Sustainable Proposal for Regulating Organophosphate Pesticides in Wastewater Treatment Plants in South Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Organophosphate Pesticides
2.2. Agrochemical Wastewater Treatment Plants
2.3. Sample Collection
2.4. Sample Analysis and Quality Control
3. Results and Discussion
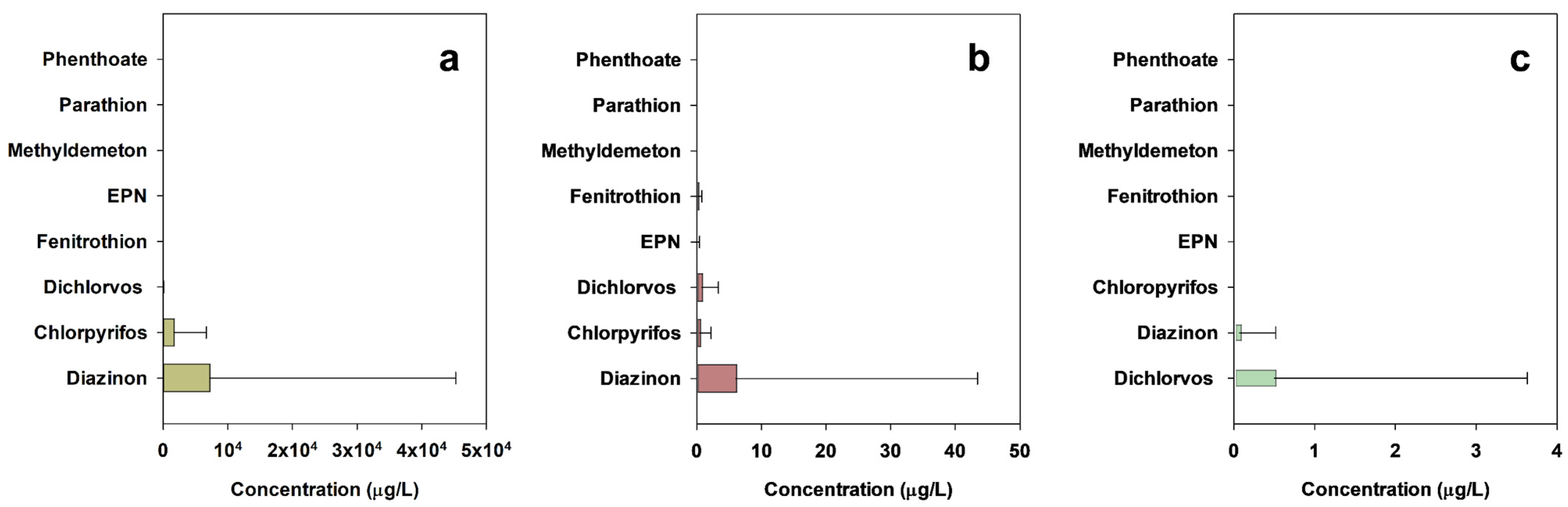
3.1. Occurrence of Organophosphate Pesticides in Agrochemical Manufacturing Facilities and Agrochemical Wastewater Treatment Plants
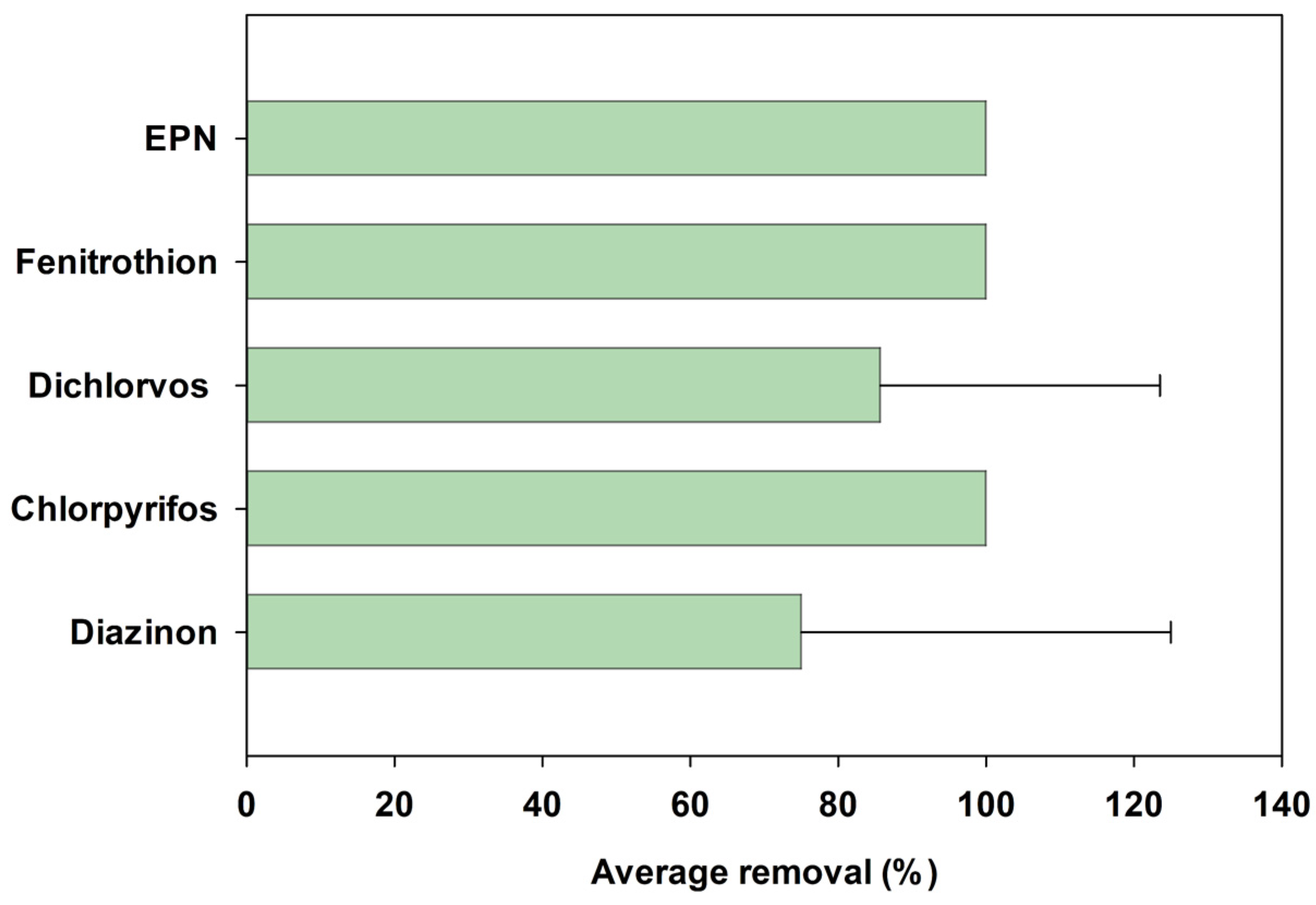
3.2. Organophosphate Pesticide Removal Characteristics in Agrochemical Wastewater Treatment Plants
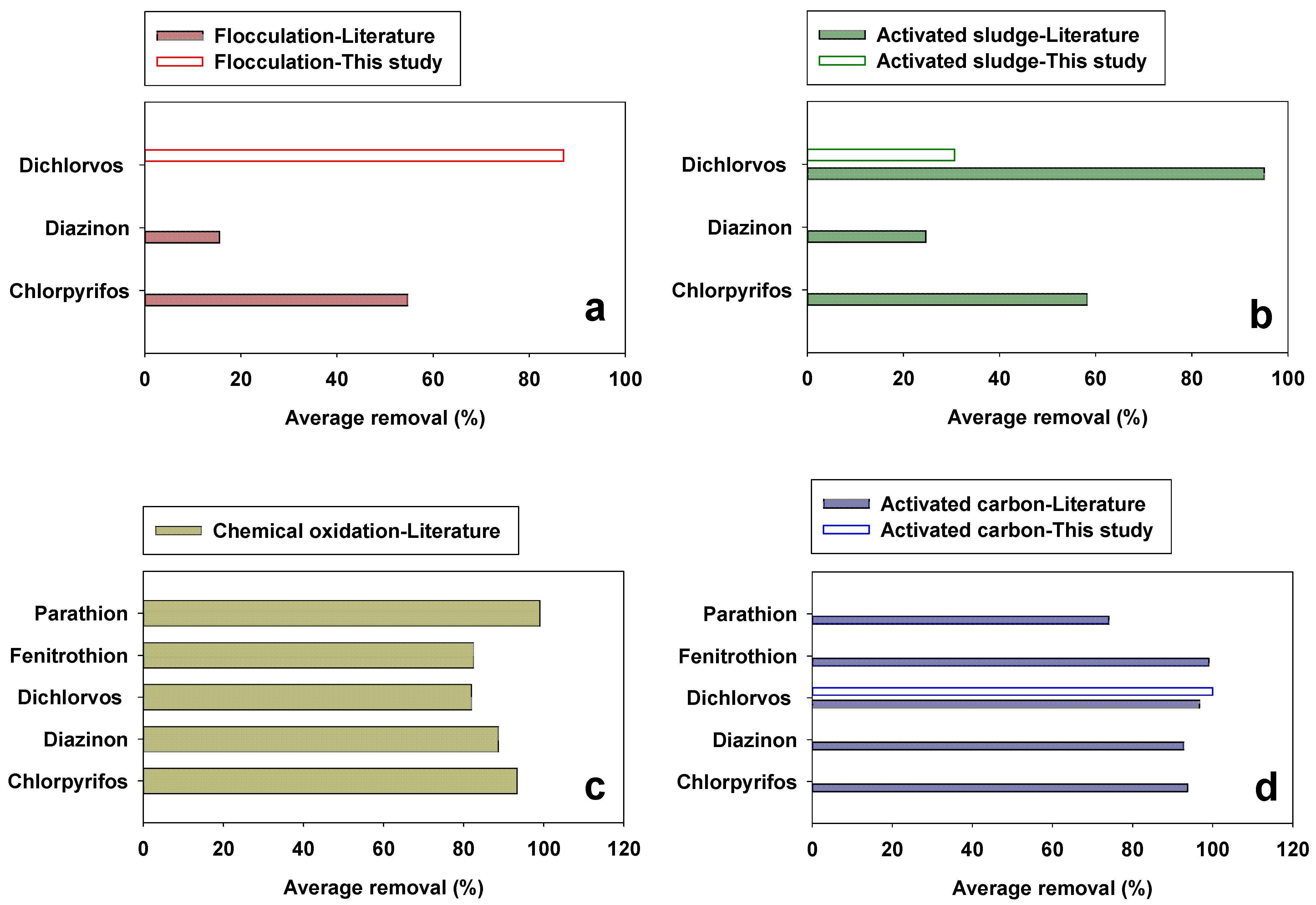
