Photosynthetic Behavior of Argania spinosa (L.) Skeels Induced under Grazed and Ungrazed Conditions
Abstract
:1. Introduction
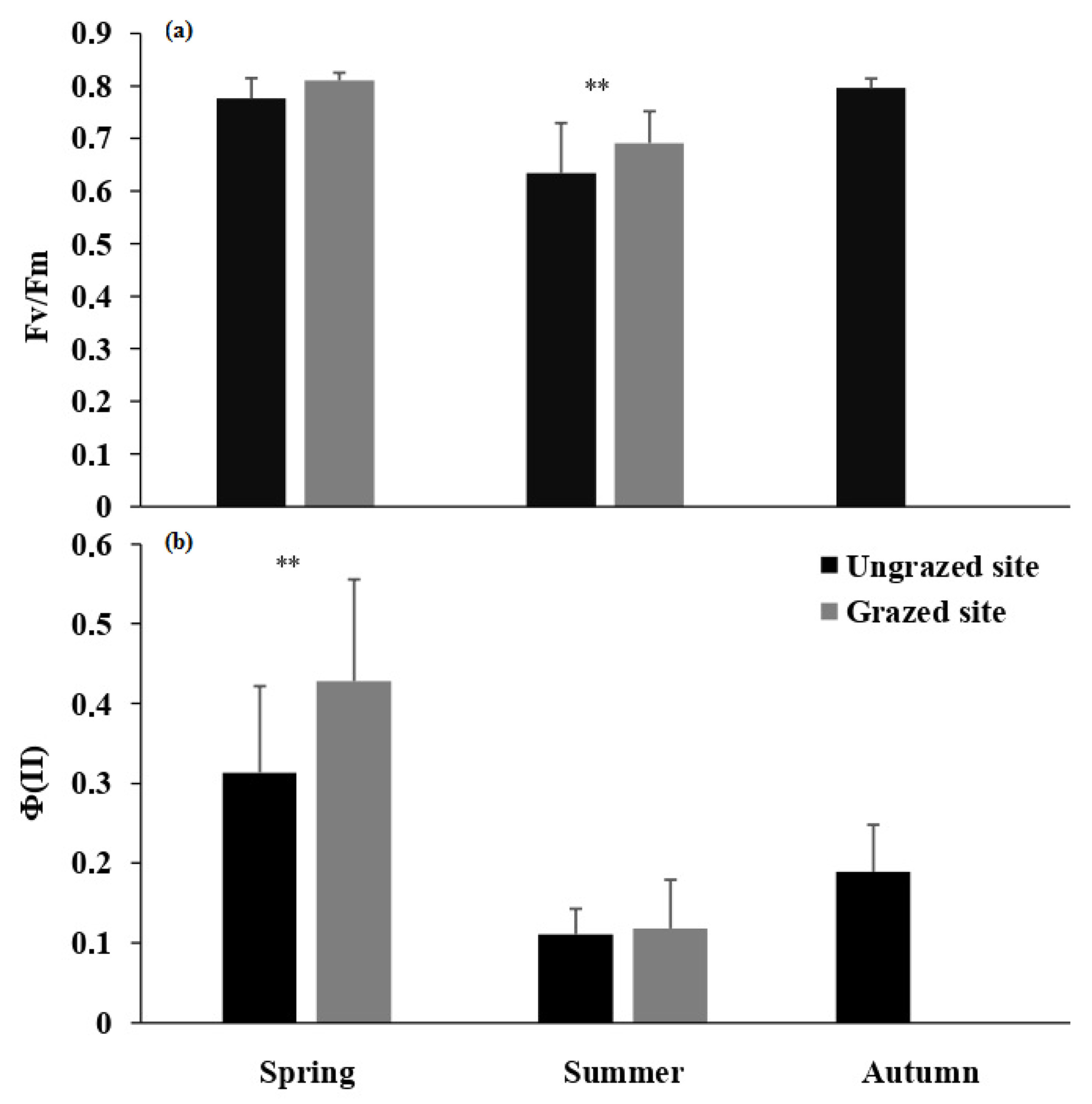
2. Results
3. Discussion
4. Materials and Methods
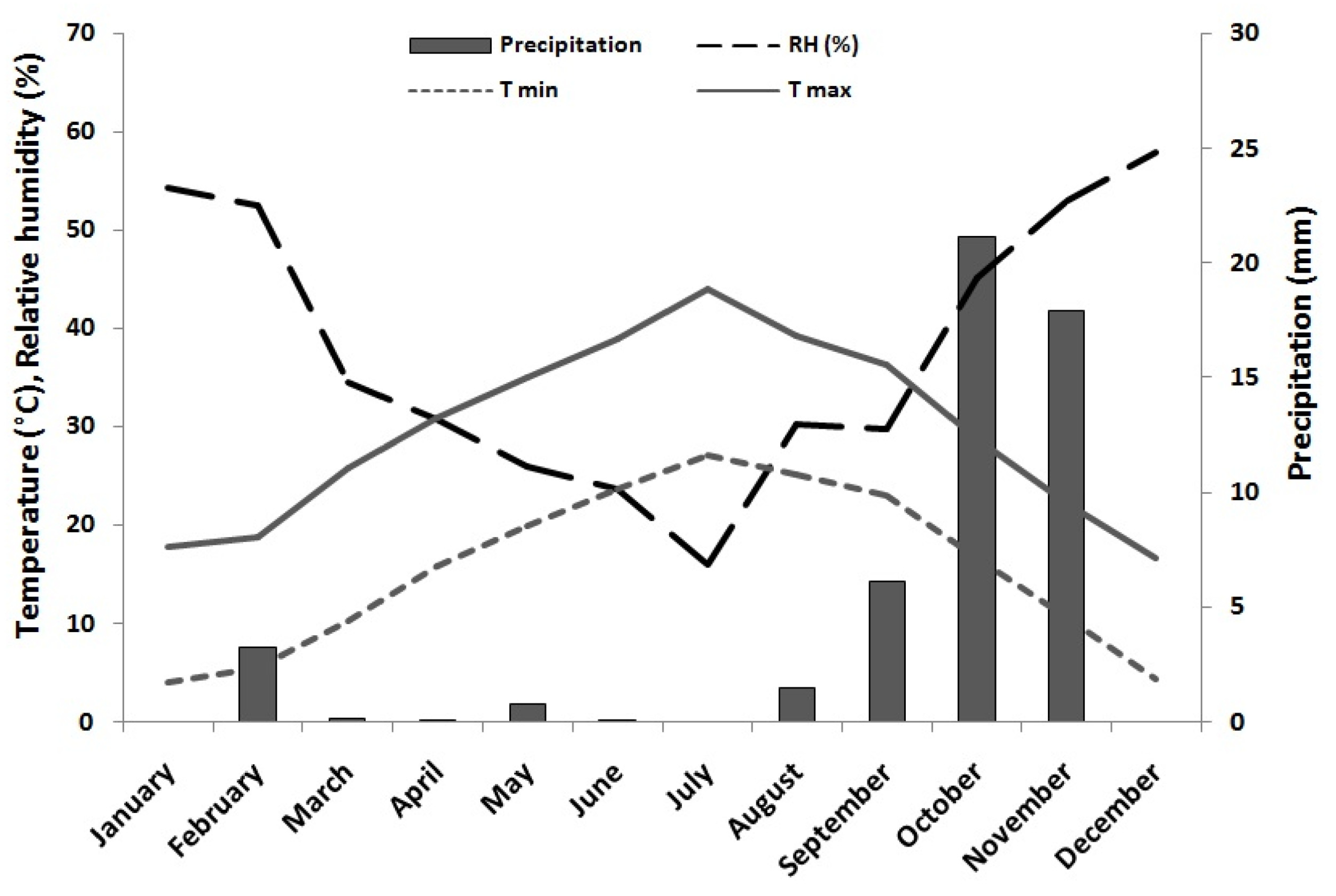
4.1. Plant Material and Experimental Conditions
4.2. Gas Exchange Measurements
4.3. Chlorophyll Fluorescence Measurements
4.4. Pigment Content
4.5. Water Status
4.6. Stomatal Features
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achour, A. Contribution à L’étude de la Phénologie de l’Arganier et de la Biodiversité au Sein D’une Parcelle Clôturée Evaluation D’un Essai de Régénération Artificielle. Ph.D. Thesis, Ibn Zohr University, Agadir, Morocco, 2014; 204p. [Google Scholar]
- Díaz Barradas, M.C.; Zunzunegui, M.; Esquivias, M.P.; Boutaleb, S.; Valera Burgos, J.; Tagma, T.; Ain-Lhout, F. Some secrets of Arganiaspinosa water economy in a semiarid climate. Nat. Prod. Commun. 2013, 8, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ait Bihi, M.; Ain-Lhout, F.; Hatimi, A.; Fahmi, F.; Tahrouch, S. Ecophysiological Response and Morphological Adjustment of Arganiaspinosa L. Skeels under Contrasting Climates: Case Study of Marginal Populations. Int. J. Plant Biol. 2021, 12, 9404. [Google Scholar] [CrossRef]
- Benabid, A. Les écosystèmes forestiers, préforestiers et pré-steppiques du Maroc: Diversité, répartition biogéographique et problèmes posés par leur aménagement. For. Méditerr. 1985, 7, 65–68. [Google Scholar]
- M’Hirit, O.; Benzyane, M.; Benchekroun, F.; El Yousfi, S.M.; Bendaanoun, M. L’Arganier: Une Espèce Fruitière-Forestière à Usages Multiples; Mardaga Press: Brussels, Belgium, 1998. [Google Scholar]
- Lefhaili, A. FAO Forest Resources Assessment: Morocco Country Report; FAO: Rome, Italy, 2010. [Google Scholar]
- Chakhchar, A.; Ben Salah, I.; El Kharrassi, Y.; Filali-Maltouf, A.; El Modafar, C.; Lamaoui, M. Agro-Fruit-Forest Systems Based on argan Tree in Morocco: A Review of Recent Results. Front. Plant Sci. 2022, 12, 783615. [Google Scholar] [CrossRef] [PubMed]
- De Waroux, Y.; Lambin, E.F. Monitoring degradation in arid and semi-arid forests and woodlands: The case of the argan woodlands (Morocco). Appl. Geogr. 2012, 32, 777–786. [Google Scholar] [CrossRef]
