Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining the Tomatoes Processing Byproducts
2.3. Phytochemical Profile of Tomatoes and Tomato Processing Byproducts
2.3.1. Preparation of the Alcoholic Extracts
2.3.2. Assessment of Total Phenolic Content (TPC)
2.3.3. Chromatographic Determination of Non-Anthocyanin Polyphenols by LC-MS
2.3.4. Spectrophotometric Determination of Lycopene
2.4. Manufacture of Sausages Formulas
2.5. Proximate Composition of Sausages
2.6. Oxidative Stability Assessment
2.6.1. Determination of Peroxide Value (PV)
2.6.2. Determination of p-Anisidine Value (p-AV)
2.6.3. Total Oxidation Value (TOTOX)
2.6.4. Thiobarbituric Acid (TBA) Test
2.7. Statistical Data Analysis
3. Results and Discussion
3.1. Phytochemical Profile of Tomatoes and Tomatoes Processing Byproducts
3.1.1. Assessment of Total Phenolic Content (TPC)
3.1.2. Chromatographic Evaluation of Individual Polyphenolic Compounds by LC-MS
3.1.3. Lycopene Content
3.2. The Proximate Composition of Sausages
3.3. Oxidative Stability Assessment
3.3.1. Peroxide Value (PV)
3.3.2. p-Anisidine Value (p-AV)
3.3.3. Total Oxidation Value (TOTOX)
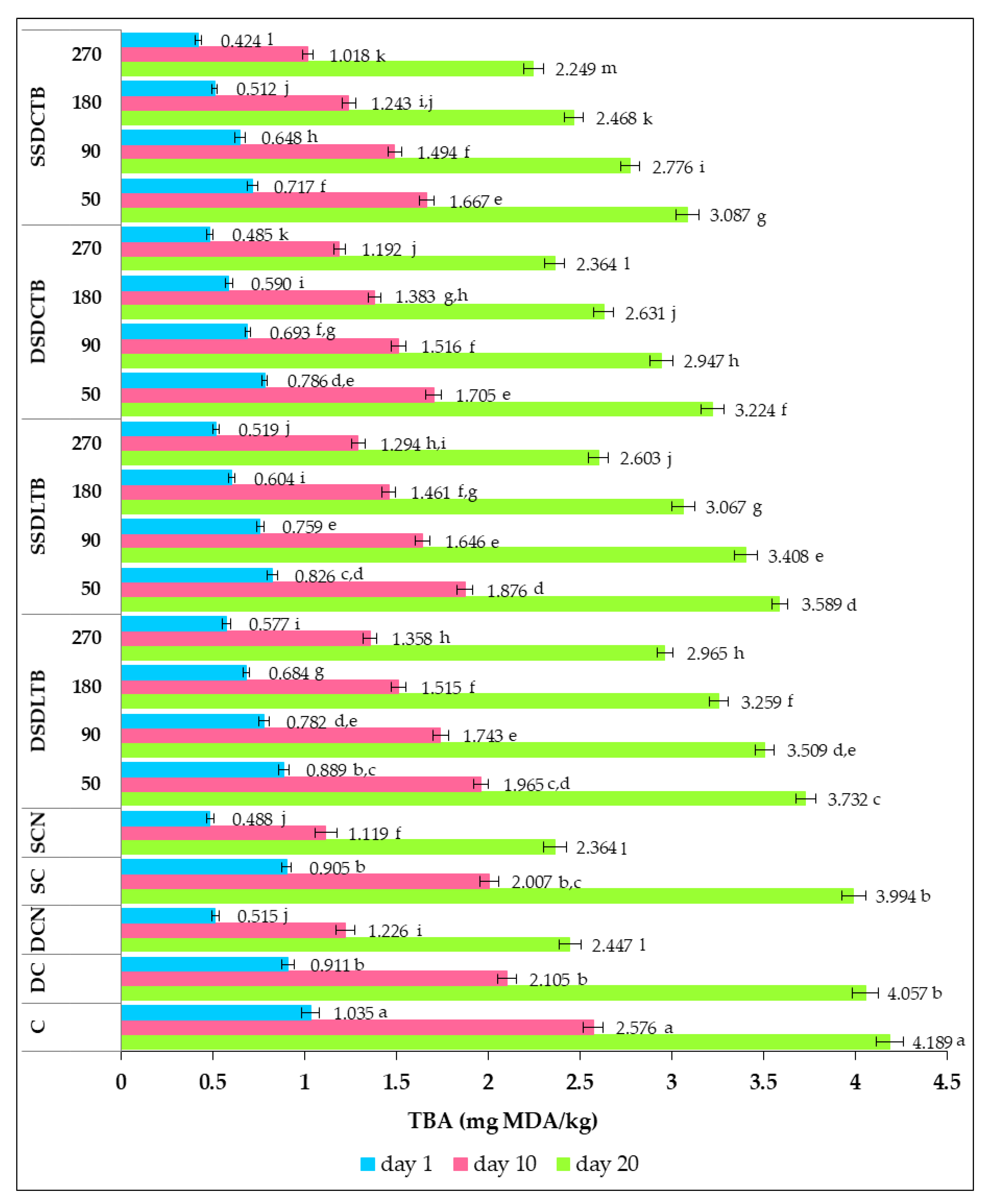
3.3.4. Thiobarbituric Acid (TBA) Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carballo, J. Sausages: Nutrition, Safety, Processing and Quality Improvement. Foods 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Safa, H.; Gatellier, P.; Lebert, A.; Picgirard, L.; Mirade, P.S. Effect of combined salt and animal fat reductions on physicochemical and biochemical changes during the manufacture of dry-fermented sausages. Food Bioprocess Technol. 2015, 8, 2109–2122. [Google Scholar] [CrossRef]
- Yim, D.G.; Seo, J.K.; Yum, H.W.; Zahid, M.A.; Park, J.Y.; Parvin, R.; Go, J.; Jin, S.K.; Koo, O.K.; Yang, H.S. Effects of Caesalpinia sappan L. extract on the color stability, antioxidant and antimicrobial activity in cooked pork sausages during cold storage. LWT—Food Sci. Technol. 2019, 112, 108235. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.A.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid stability in meat and meat products. Meat Sci. 1998, 49, S73–S86. [Google Scholar] [CrossRef]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food additives: Production of microbial pigments and their antioxidant properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of early postmortem protein oxidation on beef quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Naveena, B.M. Effect of Salt, Kinnow and Pomegranate Fruit Byproduct Powders on Color and Oxidative Stability of Raw Ground Goat Meat during Refrigerated Storage. Meat Sci. 2010, 85, 306–311. [Google Scholar] [CrossRef]