3.3. Suggestions for Sustainable Organophosphate Pesticide Regulation in Wastewater Treatment Plants
4. Conclusions
- (1)
- Five OPs (chlorpyrifos, diazinon, dichlorvos, EPN, and fenitrothion) were found at very high concentration levels in wastewaters generated from eleven AMFs and influents of ten AWWTPs.
- (2)
- Among the five OPs observed in the AWWTPs influents, chlorpyrifos, fenitrothion, and EPN were completely removed (100%). However, the average removal efficiencies of diazinon and dichlorvos were 75.0% and 85.7%, respectively, with high standard deviations of 50% and 37.8%, respectively, indicating their comparatively lower removal efficiency and treatment stability.
- (3)
- In the evaluation of four unit processes (FL, ASP, CO, and AC adsorption) for OP removal, the highest removal was observed for AC adsorption (93.4%), followed by CO (85%). In addition, AC adsorption showed higher treatment stability than CO while ASP showed a lower OP removal efficiency (48.5%).
- (4)
- For the sustainable management of OPs in AWWTPs, the additional regulation of chlorpyrifos, dichlorvos, and fenitrothion is required in South Korea. Additionally, the most effective basic process configuration for OP removal from AWWTPs includes ASP as the main treatment technology followed by AC adsorption as a polishing treatment.