- Karmaoui, A. Ecosystem services of the argan forest, the current state and trends. Adv. Res. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Genin, M.; Alifriqui, M.; Fakhech, A.; Hafidi, M.; Ouahmane, L.; Genin, D. Back to forests in pre-Saharan Morocco? When prickly pear cultivation and traditional agropastoralism reduction promote argan tree regeneration. Silva Fenn. 2017, 51, 1618. [Google Scholar] [CrossRef]
- El Aïch, A.; Bourbouze, A.; Morand-Fehr, P. La chèvre dans l’Arganeraie. In Acte de L’institut Agronomique et Vétérinaire Hassan II; Institut Agronomique et Vétérinaire Hassan II: Rabat, Morocco, 2005; p. 136. [Google Scholar]
- Carrión, J.S.; Sánchez-Gómez, P.; Mota, J.F.; Yll, R.; Chaín, C. Holocene vegetation dynamics, fire and grazing in the Sierra de Gádor, southern Spain. Holocene 2003, 13, 839–849. [Google Scholar] [CrossRef]
- Nouaim, R. L’Arganier au Maroc. Entre Mythes et Réalités: Une Civilisation Née D’un Arbre; L’Harmattan: Paris, France, 2005; pp. 17–18. [Google Scholar]
- Aafi, A. Etude de la Diversité Floristique de L’écosystème de Chêne-Liège de la Forêt de la Maamora. Ph.D. Thesis, Institut Agronomique et Vétérinaire Hassan II, Rabat, Morocco, 2007; 190p. [Google Scholar]
- Wang, S.; Duan, J.; Xu, G.; Wang, Y.; Zhang, Z.; Rui, Y.; Cui, X. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology 2012, 93, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Eekeren, N.V. EGF at 50: The future of European grasslands. In Proceedings of the 25th General Meeting of the European Grassland Federation, Aberystwyth, UK, 7–11 September 2014; IBERS, Aberystwyth University: Aberystwyth, UK, 2014; pp. 665–667. [Google Scholar]
- Davidson, K.E.; Fowler, M.S.; Skov, M.W.; Doerr, S.H.; Beaumont, N.; Griffin, J.N. Livestock grazing alters multiple ecosystem properties and services in salt marshes: A meta-analysis. J. Appl. Ecol. 2017, 54, 1395–1405. [Google Scholar] [CrossRef]