- Shan, B.Z.; Cai, Y.; Brooks, D.B.; Corke, H. Antibacterial and Antioxidant Effects of Five Spices and Herb Extracts as Natural Preservatives of Raw Pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Boeira, C.P.; Piovesan, N.; Soquetta, M.B.; Flores, D.C.B.; Lucas, B.N.; Rosa, C.S.; Terra, N.N. Extraction of bioactive compounds of lemongrass, antioxidant activity and evaluation of antimicrobial activity in fresh chicken sausage. Ciênc. Rural 2018, 48, e20180477. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrite and nitrate for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Chin, K.B. Effects of drying temperature on antioxidant activities of tomato powder and storage stability of pork patties. Korean J. Food Sci. Anim. Resour. 2016, 36, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Carotenoids as antioxidants. Nutrition 2001, 17, 815–817. [Google Scholar] [CrossRef]
- Di Mascio, P.; Murphy, M.E.; Sies, H. Antioxidant defense systems: The role of carotenoids, tocopherols and thiols. Am. J. Clin. Nutr. 1991, 53, 194S–200S. [Google Scholar] [CrossRef]
- Sahlin, E.; Savage, G.P.; Lister, C.E. Investigation of the antioxidant properties of tomatoes after processing. J. Food Compos. Anal. 2004, 17, 635–647. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, E.; Herrera-Ruiz, G.; Pedraza-Aboytes, G.; Loarca-Piña, G. Antioxidant capacity and antimutagenic activity of natural oleoresin from greenhouse grown tomatoes (Lycopersicon esculentum). Plant Foods Hum. Nutr. 2009, 64, 46–51. [Google Scholar] [CrossRef]
- Kumar, A.O.; Tata, S.S. Ascorbic acid contents in chilli peppers (Capsicum annum L.). Not. Sci. Biol. 2009, 1, 50–52. [Google Scholar] [CrossRef]
- Demirbas, A. Waste management, waste resource facilities and waste conversion processes. Energy Convers. Manag. 2011, 52, 1280–1287. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.L.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef]