- (5)
- The application of the process suggested in this study would lead to the effective control of OPs in AWWTPs, which would reduce the concentration and detection frequency of OPs in surface water.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lima, J.A.M.; Labanowski, J.; Bastos, M.C.; Zanella, R.; Prestes, O.D.; Vargas, J.P.R.; Mondamert, L.; Granado, E.; Tiecher, T.; Zafar, M.; et al. “Modern agriculture” transfers many pesticides to watercourses: A case study of a representative rural catchment of southern Brazil. Environ. Sci. Pollut. Res. 2020, 27, 10581–10598. [Google Scholar] [CrossRef] [PubMed]
- Carena, L.; Comis, S.; Vione, D. Geographical and temporal assessment of the photochemical decontamination potential of river waters from agrochemicals: A first application to the Piedmont region (NW Italy). Chemosphere 2021, 263, 127921–127930. [Google Scholar] [CrossRef] [PubMed]
- Horak, I.; Horn, S.; Pieters, R. Agrochemicals in freshwater systems and their potential as endocrine disrupting chemicals: A South African context. Environ. Pollut. 2021, 268, 115718–115730. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.M.; González-Farias, F.A.; Botello, A.V. Pollution by organochlorine pesticides in Navachiste-Macapule, Sinaloa, Mexico. Environ. Monit. Assess. 2012, 184, 1359–1369. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ma, X.Y.; Wang, Y.; Zhang, S.; Zheng, K.; Wang, X.C.; Lin, Y. Removal of trace organic pollutants (pharmaceuticals and pesticides) and reduction of biological effects from secondary effluent by typical granular activated carbon. Sci. Total Environ. 2020, 749, 141611–141620. [Google Scholar] [CrossRef] [PubMed]
- Torres-Palma, R.A.; Serna-Galvis, E.A. Sonolysis. In Advanced Oxidation Processes for Waste Water Treatment: Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: London, UK, 2018; pp. 177–213. [Google Scholar]
- Stamatis, N.; Hela, D.; Konstantinou, I. Pesticide inputs from the sewage treatment plant of Agrinio to River Acheloos, western Greece: Occurrence and removal. Water Sci. Technol. 2010, 62, 1098–1105. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Villagrasa, M.; de Alda, M.L.; Céspedes-Sánchez, R.; Ventura, F.; Barceló, D. Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci. Total Environ. 2013, 458–860, 466–476. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122–142130. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, Y.; Gu, Y. Assembly of UiO-66 onto Co-doped Fe3O4 nanoparticles to activate peroxymonosulfate for efficient degradation of fenitrothion and simultaneous in-situ adsorption of released phosphate. J. Hazard. Mater. 2022, 436, 129058–129068. [Google Scholar] [CrossRef]
- Yu, X.; Kamali, M.; Aken, P.V.; Appels, L.; Bruggen, B.V.; Dewil, R. Synergistic effects of the combined use of ozone and sodium percarbonate for the oxidative degradation of dichlorvos. J. Water Process Eng. 2021, 39, 101721–101728. [Google Scholar] [CrossRef]
- Amiri, N.A.; Amiri, F.A.; Faravardeh, L.; Eslami, A.; Ghasemi, A.; Rafiee, M. Enhancement of MBBR reactor efficiency using effective microorganism for treatment of wastewater containing diazinon by engineered Pseudomonas putida KT2440 with manganese peroxidase 2 gene. J. Environ. Manag. 2022, 316, 115293–115306. [Google Scholar] [CrossRef] [PubMed]
- Hamadeen, H.M.; Elkhatib, E.A. Nanostructured modified biochar for effective elimination of chlorpyrifos from wastewater: Enhancement, mechanisms and performance. J. Water Process Eng. 2022, 47, 102703–102712. [Google Scholar] [CrossRef]
- Pariente, M.I.; Siles, J.A.; Molina, R.; Botas, J.A.; Melero, J.A.; Martinez, F. Treatment of an agrochemical wastewater by integration of heterogeneous catalytic wet hydrogen peroxide oxidation and rotating biological contactors. Chem. Eng. J. 2013, 226, 409–415. [Google Scholar] [CrossRef]
- Guieysse, B.; Norvill, Z.N. Sequential chemical–biological processes for the treatment of industrial wastewaters: Review of recent progresses and critical assessment. J. Hazard. Mater. 2014, 267, 142–152. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Pariente, M.I.; Martinez, F.; Melero, J.A.; Molina, R. Modeling the integrated heterogeneous catalytic fixed-bed reactor and rotating biological contactor system for the treatment of poorly biodegradable industrial agrochemical wastewater. J. Environ. Chem. Eng. 2016, 4, 2313–2321. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, Y.; Han, W.; Li, J.; Sun, X.; Shen, J.; Wang, L. Advanced treatment of triazole fungicides discharged water in pilot scale by integrated system: Enhanced electrochemical oxidation, upflow biological aerated filter and electrodialysis. Chem. Eng. J. 2017, 315, 335–344. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. A Survey on the Monitoring of Potentially Hazardous Compounds and Contamination Routes in Tributary of Four Major Rivers; National Institute of Environmental Research: Incheon, Korea, 2018.