- Ain-Lhout, F.; Zunzunegui, M.; DiázBarradas, M.C.; Jáuregui, J.; Tagma, T.; Boutaleb, S. Climatic conditions and herbivory effects on morphological plasticity of Argania spinosa. Nat. Prod. Commun. 2013, 8, 5–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunzunegui, M.; Ain-Lhout, F.; Díaz Barradas, M.C.; Jáuregui, J.; Boutaleb, S.; ÁlvarezCansino, L.; Esquivias-Segura, M.P. Fruit production under different environmental and management conditions on Arganiaspinosa. J. Arid. Environ. 2010, 74, 1138–1145. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Guo, H.; Zhang, J.; Kong, L.; Ren, W. Legacy effects of historical grazing alter leaf stomatal characteristics in progeny plants. PeerJ 2020, 8, e9266. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J. Serengeti migratory wildebeest: Facilitation of energy flow by grazing. Science 1976, 191, 92–94. [Google Scholar] [CrossRef]
- McNaughton, S.J. Grazing as an optimization process: Grass–ungulate relationships in the Serengeti. Am. Nat. 1979, 113, 691–703. [Google Scholar] [CrossRef]
- Hayashi, M.; Fujita, N.; Yamauchi, A. Theory of grazing optimization in which herbivory improves photosynthetic ability. J. Theor. Biol. 2007, 248, 367–376. [Google Scholar] [CrossRef]
- Peng, Y.; Jiang, G.M.; Liu, X.H.; Niu, S.L.; Liu, M.Z.; Biswas, D.K. Photosynthesis, transpiration and water use efficiency of four plant species with grazing intensities in Hunshandak Sandland, China. J. Arid. Environ. 2007, 70, 304–315. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mancilla-Leytón, J.M.; Mateos-Naranjo, E.; Cambrollé, J.; Martín-Vicente, A. Differential photosynthetic performance of three Mediterranean shrubs under grazing by domestic goats. Photosynthetica 2010, 48, 348–354. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Mi, X.; Bai, Y.; Zhang, M.; Li, X. Grazing every month minimizes size but boosts photosynthesis in Stipagrandis in the steppe of Inner Mongolia, China. J. Arid. Land 2018, 10, 601–611. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Chludil, H.D.; Leicach, S.R.; Corbino, G.B.; Barriga, L.G.; Vilariño, M.P. Genistin and quinolizidine alkaloid induction in L. angustifolius aerial parts in response to mechanical damage. J. Plant Interact. 2013, 8, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Obeso, J.R. Effects of defoliation and girdling on fruit production in Ilex aquifolium. Funct. Ecol. 1998, 12, 486–491. [Google Scholar] [CrossRef]