- Duca, A.; Sturza, A.; Moacă, E.-A.; Negrea, M.; Lalescu, V.-D.; Lungeanu, D.; Dehelean, C.-A.; Muntean, D.-M.; Alexa, E. Identification of Resveratrol as Bioactive Compound of Propolis from Western Romania and Characterization of Phenolic Profile and Antioxidant Activity of Ethanolic Extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Le Marguer, M. Lycopene in tomatoes and tomato pulp fractions. Ital. J. Food Sci. 1996, 8, 107–113. [Google Scholar]
- SR ISO 91-2007; Meat and Meat Products—Determination of NaCl. ISO: Geneva, Switzerland, 2007.
- SR ISO 1443:2008; Meat and Meat Products—Determination of Total Fat Content. ISO: Geneva, Switzerland, 2008.
- SR ISO 937:2007; Meat and Meat Products—Determination of Nitrogen Content (Reference Method). ISO: Geneva, Switzerland, 2007.
- SR ISO 936:2009; Meat and Meat Products—Determination of Total Ash. ISO: Geneva, Switzerland, 2009.
- SR ISO 1442:2010; Meat and Meat Products—Determination of Moisture Content (Reference Method). ISO: Geneva, Switzerland, 2010.
- Association of Coaching Supervisors. Official and Recommended Practices of the American Oil Chemists’ Society, Official Methods and Recommended Practices, 5th ed.; Firestone, D., Ed.; AOAC Press: Champaign, IL, USA, 1998. [Google Scholar]
- AOCS. Official Method Cd 18–90, p-Anisidine Value, Official Methods and Recommended Practices of the AOCS; AOCS: Urbana, IL, USA, 2017. [Google Scholar]
- Cocan, I.; Negrea, M.; Cozma, A.; Alexa, E.; Poiana, M.-A.; Raba, D.; Danciu, C.; Popescu, I.; Cadariu, A.I.; Obistioiu, D.; et al. Chili and Sweet Pepper Seed Oil Used as a Natural Antioxidant to Improve the Thermo-Oxidative Stability of Sunflower Oil. Agronomy 2021, 11, 2579. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.T.; Le, V.V.M.; Sajeev, D.; Schilling, M.W.; Dinh, T.T.N. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Sci. 2020, 165, 108106. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Various Typologies of Canned Tomatoes. Front. Nutr. 2022, 9, 849163. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Chaidez, C.; Ornelas-Paz, J.J.; López-Mata, M.A.; Márquez-Ríos, E.; Estrada, M.I. Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res. 2015, 25, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codin, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Abdul-Hafeez, E.; Karamova, N.; Ilinskaya, O. Antioxidant activity and total phenolic compound content of certain medicinal plants. Int. J. Biosci. 2014, 5, 213–222. [Google Scholar]
