnicer, J.; Coll, M.; Barbeta, A.; Rivas-Ubach, A.; Llusià, J.; Garbulsky, M.; et al. Evidence of current impact of climate change on life: A walk from genes to the biosphere. Glob. Chang. Biol. 2013, 19, 2303–2338. [Google Scholar] [CrossRef] [PubMed]
- Theurillat, J.-P.; Guisan, A. Potential impact of climate change on vegetation in the European alps: A review. Clim. Chang. 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Popp, A.; Dietrich, J.P.; Lotze-Campen, H.; Klein, D.; Bauer, N.; Krause, M.; Beringer, T.; Gerten, D.; Edenhofer, O. The economic potential of bioenergy for climate change mitigation with special attention given to implications for the land system. Environ. Res. Lett. 2011, 6, 034017. [Google Scholar] [CrossRef]
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the united states. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef]
- Shuckburgh, E.; Mitchell, D.; Stott, P. Hurricanes harvey, irma and maria: How natural were these ‘natural disasters’? Weather 2017, 72, 353–354. [Google Scholar] [CrossRef]
- Shirk, A.J.; Cushman, S.A.; Waring, K.M.; Wehenkel, C.A.; Leal-Sáenz, A.; Toney, C.; Lopez-Sanchez, C.A. Southwestern white pine (Pinus strobiformis) species distribution models project a large range shift and contraction due to regional climatic changes. For. Ecol. Manag. 2018, 411, 176–186. [Google Scholar] [CrossRef]
- Peterson, A.T.; Sánchez-Cordero, V.; Soberon, J.; Bartley, J.; Buddemeier, R.W.; Navarro-Sigüenza, A.G. Effects of global climate change on geographic distributions of Mexican Cracidae. Ecol. Model. 2001, 144, 21–30. [Google Scholar] [CrossRef]
- de Siqueira, M.F.; Peterson, A.T. Consequences of global climate change for geographic distributions of Cerrado tree species. Biota Neotrop. 2003, 3, 1–14. [Google Scholar] [CrossRef]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef] [PubMed]
- Stoms, D.M.; Davis, F.W.; Cogan, C.B. Sensitivity of wildlife habitat models to uncertainties in GIS data. Photogramm. Eng. Remote Sens. 1992, 58, 843–850. [Google Scholar]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Lessons Conserv. 2010, 3, 54–89. [Google Scholar]
- El Alqamy, H.; Ismael, A.; Abdelhameed, A.; Nagy, A.; Hamada, A.; Rashad, S.; Kamel, M. Predicting the status and distribution of the Nubian ibex (Capra nubiana) in the high-altitude mountains of South Sinai (Egypt). Galemys 2010, 22, 517–530. [Google Scholar]
- Akhter, S.; McDonald, M.A.; van Breugel, P.; Sohel, S.; Kjaer, E.D.; Mariott, R. Habitat distribution modelling to identify areas of high conservation value under climate change for Mangifera sylvatica Roxb of bangladesh. Land Use Policy 2017, 60, 223–232. [Google Scholar] [CrossRef]
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. The impact of climate change on the distribution of two threatened dipterocarp trees. Ecol. Evol. 2017, 7, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.A.; Bugalho, M.N.; Franco, A.M.A.; Palmeirim, J.M. Contribution of spatially explicit models to climate change adaptation and mitigation plans for a priority forest habitat. Mitig. Adapt. Strateg. Glob. Chang. 2017, 23, 371–386. [Google Scholar] [CrossRef]
- Örücü, Ö.K. Phoenix theophrasti gr.’nin iklim değişimine bağli günümüz ve gelecekteki yayiliş alanlarinin maxent modeli ile tahmini ve bitkisel tasarimda kullanimi. Türkiye Orman. Derg. 2019, 20, 274–283. [Google Scholar] [CrossRef]
- Sarikaya, A.G.; Orucu, O.K. Prediction of potential and future distribution areas of Anatolian Chesnut (Castanea Sativa Mill.) by using maximum entropy (maxent) modeling depending on climate change in Turkey. Int J. Ecosyst. Ecol. Sci. 2019, 9, 699–708. [Google Scholar] [CrossRef]
- Holt, R.D. The microevolutionary consequences of climate change. Trends Ecol. Evol. 1990, 5, 311–315. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. Riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten european forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Silva, C.; Leiva, F.; Lastra, J. Predicting the current and future suitable habitat distributions of the anchovy (Engraulis Ringens) using the maxent model in the coastal areas off Central-Northern Chile. Fish. Oceanogr. 2019, 28, 171–182. [Google Scholar] [CrossRef]