- Ministry of Environment. Study on the Investigation System Construction of Unregulated Water Pollutants; Ministry of Environment: Sejong, Korea, 2020.
- U.S. Environmental Protection Agency. Method 549.2: Determination of Diquat and Paraquat in Drinking Water by Liquid-Solid Extraction and High Performance Liquid Chromatography with Ultraviolet Detection, Revision 1.0; U.S. EPA: Cincinnati, OH, USA, 1997.
- U.S. Environmental Protection Agency. Method 540: Determination of Selected Organic Chemicals in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS), Version 1.0; U.S. EPA: Cincinnati, OH, USA, 2013.
- Ministry of Environment. Korea Standard Methods for the Examination of Water and Wastewater; National Institute of Environmental Research: Incheon, Korea, 2017.
- Pham, T.V.; Phuoc, V.M.; Nguyen, D.V.; Koyama, J. Treatment efficiency of a combination of alternative technologies in removing pollutants from pesticide containing wastewater. Environ. Eng. Res. 2021, 26, 200263–200271. [Google Scholar] [CrossRef]
- Golash, N.; Gogate, P.R. Degradation of dichlorvos containing wastewaters using sonochemical reactors. Ultrason. Sonochem. 2012, 19, 1051–1060. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Pang, J.L.S.; Mishra, S.; Bhatt, P.; Zeng, D.; Chen, S. Emerging technologies for degradation of dichlorvos: A review. Int. J. Environ. Res. Public Health 2021, 18, 5789–5801. [Google Scholar] [CrossRef]
- Patil, P.N.; Gogate, P.R. Degradation of dichlorvos using hybrid advanced oxidation processes based on ultrasound. J. Water Process Eng. 2015, 8, e58–e65. [Google Scholar] [CrossRef]
- Aminu, A.S.; Gimba, C.E.; Kagbu, J.; Turoti, M.; Itodo, A.U.; Sariyya, A.I. Sorption efficiency study of pesticide adsorption on granulated activated carbon from groundnut shell using GC/MS. Electron. J. Environ. Agric. Food. Chem. 2010, 9, 1222–1231. [Google Scholar]
- Ogwuche, E.O.; Gimba, C.E.; Abechi, E.S. An evaluation of the adsorptive behaviour of activated carbon derived from Hyphaene thebaica nut shells for the removal of dichlorvos from wastewater. Int. J. Sci. Technol. 2015, 3, 274–285. [Google Scholar]
- Binh, Q.A.; Tungtakanpoung, D.; Kajitvichyanukul, P. Similarities and differences in adsorption mechanism of dichlorvos and pymetrozine insecticides with coconut fiber biowaste sorbent. J. Environ. Sci. Health B 2020, 55, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sancho, F.; Molinos-Senante, M.; Sala-Garrido, R. Cost modelling for wastewater treatment processes. Desalination 2011, 268, 1–5. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, G.; Molinos-Senante, M.; Hospido, A.; Hernandez-Sancho, F.; Moreira, M.T.; Feijoo, G. Environmental and economic profile of six typologies of wastewater treatment plants. Water Res. 2011, 45, 5997–6010. [Google Scholar] [CrossRef]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.Q.; Leung, K.T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Ormad, M.P.; Miguel, N.; Claver, A.; Matesanz, J.M.; Ovelleiro, J.L. Pesticides removal in the process of drinking water production. Chemosphere 2008, 71, 97–106. [Google Scholar] [CrossRef]
- Leoni, V.; Cremisini, C.; Giovinazzo, R.; Puccetti, G.; Vitali, M. Activated sludge biodegradation test as a screening method to evaluate persistence of pesticides in soil. Sci. Total Environ. 1992, 123–124, 279–289. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.J.; Khan, M.S.I.; Na, Y.C. Identification and determination of by-products originating from ozonation of chlorpyrifos and diazinon in water by liquid chromatography–mass spectrometry. J. Sep. Sci. 2020, 43, 4047–4057. [Google Scholar] [CrossRef]