- Perea-Domínguez, X.P.; Hernández-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato byproduct: Free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. Can. Med. Assoc. J. 2000, 163, 739–744. [Google Scholar]
- Górecka, D.; Wawrzyniak, A.; Jędrusek-Golińska, A.; Dziedzic, K.; Hamułka, J.; Kowalczewski, P.Ł.; Walkowiak, J. Lycopene in tomatoes and tomato products. Open Chem. 2020, 18, 752–756. [Google Scholar] [CrossRef]
- Østerlie, M.; Lerfall, J. Lycopene from tomato products added minced meat: Effect on storage quality and colour. Food Res. Int. 2005, 38, 925–929. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 223–230. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.W.; Cho, S.H.; Kim, Y.S.; Kim, J.H.; Ham, J.S.; Park, B.Y.; Pil-Nam, S. Effects of extraction methods of shiitake byproducts on their antioxidant and antimicrobial activities in fermented sausages during storage. Food Control 2017, 79, 109–118. [Google Scholar]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I.B.; Rodriguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of phenols extracted from a byproduct of the oil mill on the shelf-life of raw and cooked fresh pork sausages in the absence of chemical additives. LWT—Food Sci. Technol. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Sam, F.E.; Ma, T.-Z.; Atuna, R.A.; Salifu, R.; Nubalanaan, B.-A.; Amagloh, F.K.; Han, S.-Y. Physicochemical, Oxidative Stability and Sensory Properties of Frankfurter-Type Sausage as Influenced by the Addition of Carrot (Daucus carota) Paste. Foods 2021, 10, 3032. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, A.; Pacheco-Delahaye, E.; Hevia, P. Value of a tomato byproduct as a source of dietary fiber in rats. Plant Foods Hum. Nutr. 2001, 56, 335–348. [Google Scholar] [CrossRef]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Alvarez, J.A. Effect of Storage Conditions on Quality Characteristics of Bologna Sausages Made with Citrus Fiber. J. Food Sci. 2003, 68, 710–714. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, Z.; Li, G.; Zhao, X.; Wu, H.; Ren, Y. Tomato peel powder as fat replacement in low-fat sausages: Formulations with mechanically crushed powder exhibit higher stability than those with airflow ultra-micro crushed powder. Eur. J. Lipid Sci. Technol. 2016, 118, 175–184. [Google Scholar] [CrossRef]
- Ahmad, S.; Jafarzadeh, S.; Ariffin, F.; Zainul Abidin, S. Evaluation of Physicochemical, Antioxidant and Antimicrobial Properties of Chicken Sausage Incorporated with Different Vegetables. Ital. J. Food Sci. 2020, 32, 75–90. [Google Scholar]