- Rosenthal, J.P.; Kotanen, P.M. Terrestrial plant tolerance to herbivory. Trends Ecol. Evol. 1994, 9, 145–148. [Google Scholar] [CrossRef]
- El Mousadik, A.; Petit, R.J. High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L. Skeels)] endemic to Morocco. Theor. Appl. Genet. 1996, 92, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Dong, S.; Li, S.; Xiao, J.; Han, Y.; Yang, M.; Zhang, J.; Gao, X.; Xu, Y.; Li, Y.; et al. Grazing enhances plant photosynthetic capacity by altering soil nitrogen in alpine grasslands on the Qinghai-Tibetan plateau. Agric. Ecosyst. Environ. 2019, 280, 161–168. [Google Scholar] [CrossRef]
- Fornara, D.A.; du Toit, J.T. Community-level interactions between ungulate browsers and woody plants in an African savanna dominated by palatable-spinescent Acacia trees. J. Arid. Environ. 2008, 72, 534–545. [Google Scholar] [CrossRef]
- Nyamukanza, C.C.; Sebata, A. Response of Ziziphusmucronata and Acacia nilotica saplings to increasing clipping intensity in a southern African savanna. Plant Ecol. 2020, 221, 1167–1176. [Google Scholar] [CrossRef]
- Layne, D.R.; Flore, J.A. Photosynthetic compensation to partial leaf area reduction in Sour Cherry. J. Am. Soc. Hortic. Sci. 1992, 117, 279–286. [Google Scholar] [CrossRef]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Liu, H.; Zang, R.; Chen, H. Effects of grazing on photosynthetic features and soil respiration of rangelands in the Tianshan Mountains of Northwest China. Sci. Rep. 2016, 6, 30087. [Google Scholar] [CrossRef]
- Wang, Z.B.; Wang, Y.F.; Zhao, J.J.; Ma, L.; Wang, Y.J.; Zhang, X.; Zhao, Z.Y. Effects of GeO2 on chlorophyll fluorescence and antioxidant enzymes in apple leaves under strong light. Photosynthetica 2018, 56, 1081–1092. [Google Scholar] [CrossRef]
- Díaz-Barradas, M.C.; Zunzunegui, M.; Ain-Lhout, F.; Jáuregui, J.; Boutaleb, S.; Álvarez-Cansino, L.; Esquivias, M.P. Seasonalphysiologicalresponses of Arganiaspinosa tree from Mediterranean to semi-arid climate. Plant Soil 2010, 337, 217–231. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2, evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The xanthophyll cycle is induced by light irrespective of water status in field-grown lavender (Lavandulastoechas) plants. Physiol. Plant. 2000, 108, 147–151. [Google Scholar] [CrossRef]