- Bourgeois, A.; Klinkhamer, E.; Price, J. Pesticide Removal from Water. Bachelor’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2012. [Google Scholar]
- Badawy, M.I.; Ghaly, M.Y.; Gad-Allah, T.A. Advanced oxidation processes for the removal of organophosphorus pesticides from wastewater. Desalination 2006, 194, 166–175. [Google Scholar] [CrossRef]
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Gonzalez, M. Removal of diazinon by various advanced oxidation processes. J. Chem. Technol. Biotechnol. 2007, 82, 566–574. [Google Scholar] [CrossRef]
- Toolabi, A.; Malakootian, M.; Ghaneian, M.T.; Esrafili, A.; Ehrampoush, M.H.; AskarShahi, M.; Tabatabaei, M.; Khatami, M. Optimizing the photocatalytic process of removing diazinon pesticide from aqueous solutions and effluent toxicity assessment via a response surface methodology approach. Rend. Lincei Sci. Fis. Nat. 2019, 30, 155–165. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Gharanjig, K.; Nourmohammadian, F.; Bidokhti, A.Y. Purification of water containing agricultural organophosphorus pollutant using titania nanophotocatalysis: Laboratory studies and numerical modeling. Desalination 2008, 230, 183–192. [Google Scholar] [CrossRef]
- Barbusiński, K.; Filipek, K. Use of Fenton’s reagent for removal of pesticides from industrial wastewater. Pol. J. Environ. Stud. 2001, 10, 207–212. [Google Scholar]
- Goodwin, L.; Carra, I.; Campo, P.; Soares, A. Treatment options for reclaiming wastewater produced by the pesticide industry. Int. J. Water Wastewater Treat. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Beduk, F.; Aydin, M.E.; Ozcan, S. Degradation of malathion and parathion by ozonation, photolytic ozonation, and heterogeneous catalytic ozonation processes. Clean—Soil Air Water 2012, 40, 179–187. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Combined solar advanced oxidation and PAC adsorption for removal of pesticides from industrial wastewater. J. Mater. Environ. Sci. 2015, 6, 800–809. [Google Scholar]
- Moussavi, G.; Hosseini, H.; Alahabadi, A. The investigation of diazinon pesticide removal from contaminated water by adsorption onto NH4Cl-induced activated carbon. Chem. Eng. J. 2013, 214, 172–179. [Google Scholar] [CrossRef]
- Akbarlou, Z.; Alipour, V.; Heidari, M.; Dindarloo, K. Adsorption of diazinon from aqueous solutions onto an activated carbon sample produced in Iran. Environ. Health Eng. Manag. J. 2017, 4, 93–99. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Dargahi, A.; Hazrati, S.; Fazlzadehdavil, M. Removal of diazinon and 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solutions by granular-activated carbon. Desalination Water Treat. 2014, 52, 4350–4355. [Google Scholar] [CrossRef]
- Lee, D.I.; Chun, S.U.; Joo, Y.K. Sorption and leaching studies of fenitrothion and tebuconazole in granular activated carbon and charcoal. Asian J. Turfgrass Sci. 2006, 20, 47–55. [Google Scholar]
- Sigworth, E.A. Identification and removal of herbicides and pesticides. J. Am. Water Works Assoc. 1965, 57, 1016–1022. [Google Scholar] [CrossRef]
- Effluent Limitations Guidelines and Standards (ELG) Database. Available online: https://owapps.epa.gov/elg/ (accessed on 4 July 2022).