- Ellekjær, M.R.; Hildrum, K.I.; Næs, T.; Isaksson, T. Determination of the Sodium Chloride Content of Sausages by near Infrared Spectroscopy. J. Near Infrared Spectrosc. 1993, 1, 65–75. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Vestergaard, C.; Koch, A.G. The effect of salt reduction on sensory quality and microbial growth in hotdog sausages, bacon, ham and salami. Meat Sci. 2014, 96, 47–55. [Google Scholar] [CrossRef]
- Romero, M.C.; Romero, A.M.; Doval, M.M.; Judis, M.A. Nutritional value and fatty acid composition of some traditional Argentinean meat sausages. Food Sci. Technol. 2013, 33, 161–166. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ktari, N.; Triki, M.; Bkhairia, I.; Slima, S.B.; Aydi, S.S.; Aydi, S.; Abdeslamf, A.; Salah, R.B. Physicochemical, techno-functional, and antioxidant properties of a novel bacterial exopolysaccharide in cooked beef sausage. Int. J. Biol. Macromol. 2018, 111, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Jouki, M.; Rabbani, M.; Shakour, M.J. Effects of pectin and tomato paste as a natural antioxidant on inhibition of lipid oxidation and production of functional chicken breast sausage. Food Sci. Technol. 2020, 40, 521–527. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT—Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Nacak, B.; Kavuşan, H.S.; Serdaroğlu, M. Effect of α-tocopherol, rosemary extract and their combination on lipid and protein oxidation in beef sausages. IOP Conf. Ser. Earth Environ. Sci. 2021, 854, 012062. [Google Scholar] [CrossRef]
- Chouliara, E.; Badeka, A.; Savvaidis, I.; Kontominas, M. Combined effect of irradiation and modified atmosphere packaging on shelf-life extension of chicken breast meat: Microbiological, chemical and sensory changes. Eur. Food Res. Technol. 2008, 226, 877–888. [Google Scholar] [CrossRef]
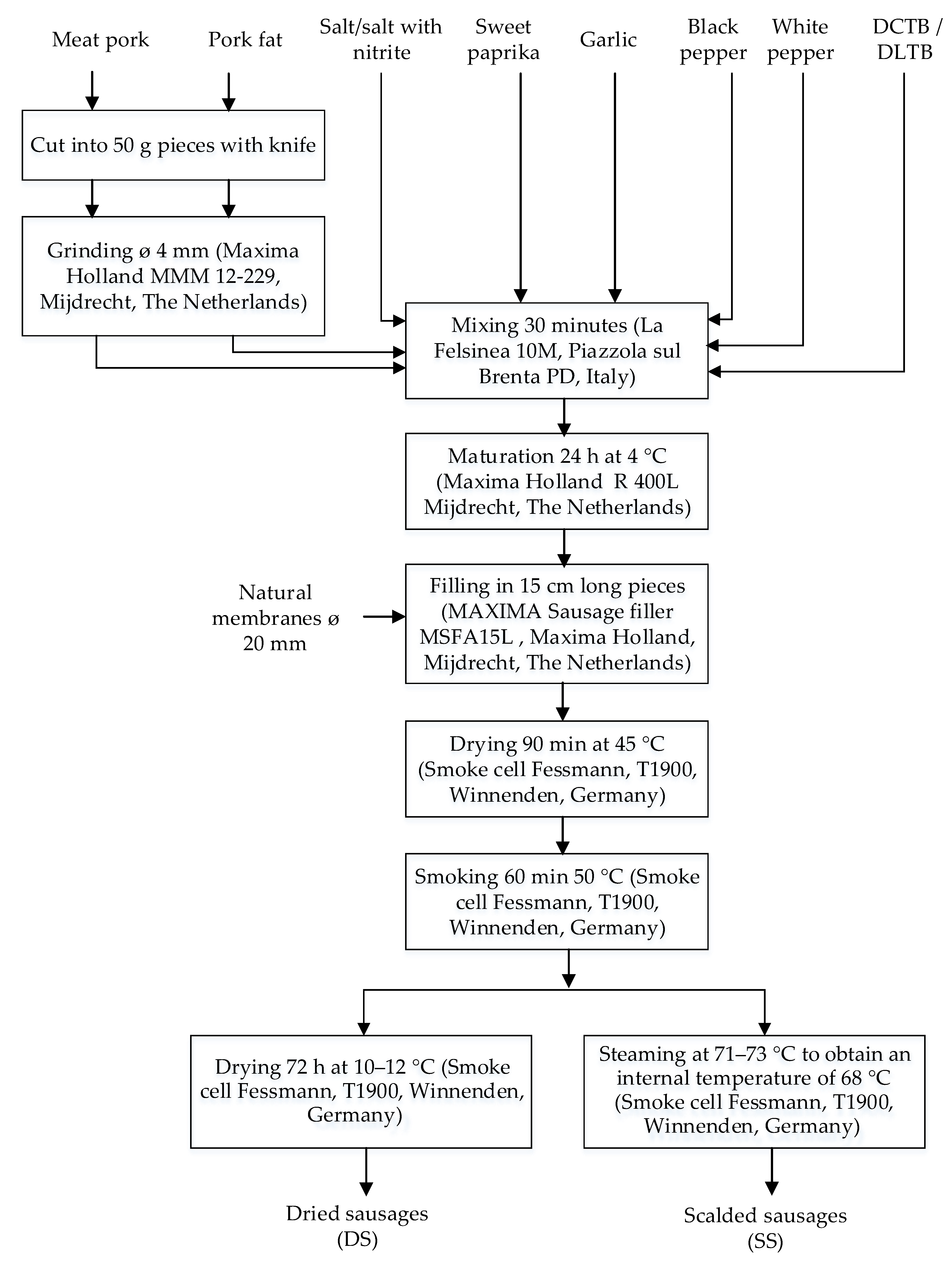





| Sample | Pork Meat (g) | Pork Fat (g) | Salt (g) | Salt + 0.5% (w/w) Sodium Nitrite (g) | Sweet Paprika (g) | Garlic (g) | White Pepper (g) | Black Pepper (g) | DLTB (g) | DCTB (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| SC | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | - |
| SDC | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | - |
| SDCN | 800 | 200 | - | 18 | 6 | 16 | 2 | 2 | - | - |