- Richards, C.L.; Carstens, B.C.; Lacey Knowles, L. Distribution modelling and statistical phylogeography: An integrative framework for generating and testing alternative biogeographical hypotheses. J. Biogeogr. 2007, 34, 1833–1845. [Google Scholar] [CrossRef] [Green Version]
- Gür, H. Geç kuvaterner buzul buzullararasi döngülerinin anadolu’nun biyolojik çeşitliliği üzerine etkileri. Türkiye Jeol. Bülteni/Geol. Bull. Turk. 2017, 60, 507–528. [Google Scholar] [CrossRef]
- Choudhary, J.S.; Kumari, M.; Mali, S.S.; Dhakar, M.K.; Das, B.; Singh, A.K.; Bhatt, B.P. Predicting impact of climate change on habitat suitability of guava fruit fly, bactrocera correcta (Bezzi) using maxent modeling in India. J. Agrometeorol. 2019, 21, 24–30. [Google Scholar] [CrossRef]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 3061. [Google Scholar] [CrossRef] [PubMed]
- Jaynes, E.T. Probability Theory: The Logic of Science; Cambridge University Press: Cambridge, UK, 2003; ISBN 0-521-59271-2. [Google Scholar]
- Dudik, M.; Phillips, S.J.; Schapire, R.E. Performance Guarantees for Regularized Maximum Entropy Density Estimation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 472–486. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of maxent for ecologists. Divers Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Rangel, T.F.; Loyola, R.D. Labeling ecological niche models. Nat. Conserv. 2012, 10, 119–126. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H. Exploring the advantages of the maximum entropy model in calibrating cellular automata for urban growth simulation: A comparative study of four methods. GISci. Remote Sens. 2022, 59, 71–95. [Google Scholar] [CrossRef]
- Royle, J.A.; Chandler, R.B.; Yackulic, C.; Nichols, J.D. Likelihood analysis of species occurrence probability from presence-only data for modelling species distributions. Methods Ecol. Evol. 2012, 3, 545–554. [Google Scholar] [CrossRef]
- Fitzpatrick, M.C.; Gotelli, N.J.; Ellison, A.M. MaxEnt versus MaxLike: Empirical comparisons with ant species distributions. Ecosphere 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M. The importance of correcting for sampling bias in maxent species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-only modelling using MAXENT: When can we trust the inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernandez, I.C.; Baca-Gonzalez, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. Peerj 2017, 5, e3093. [Google Scholar] [CrossRef]
- Fois, M.; Bacchetta, G.; Cogoni, D.; Fenu, G. Current and future effectiveness of the natura 2000 network for protecting plant species in Sardinia: A nice and complex strategy in its raw state? J. Environ. Plann. Man. 2018, 61, 332–347. [Google Scholar] [CrossRef]
- Brotons, L.; Thuiller, W.; Araújo, M.B.; Hirzel, A.H. Presence-absence versus presence-only modelling methods for predicting bird habitat suitability. Ecography 2004, 27, 437–448. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. NCEAS predicting species distribut effects of sample size on the performance of species distribution models. Divers Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Tutin, T.; Burges, N.; Chater, A.; Edmondson, J.; Heywood, V.; Moore, D. Cruciferae; Flora Europea Ed. 2; Cambridge University Press: Cambridge, UK, 1993; Volume 1, pp. 313–416. [Google Scholar]
- Diamandis, S. Platanus orientalis a Divine Gift for Greece. 2004, pp. 52–53. Available online: http://www.dendrology.org/site/images/web4events/pdf/Tree%20info%20IDS_04_pp52_53_Platanus.pdf (accessed on 29 May 2014).
- Bıçakçı, A.; Saatçioğlu, G.; Tosunoğlu, A. Allerjenik betula (huş ağaci) polenlerinin türkiye’deki dağilimlari. Asthma Allergy Immunol. 2017, 15, 142–153. [Google Scholar] [CrossRef]
- Akgün, B.; Okumuş, A.; Yazar, E. Gaziantep Yöresinde bulunan ve anit ağaç niteliği taşiyan doğu çinari (Platanus orientalis L.)’nin fiziksel özelliklerinin belirlenmesi. Kahramanmaraş Sütçü İmam Üniversitesi Mühendislik Bilimleri Derg. 2018, 21, 305–311. [Google Scholar] [CrossRef]
- Seker, D.Z.; Sivri, N.; Demir, M.; Baytimur, A.; Gunduz, E.; Eroglu, M.F. GIS based inventory and documentation of monumental trees in black sea region of Turkey. FEB Fresenius Environ. Bull. 2019, 28, 524. [Google Scholar]
- OGM Doğu Çınarı. Available online: https://www.ogm.gov.tr/tr/yararli-bilgiler/haftanin-agaci/do%C4%9Fu-%C3%A7%C4%B1nar%C4%B1 (accessed on 10 June 2022).
