Bio-Acidification of Cattle Slurry with Whey Reduces Gaseous Emission during Storage with Positive Effects on Biogas Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Chemical Characterization of Dairy Cattle Slurry and Whey
2.2. Preliminary Experiments
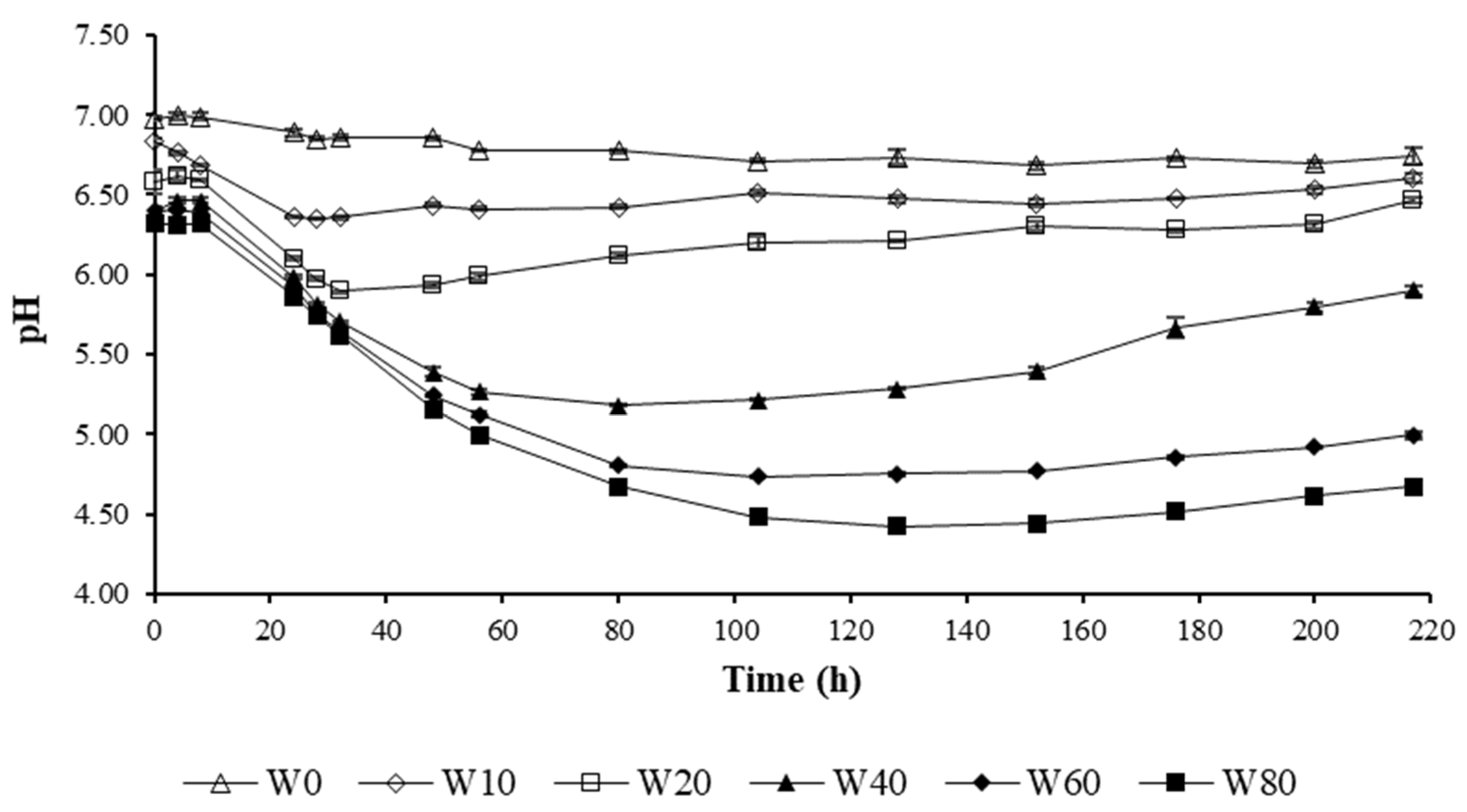
2.2.1. Phase 1: Assessment of the Amount of Whey Needed for Acidification
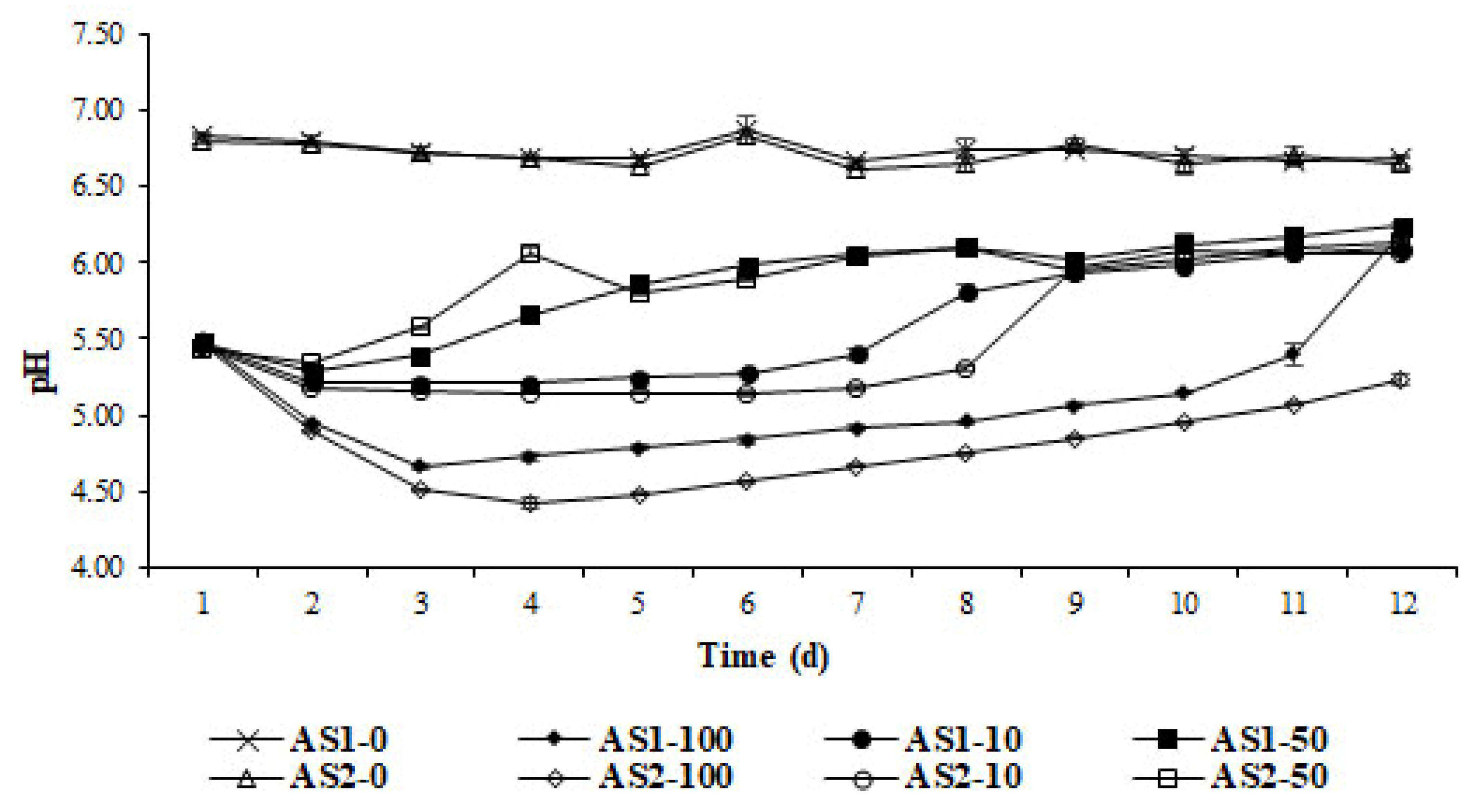
2.2.2. Phase 2: Assessment of Cattle Slurry