| SSC | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | - |
| SSCN | 800 | 200 | - | 18 | 6 | 16 | 2 | 2 | - | - |
| DSDLTB50 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 9.527 | - |
| DSDLTB90 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 17.149 | - |
| DSDLTB180 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 34.299 | - |
| DSDLTB270 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 51.448 | - |
| SSDLTB50 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 9.527 | - |
| SSDLTB90 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 17.149 | - |
| SSDLTB180 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 34.299 | - |
| SSDLTB270 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | 51.448 | - |
| DSDCTB50 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 8.731 |
| DSDCTB90 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 15.715 |
| DSDCTB180 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 31.430 |
| DSDCTB270 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 47.145 |
| SSDCTB50 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 8.731 |
| SSDCTB90 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 15.715 |
| SSDCTB180 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 31.430 |
| SSDCTB270 | 800 | 200 | 18 | - | 6 | 16 | 2 | 2 | - | 47.145 |
| Sample | Smoking | Drying | Scalding |
|---|---|---|---|
| SC | - | - | - |
| SDC | + | + | - |
| SDCN | + | + | - |
| SSC | + | - | + |
| SSCN | + | - | + |
| DSDLTB50 | + | + | - |
| DSDLTB90 | + | + | - |
| DSDLTB180 | + | + | - |
| DSDLTB270 | + | + | - |
| SSDLTB50 | + | - | + |
| SSDLTB90 | + | - | + |
| SSDLTB180 | + | - | + |
| SSDLTB270 | + | - | + |
| DSDCTB50 | + | + | - |
| DSDCTB90 | + | + | - |
| DSDCTB180 | + | + | - |
| DSDCTB270 | + | + | - |
| SSDCTB50 | + | - | + |
| SSDCTB90 | + | - | + |
| SSDCTB180 | + | - | + |
| SSDCTB270 | + | - | + |
| Polyphenolic Compound | RT (Min) | Compound Content (mg/g d.s) | |||||
|---|---|---|---|---|---|---|---|
| CT | LT | CTB | LTB | DCTB | DLTB | ||
| Gallic acid | 5.694 | 3.481 ± 0.132 a | 3.074 ± 0.124 b | 3.350 ± 0.126 a | 2.940 ± 0.118 b | 2.056 ± 0.094 c | 1.960 ± 0.091 c |
| Protocatechuic acid | 12.631 | 3.579 ± 0.141 d | 6.542 ± 0.255 a | 3.132 ± 0.152 e | 5.234 ± 0.201 b | 2.666 ± 0.125 f | 4.426 ± 0.191 c |
| Caffeic acid | 18.747 | 3.450 ± 0.140 a | 2.584 ± 0.122 c | 3.336 ± 0.139 a | 2.013 ± 0.092 d | 3.117 ± 0.118 b | 1.973 ± 0.095 d |