- Güler, Z.; Dursun, A.; Özkan, D. Volatile compounds in the leaf of plane tree (Platanus orientalis) with solid phase microextraction (SPME) technique. Int. J. Second. Metab. 2017, 4, 167–176. [Google Scholar] [CrossRef]
- Öztürk, M.; Uysal, I.; Yücel, E.; Altay, V.; Karabacak, E. Soil-Plant Interactions in the Monumental Plane Trees (Platanus orientalis) Grove-çanakkale-Turkey. 2017. Available online: https://hdl.handle.net/11421/16340 (accessed on 1 May 2022).
- Bazos, I.; Matevski, V.; Montagnani, C.; Petrova, A.; Shuka, A.; Troia, A. Platanus orientalis. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/33951/103192536 (accessed on 1 July 2022).
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.nj49bd (accessed on 9 August 2022).
- Davis, P.H. Flora of Turkey and The East Aegean Islands—I; David, P.H., Ed.; Edinburgh University Press: Edinburgh, UK, 1965; Volume 1. [Google Scholar]
- BIYOD. Biyolojik Çeşitlilik ve Odun DIşı Orman Ürünleri Veri Tabanı; Tarım ve Orman Bakanlığı Orman Genel Müdürlüğü: Ankara, Turkey, 2020. [Google Scholar]
- WorldClim. Global Climate and Weather Data—WorldClim. Available online: https://worldclim.org/data/index.html (accessed on 1 December 2020).
- Zhang, J.; Li, S. A review of machine learning based species’ distribution modelling. In Proceedings of the 2017 International Conference on Industrial Informatics—Computing Technology, Intelligent Technology, Industrial Information Integration (ICIICII), Wuhan, China, 2–3 December 2017; pp. 199–206. [Google Scholar]