- Crane, M.; Babut, M. Environmental quality standards for water framework directive priority substances: Challenges and opportunities. Integr. Environ. Assess. Manag. 2007, 3, 290–296. [Google Scholar] [CrossRef]
- Guillossou, R.; Roux, J.L.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef]
- Han, T.; Zheng, J.; Han, Y.; Xu, X.; Li, M.; Schwarz, C.; Zhu, L. Comprehensive insights into core microbial assemblages in activated sludge exposed to textile-dyeing wastewater stress. Sci. Total Environ. 2021, 791, 148145–148155. [Google Scholar] [CrossRef]
- Manna, M.; Sen, S. Advanced oxidation process: A sustainable technology for treating refractory organic compounds present in industrial wastewater. Environ. Sci. Pollut. Res. 2022, 14, 1–29. [Google Scholar] [CrossRef]
- Al-Khafaji, S.A.; Al-Rekabi, W.S.; mawat, M.J. Apply membrane biological reactor (MBR) in industrial wastewater treatment: A mini review. Eurasian J. Eng. Technol. 2022, 7, 98–106. [Google Scholar]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109–100123. [Google Scholar] [CrossRef]


| OP | Percent Frequency of NMS detection in South Korean Rivers 1 |
|---|---|
| Chlorpyrifos | 4 (68/1733) 2 |
| Diazinon | 2 (25/1378) |
| Dichlorvos | 4 (39/954) |
| EPN | 6 (66/1050) |
| Fenitrothion | 0.2 (1/414) |
| Methyldemeton | n.a. |
| Parathion | n.a. |
| Phenthoate | 5 (40/858) |
| AWWTPs | Main Treatment Processes | Quantity of Effluent (m3/d) |
|---|---|---|
| W1 | In → FL 1 → ASP 2 → AC 3 → Out | 18 |
| W2 | In → FL → ASP → FL → Out | 149 |
| W3 | In → FL → AC → AF 4 → ASP → Out | 36 |
| W4 | In → FL → ASP → AC → Out | 254 |
| W5 | In → ASP → Out | 173 |
| W6 | In → CO 5 (NaOCl) → FL → Filtration → AC → Out | 367 |
| W7 | In → ASP → CO (Fenton) → Out | 537 |
| W8 | In → ASP → FL → Out | 4000 |
| W9 | In → FL → ASP → FL → Out | 200 |
| W10 | In → FL → ASP → FL → AC → Out | 200 |
| W11 | In → FL → ASP → Filtration → AC → Out | 270 |
| W12 | In → FL → ASP → Out | 169 |
| W13 | In → FL → Out | 60 |
| OP | AMF Wastewater | AWWTPs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | ||||||||
| Min. (μg/L) | Max. (μg/L) | Freq. (%) | Min. (μg/L) | Max. (μg/L) | Freq. (%) | Min. (μg/L) | Max. (μg/L) | Freq. (%) | |
| Chlorpyrifos | n.d. | 24,680 | 51.7 | n.d. | 10.04 | 7.7 | n.d. | n.d. | 0 |
| Diazinon | n.d. | 205,200 | 13.8 | n.d. | 233.6 | 10.3 | n.d. | 2.79 | 2.6 |
| Dichlorvos | n.d. | 378.6 | 17.2 | n.d. | 15.29 | 17.9 | n.d. | 19.56 | 2.6 |
| EPN | n.d. | 3.91 | 3.4 | n.d. | 2.27 | 2.6 | n.d. | n.d. | 0 |
| Fenitrothion | n.d. | 24.8 | 3.4 | n.d. | 3.25 | 7.7 | n.d. | n.d. | 0 |
| Methyldemeton | n.d. | n.d. | 0 | n.d. | n.d. | 0 | n.d. | n.d. | 0 |
| Parathion | n.d. | n.d. | 0 | n.d. | n.d. | 0 | n.d. | n.d. | 0 |
| Phenthoate | n.d. | n.d. | 0 | n.d. | n.d. | 0 | n.d. | n.d. | 0 |
| Unit Process | Average Removal (%) | ||
|---|---|---|---|
| This Study | Literature | Overall | |
| Flocculation | 87.2 | 41.6 (±26.9 1) [24,34] | 53.0 (±31.6) |
| Biological removal with activated sludge | 30.7 | 52.1 (±40.9) [8,9,35] | 48.5 (±37.6) |
| Chemical oxidation | n.a. | 87.0 (±16.8) [25,27,34,36,37,38,39,40,41,42,43,44] | 87.0 (±16.8) |
| Activated carbon adsorption | 100 | 92.9 (±7.8) [6,28,29,30,31,32,33,34,45,46,47,48,49,50] | 93.5 (±7.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.-D.; Han, H.; Park, J.-H.; Kim, Y.S. Sustainable Proposal for Regulating Organophosphate Pesticides in Wastewater Treatment Plants in South Korea. Sustainability 2022, 14, 11979. https://doi.org/10.3390/su141911979
Ryu H-D, Han H, Park J-H, Kim YS. Sustainable Proposal for Regulating Organophosphate Pesticides in Wastewater Treatment Plants in South Korea. Sustainability. 2022; 14(19):11979. https://doi.org/10.3390/su141911979
Chicago/Turabian StyleRyu, Hong-Duck, Hyeyeol Han, Ji-Hyoung Park, and Yong Seok Kim. 2022. "Sustainable Proposal for Regulating Organophosphate Pesticides in Wastewater Treatment Plants in South Korea" Sustainability 14, no. 19: 11979. https://doi.org/10.3390/su141911979