| Epicatechin | 23.417 | 2.332 ± 0.124 b | 2.576 ± 0.126 a | 2.213 ± 0.121 c | 2.235 ± 0.125 c | 1.805 ± 0.920 e | 1.951 ± 0.954 d |
| p-Coumaric acid | 24.952 | 0.376 ± 0.012 a | 0.260 ± 0.011 b | 0.188 ± 0.010 c | 0.133 ± 0.009 c | 0.074 ± 0.009 d | 0.065 ± 0.008 d |
| Ferulic acid | 23.521 | 4.272 ± 0.022 a | 2.544 ± 0.015 d | 4.011 ± 0.021 b | 2.052 ± 0.014 e | 3.152 ± 0.012 c | 1.252 ± 0.010 f |
| Rutin | 25.837 | 24.105 ± 1.075 a | 9.311 ± 0.335 d | 22.280 ± 1.005 b | 8.210 ± 0.257 e | 14.852 ± 0.662 c | 7.162 ± 0.324 f |
| Rosmarinic acid | 28.631 | 1.813 ± 0.090 a | 0.615 ± 0.028 d | 1.638 ± 0.062 b | 0.419 ± 0.020 e | 1.282 ± 0.051 c | 0.218 ± 0.088 f |
| Resveratrol | 29.200 | 3.091 ± 0.138 a | 1.639 ± 0.071 c | 2.587 ± 0.132 b | 1.400 ± 0.603 d | 1.598 ± 0623 c | 1.351 ± 0.516 d |
| Quercetin | 31.871 | 2.361 ± 0.128 a | 1.726 ± 0.746 c | 2.096 ± 0.904 b | 1.563 ± 0.615 d | 1.548 ± 0.557 d | 1.302 ± 0.413 e |
| Kaempferol | 34.644 | 0.750 ± 0.025 c | 0.998 ± 0.032 a | 0.631 ± 0.022 d | 0.892 ± 0.030 b | 0.439 ± 0.012 e | 0.414 ± 0.010 e |
| Sample | Chemical Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Moisture (g/100 g) | Protein (g/100 g) | Lipids (g/100 g) | Ash (g/100 g) | NaCl (g/100 g) | Carbohy-Drates (g/100 g) | Energy Value (kcal/100 g) | |
| SC | 49.583 ± 1.354 a | 14.670 ± 0.361 l | 30.662 ± 0.761 d | 2.290 ± 0.051 h,i | 2.110 ± 0.055 a | 0.685 | 337.378 |
| SDC | 48.982 ± 1.300 b,d | 15.430 ± 0.379 k | 30.540 ± 0.768 a | 2.380 ± 0.050 h,i | 2.120 ± 0.058, a | 0.548 | 338.772 |
| SDCN | 48.974 ± 1.299 b,d | 15.410 ± 0.352 k | 30.482 ± 0.755 a,b | 2.360 ± 0.048 i | 2.130 ± 0.056 a | 0.644 | 338.554 |
| SSC | 48.758 ± 1.315 b,d,e | 15.350 ± 0.344 k | 31.031 ± 0.723 c | 2.450 ± 0.055 f,g | 2.120 ± 0.055 a | 0.291 | 341.843 |
| SSCN | 48.687 ± 1.218 b,d,f | 15.400 ± 0.358 k | 31.102 ± 0.729 b,c | 2.410 ± 0.050 g,h | 2.140 ± 0.056 a | 0.261 | 342.562 |
| DSDLTB50 | 48.933 ± 1.323 b | 16.180 ± 0.361 j | 29.242 ± 0.705 e | 2.570 ± 0.058 e,f | 2.130 ± 0.065 a | 0.945 | 331.677 |
| DSDLTB90 | 48.837 ± 1.315 b,d | 16.540 ± 0.375 i | 28.971 ± 0.695 e,f | 2.660 ± 0.060 d | 2.110 ± 0.063 a | 0.881 | 330.427 |
| DSDLTB180 | 48.573 ± 1.309 b,d,f | 17.460 ± 0.385 f,g | 28.349 ± 0.694 g,h | 2.700 ± 0.065 c | 2.140 ± 0.055 a | 0.778 | 328.093 |
| DSDLTB270 | 48.312 ± 1.297 e,f | 18.110 ± 0.388 c | 27.974 ± 0.681 h | 2.890 ± 0.074 b | 2.120 ± 0.054 a | 0.594 | 326.580 |