- Li, J.; Hou, F.; Ren, J. Grazing Intensity Alters Leaf and Spike Photosynthesis, Transpiration, and Related Parameters of Three Grass Species on an Alpine Steppe in the Qilian Mountains. Plants 2021, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Kyparissis, A.; Drilias, P.; Manetas, Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Aust. J. Plant Physiol. 2000, 27, 265–272. [Google Scholar] [CrossRef]
- Liu, M.; Gong, J.; Li, Y.; Li, X.; Yang, B.; Zhang, Z.; Yang, L.; Hou, X. Growth–defense trade-off regulated by hormones in grass plants growing under different grazing intensities. Physiol. Plant. 2019, 166, 553–569. [Google Scholar] [CrossRef]
- Limin, Y.; Mei HGuangsheng, Z.; Jiandong, L. The changes in water-use efficiency and stoma density of Leymuschinensis along Northeast China Transect. Acta Ecol. Sin. 2007, 27, 16–23. [Google Scholar] [CrossRef]
- Lang, B.; Ahlborn, J.; Oyunbileg, M. Grazing effects on intraspecific trait variability vary with changing precipitation patterns in Mongolian rangelands. Ecol. Evol. 2020, 10, 678–691. [Google Scholar] [CrossRef]
- Yin, Q.; Tian, T.; Kou, M.; Liu, P.; Wang, L.; Hao, Z.; Yue, M. The relationships between photosynthesis and stomatal traits on the Loess Plateau. Glob. Ecol. Conserv. 2020, 23, e01146. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef]
- Wang, R.; Yu, G.; He, N.; Wang, Q.; Zhao, N.; Xu, Z.; Ge, J. Latitudinal variation of leaf stomatal traits from species to community level in forests: Linkage with ecosystem productivity. Sci. Rep. 2015, 5, 14454. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Scutt, C.P.; Douthe, C.; Marino, G.; Gomes, M.T.G.; Loreto, F.; Flexas, J.; Centritto, M. Allocation of the epidermis to stomata relates to stomatalphysiological control: Stomatal factors involved in the evolutionarydiversification of the angiosperms and development of amphistomaty. Environ. Exp. Bot. 2018, 151, 55–63. [Google Scholar] [CrossRef]
- NASA. Power Data Access Viewer. Natural. 2020. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 30 April 2020).