- Sillero, N. What does ecological modelling model? A proposed classification of ecological niche models based on their underlying methods. Ecol. Model. 2011, 222, 1343–1346. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Cao, B.; Bai, C.; Zhang, L.; Li, G.; Mao, M. Modeling habitat distribution of Cornus officinal is with maxent modeling and fuzzy logics in China. J. Plant Ecol. 2016, 9, 742–751. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC plots: Calibrating species distribution models with presence-only data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Gassó, N.; Thuiller, W.; Pino, J.; Vilà, M. Potential distribution range of invasive plant species in Spain. NeoBiota 2012, 12, 25–40. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398, ISBN 0-470-58247-2. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Çoban, H.O.; Örücü, Ö.K.; Arslan, E.S. MaxEnt Modeling for predicting the current and future potential geographical distribution of Quercus libani Olivier. Sustainability 2020, 12, 2671. [Google Scholar] [CrossRef]
- Crawshaw, L.; Buchanan, T.; Shirose, L.; Palahnuk, A.; Cai, H.Y.; Bennett, A.M.; Jardine, C.M.; Davy, C.M. Widespread occurrence of batrachochytrium dendrobatidis in ontario, canada, and predicted habitat suitability for the emerging batrachochytrium salamandrivorans. Ecol. Evol. 2022, 12, e8798. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wu, W.; Ji, L.; Tian, S.; Yang, B.; Guan, B.; Wu, D. MaxEnt model-based prediction of potential distributions of parnassiawightiana (celastraceae) in China. Biodivers. Data J. 2022, 10, e81073. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.S.; Örücü, Ö.K. Present and future potential distribution of the Pinus nigra Arnold. and Pinus sylvestris l. using maxent model. Int. J. Ecosyst. Ecol. Sci. 2019, 9, 787–798. [Google Scholar]
- Khanum, R.; Mumtaz, A.S.; Kumar, S. Predicting impacts of climate change on medicinal asclepiads of pakistan using maxent modeling. Acta Oecol. 2013, 49, 23–31. [Google Scholar] [CrossRef]
- Akyol, A.; Örücü, Ö.K. İklim değişimi senaryolari ve tür dağilim modeline göre kizilcik türünün (cornus mas l.) odun dişi orman ürünleri kapsaminda değerlendirilmesi. Eur. J. Sci. Technol. 2019, 17, 224–233. [Google Scholar] [CrossRef]
- Sarikaya, A.G.; Orucu, O.K. Maxent modeling for predicting the potential distribution of Arbutus andrachne L. in Turkey. Kuwait J. Sci. 2021, 48, 1–15. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H. Conserving biodiversity in the face of climate change. Agenda—J. Policy Anal. Reform 2007, 14, 157–170. [Google Scholar] [CrossRef]
- Pimm, S.L. Biodiversity: Climate change or habitat loss—Which will kill more species? Curr. Biol. 2008, 18, R117–R119. [Google Scholar] [CrossRef] [PubMed]
- Demir, A. Küresel iklim değişikliğinin biyolojik çeşitlilik ve ekosistem kaynaklari üzerine etkisi. Ank. Üniversitesi Çevrebilimleri Derg. 2009, 1, 37–54. [Google Scholar] [CrossRef]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of european higher plants for 2050. Glob. Chang. Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Yalçın, S. Modeling the Current and Future Ranges of Turkish pine (Pinus brutia) and Oriental beech (Fagus orientalis) in Turkey in the Face of Climate Change. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2012. [Google Scholar]
- López-Tirado, J.; Hidalgo, P.J. Ecological niche modelling of three Mediterranean pine species in the south of Spain: A tool for afforestation/reforestation programs in the twenty-first century. New For. 2016, 47, 411–429. [Google Scholar] [CrossRef]
- Akyol, A.; Örücü, Ö.K.; Arslan, E.S. Habitat suitability mapping of stone pine (Pinus pinea L.) under the effects of climate change. Biologia 2020, 75, 2175–2187. [Google Scholar] [CrossRef]
- Grueva, M.; Zhelev, P. Population genetic structure of Platanus orientalis L. in Bulgaria. Iforest—Biogeosciences For. 2011, 4, 186–189. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Tattini, M.; Arena, C.; Krumova, S.; Koleva, D.; Peeva, V.; Stojchev, S.; Todinova, S.; Izzo, L.G.; et al. Physiological and structural adjustments of two ecotypes of Platanus orientalis L. from different habitats in response to drought and re-watering. Conserv Physiol 2018, 6, coy073. [Google Scholar] [CrossRef] [PubMed]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The effects of climate change scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
- Uzun, A.; Örücü, Ö.K. Adenocarpus complicatus (L.) Gay türünün iklim değişkenlerine bağli günümüz ve gelecekteki yayiliş alanlarinin tahmini. Turk. J. For. 2020, 21, 498–508. [Google Scholar] [CrossRef]