| SSDLTB50 | 48.831 ± 1.285 b,d | 16.460 ± 0.399 i | 29.213 ± 0.709 e | 2.480 ± 0.056 e,f | 2.140 ± 0.052 a | 0.876 | 332.261 |
| SSDLTB90 | 48.563 ± 1.313 b,d,f | 16.980 ± 0.325 h | 28.748 ± 0.676 f | 2.690 ± 0.059 d | 2.130 ± 0.055 a | 0.889 | 330.208 |
| SSDLTB180 | 48.210 ± 1.334 c,f | 17.680 ± 0.314 d | 28.342 ± 0.655 g,h | 2.840 ± 0.066 c | 2.120 ± 0.058 a | 0.808 | 329.031 |
| SSDLTB270 | 47.947 ± 1.275 c | 18.070 ± 0.379 c | 27.952 ± 0.611 h | 2.910 ± 0.075 a,b | 2.140 ± 0.052 a | 0.981 | 327.772 |
| DSDCTB50 | 48.746 ± 1.319 b,d | 17.080 ± 0.305 g,h | 28.941 ± 0.714 e,f | 2.410 ± 0.060 e | 2.110 ± 0.050 a | 0.713 | 331.641 |
| DSDCTB90 | 48.689 ± 1.307 b,d,f | 17.370 ± 0.311 e,f | 28.754 ±0.707 f | 2.680 ± 0.061 d | 2.140 ± 0.057 a | 0.367 | 329.734 |
| DSDCTB180 | 48.358 ± 1.296 c,d,e | 17.960 ± 0.365 c | 28.069 ± 0.653 h | 2.750 ± 0.066 c | 2.130 ± 0.052 a | 0.733 | 327.395 |
| DSDCTB270 | 48.024 ± 1.285 c | 18.930 ± 0.391 a | 27.164 ± 0.587 i | 2.920 ± 0.077 a,b | 2.120 ± 0.054 a | 0.842 | 323.564 |
| SSDCTB50 | 48.970 ± 1.324 b,d | 16.890 ± 0.358 h | 28.856 ± 0.713 e,f | 2.560 ± 0.057 e,f | 2.110 ± 0.055 a | 0.614 | 329.720 |
| SSDCTB90 | 48.537 ± 1.316 b,d,f | 17.480 ± 0.322 d,e | 28.564 ± 0.706 f,g | 2.670 ± 0.060 d | 2.140 ± 0.053 a | 0.609 | 329.430 |
| SSDCTB180 | 48.126 ± 1.303 c,f | 17.990 ± 0.368 c | 28.221 ± 0.641 g,h | 2.820 ± 0.068 c | 2.120 ± 0.055 a | 0.723 | 328.841 |
| SSDCTB270 | 47.958 ± 1.288 c | 18.440 ± 0.381 b | 27.996 ± 0.628 h | 2.950 ± 0.080 a | 2.130 ± 0.058 a | 0.526 | 327.828 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadariu, A.I.; Cocan, I.; Negrea, M.; Alexa, E.; Obistioiu, D.; Hotea, I.; Radulov, I.; Poiana, M.-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability 2022, 14, 11802. https://doi.org/10.3390/su141911802
Cadariu AI, Cocan I, Negrea M, Alexa E, Obistioiu D, Hotea I, Radulov I, Poiana M-A. Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages. Sustainability. 2022; 14(19):11802. https://doi.org/10.3390/su141911802
Chicago/Turabian StyleCadariu, Andreea I., Ileana Cocan, Monica Negrea, Ersilia Alexa, Diana Obistioiu, Ionela Hotea, Isidora Radulov, and Mariana-Atena Poiana. 2022. "Exploring the Potential of Tomato Processing Byproduct as a Natural Antioxidant in Reformulated Nitrite-Free Sausages" Sustainability 14, no. 19: 11802. https://doi.org/10.3390/su141911802