- Ordiales-Plaza, R. Midebmp, ver 4.2; Estación Experimental de Zonas Áridas, CSIC, Consejo Superior de Investigaciones Científicas: Almería, Spain, 2000.
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Techniques for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Sack, L.; Grubb, P.J.; Maranon, T. The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol. 2003, 168, 247–259. [Google Scholar] [CrossRef]




| Variables | Site | Season | Site × Season | ||||||
|---|---|---|---|---|---|---|---|---|---|
| fd | F | p | fd | F | p | fd | F | p | |
| ANet | 1 | 31.08 | 0.001 * | 2 | 46.94 | 0.001 * | 2 | 8.48 | 0.001 * |
| gs | 1 | 8.39 | 0.05 * | 2 | 186.78 | 0.001 * | 2 | 49.67 | 0.001 * |
| WUEi | 1 | 10.31 | 0.05 * | 2 | 29.13 | 0.001 * | 2 | 4.17 | 0.05 * |
| Ci | 1 | 13.38 | 0.001 * | 2 | 3.83 | 0.05 * | 2 | 2.35 | 0.098 |
| Fv/Fm | 1 | 24.37 | 0.001 * | 2 | 2130.83 | 0.001 * | 1 | 1.484 | 0.224 |
| ΦPS(II) | 1 | 21.23 | 0.001 * | 2 | 221.71 | 0.001 * | 1 | 16.350 | 0.001 * |
| Chla+b | 1 | 13.345 | 0.001 * | 2 | 188.755 | 0.001 * | 2 | 24.073 | 0.001 * |
| Chla/b | 1 | 1.029 | 0.312 | 2 | 7.4137 | 0.001 * | 2 | 1.508 | 0.224 |
| Car | 1 | 16.32 | 0.001 * | 2 | 24.22 | 0.001 * | 2 | 15.33 | 0.001 * |
| Chla+b/Car | 1 | 116.79 | 0.001 * | 2 | 79.29 | 0.001 * | 2 | 7.32 | 0.001 * |
| RWC | 1 | 0.06 | 0.106 | 2 | 25.98 | 0.001 * | 2 | 3.774 | 0.08 |
| SD | 1 | 21.622 | 0.001 * | 2 | 4.457 | 0.05 * | 2 | 8.004 | 0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nait Douch, A.; Boukhalef, L.; El Asbahani, A.; Al-Namazi, A.A.; El Mehrach, K.; Bouqbis, L.; Touaf, M.; Ain-Lhout, F. Photosynthetic Behavior of Argania spinosa (L.) Skeels Induced under Grazed and Ungrazed Conditions. Sustainability 2022, 14, 12081. https://doi.org/10.3390/su141912081
Nait Douch A, Boukhalef L, El Asbahani A, Al-Namazi AA, El Mehrach K, Bouqbis L, Touaf M, Ain-Lhout F. Photosynthetic Behavior of Argania spinosa (L.) Skeels Induced under Grazed and Ungrazed Conditions. Sustainability. 2022; 14(19):12081. https://doi.org/10.3390/su141912081
Chicago/Turabian StyleNait Douch, Aicha, Laila Boukhalef, Abdelhafed El Asbahani, Ali A. Al-Namazi, Khadija El Mehrach, Laila Bouqbis, Mourad Touaf, and Fatima Ain-Lhout. 2022. "Photosynthetic Behavior of Argania spinosa (L.) Skeels Induced under Grazed and Ungrazed Conditions" Sustainability 14, no. 19: 12081. https://doi.org/10.3390/su141912081