- Wei, B.; Wang, R.L.; Hou, K.; Wang, X.Y.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using maxent model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Dalfes, H.; Karaca, M.; Sen, O. Climate Change Scenarios for Turkey in Climate Change & Turkey: Impact, Sectoral Analyses, Socio-Economic Dimensions; United Nations Development Programme (UNDP) Turkey Office: Ankara, Turke, 2007. [Google Scholar]


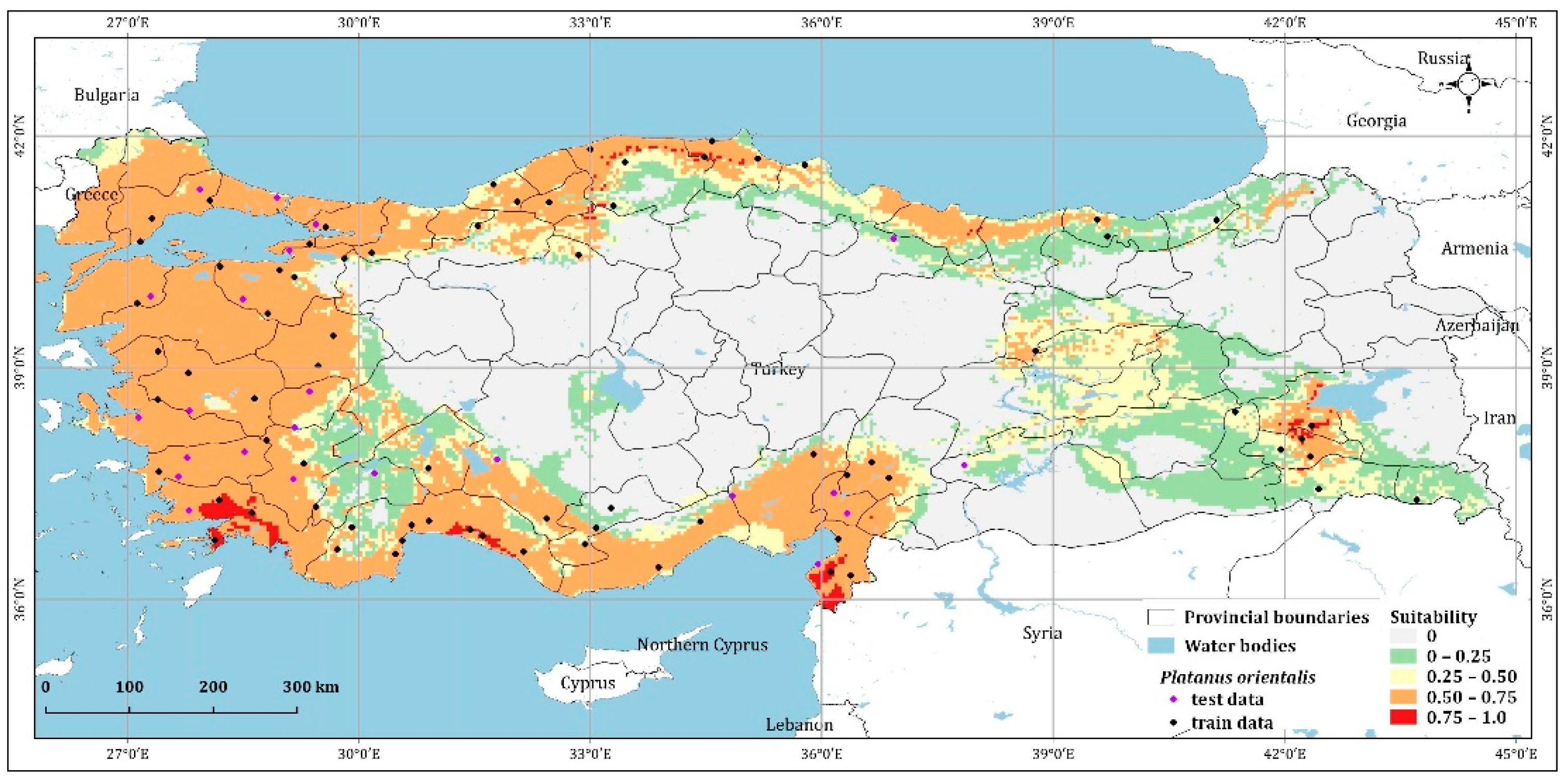
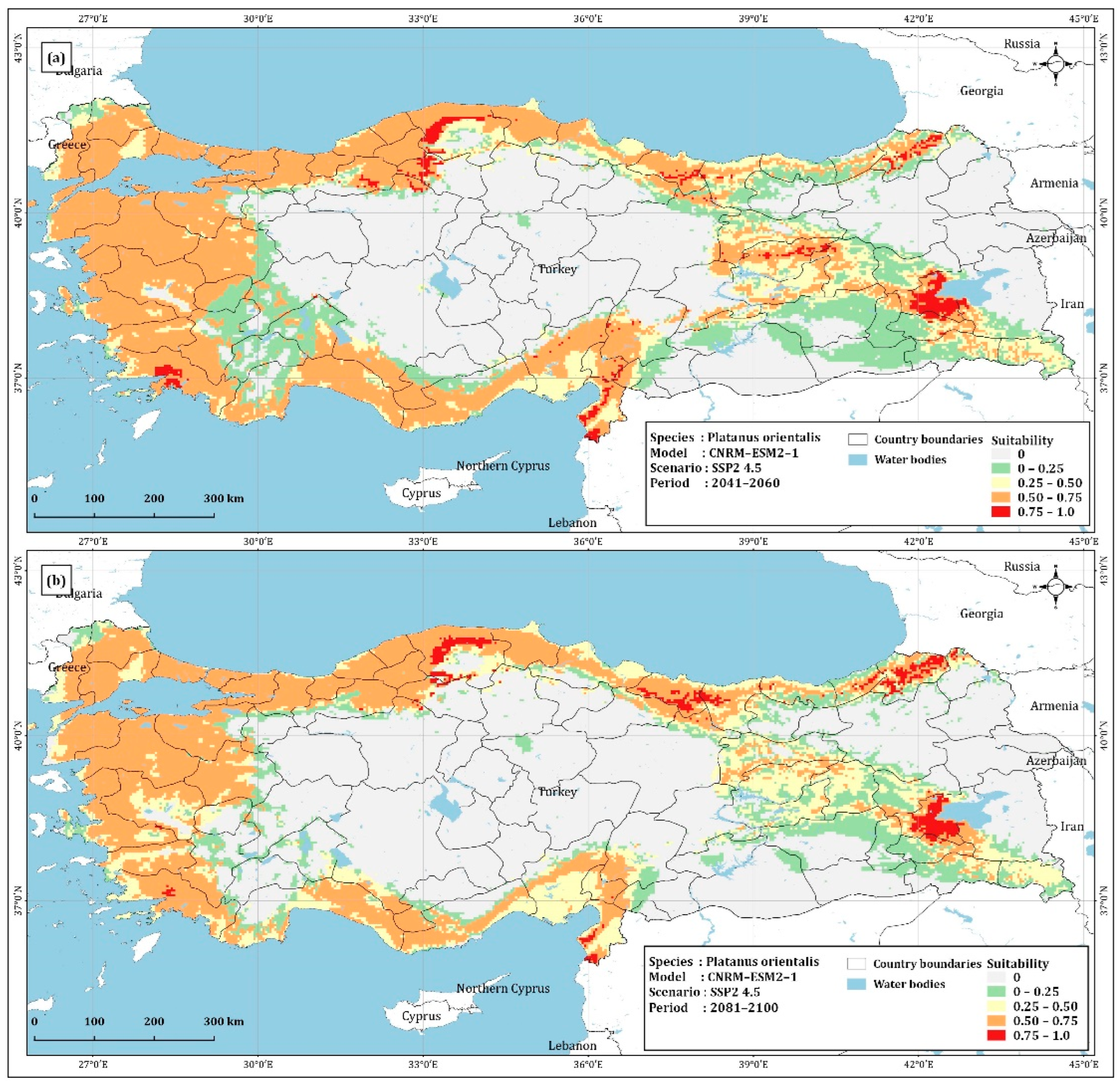
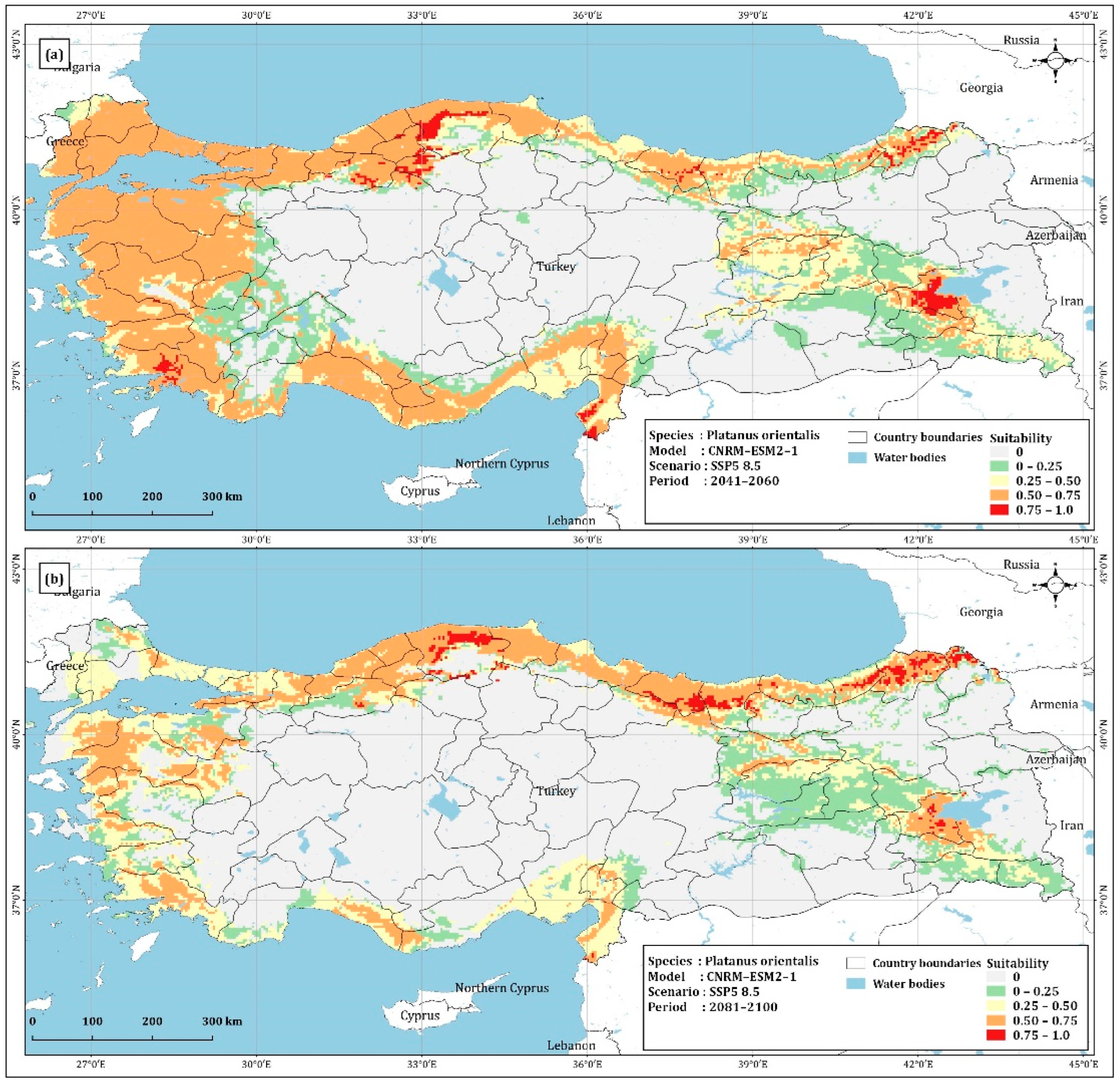
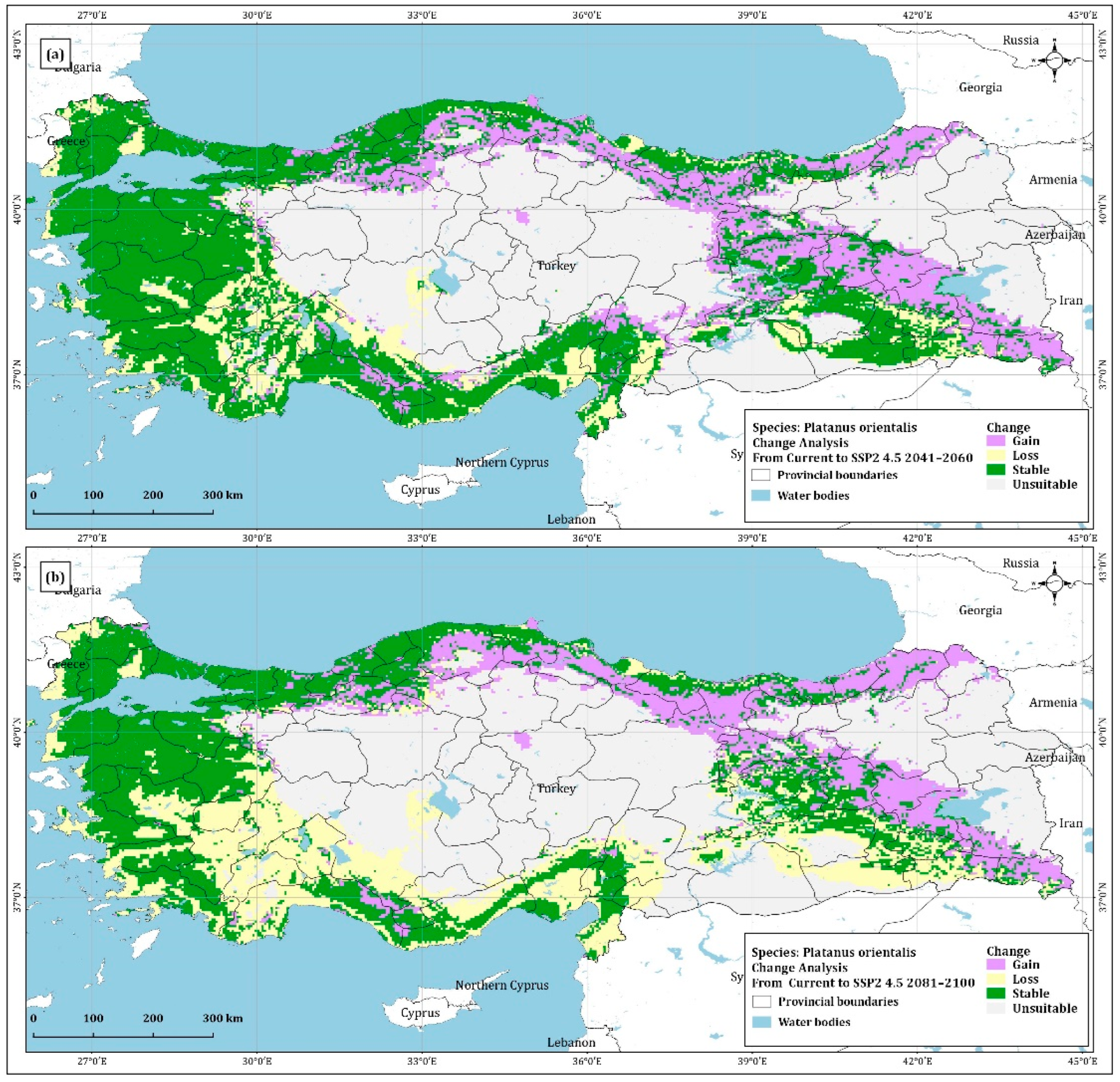
| Codes | Descriptions |
|---|---|
| BIO1 | Annual Mean Temperature |
| BIO2 | Mean Diurnal Range (Mean of monthly (max temp min temp)) |
| BIO3 | Isothermality (BIO2/BIO7) (×100) |
| BIO4 | Temperature Seasonality (standard deviation ×100) |
| BIO5 | Max Temperature of Warmest Month |
| BIO6 | Min Temperature of Coldest Month |
| BIO7 | Temperature Annual Range (BIO5–BIO6) |
| BIO8 | Mean Temperature of Wettest Quarter |
| BIO9 | Mean Temperature of Driest Quarter |
| BIO10 | Mean Temperature of Warmest Quarter |
| BIO11 | Mean Temperature of Coldest Quarter |
| BIO12 | Annual Precipitation |
| BIO13 | Precipitation of Wettest Month |
| BIO14 | Precipitation of Driest Month |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) |
| BIO16 | Precipitation of Wettest Quarter |
| BIO17 | Precipitation of Driest Quarter |
| BIO18 | Precipitation of Warmest Quarter |
| BIO19 | Precipitation of Coldest Quarter |
| Platanus orientalis L. | SSP2 4.5 | SSP5 8.5 | |||
|---|---|---|---|---|---|
| Suitability | Current | 2041–2060 | 2081–2100 | 2041–2060 | 2081–2100 |
| Unsuitable | 354,002.60 | 344,905.54 | 395,213.60 | 385,265.82 | 481,208.68 |
| 0–0.25 | 108,146.17 | 107,217.77 | 80,215.39 | 87,428.39 | 81,430.03 |
| 0.25–0.50 | 88,940.72 | 84,551.40 | 106,679.36 | 93,944.35 | 117,764.51 |
| 0.50–0.75 | 222,810.45 | 230,500.35 | 186,405.97 | 203,567.35 | 91,237.57 |
| 0.75–1 | 6560.52 | 13,282.58 | 11,944.65 | 10,252.75 | 8817.95 |
| Platanus orientalis L. | SSP2 4.5 | SSP5 8.5 | ||
|---|---|---|---|---|
| Change Type. | 2041–2060 | 2081–2100 | 2041–2060 | 2081–2100 |
| Gain | 109,876.19 | 96,034.28 | 77,817.92 | 77,756.11 |
| Loss | 79,596.06 | 157,362.45 | 122,315.83 | 282,462.32 |
| Stable | 265,876.89 | 198,902.42 | 242,953.87 | 91,154.09 |
| Chart | 325,108.37 | 328,158.53 | 337,370.55 | 329,084.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamer Aksoy, Ö. Predicting the Potential Distribution Area of the Platanus orientalis L. in Turkey Today and in the Future. Sustainability 2022, 14, 11706. https://doi.org/10.3390/su141811706
Kamer Aksoy Ö. Predicting the Potential Distribution Area of the Platanus orientalis L. in Turkey Today and in the Future. Sustainability. 2022; 14(18):11706. https://doi.org/10.3390/su141811706
Chicago/Turabian StyleKamer Aksoy, Özgür. 2022. "Predicting the Potential Distribution Area of the Platanus orientalis L. in Turkey Today and in the Future" Sustainability 14, no. 18: 11706. https://doi.org/10.3390/su141811706