Acidification Using Different Frequency of Whey Addition
2.3. Measurement of Ammonia and GHG Emissions
2.4. Biogas Yield of Acidified Slurry
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Experiments
3.1.1. Phase 1: Assessment of the Amount of Whey Needed for Acidification
3.1.2. Phase 2: Assessment of Slurry Acidification under Dynamic Slurry Storage Conditions
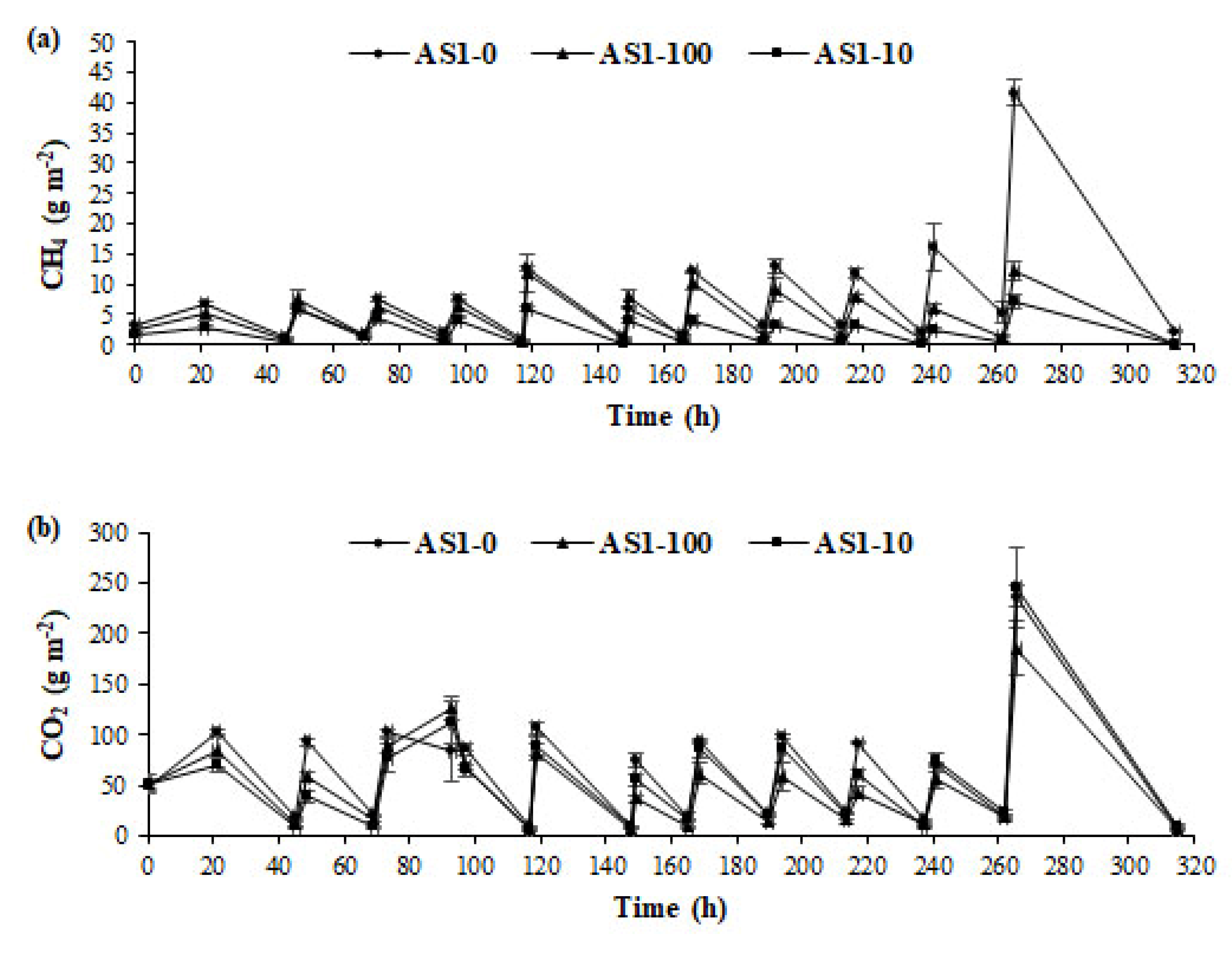
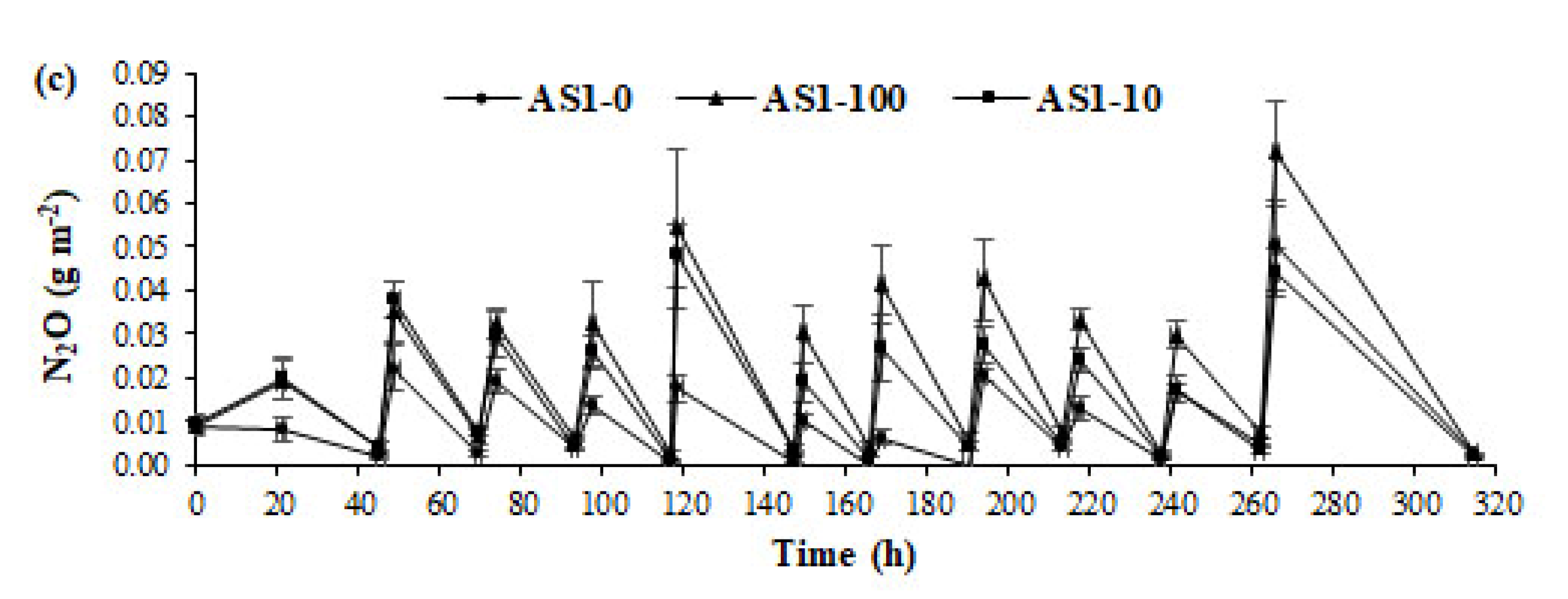
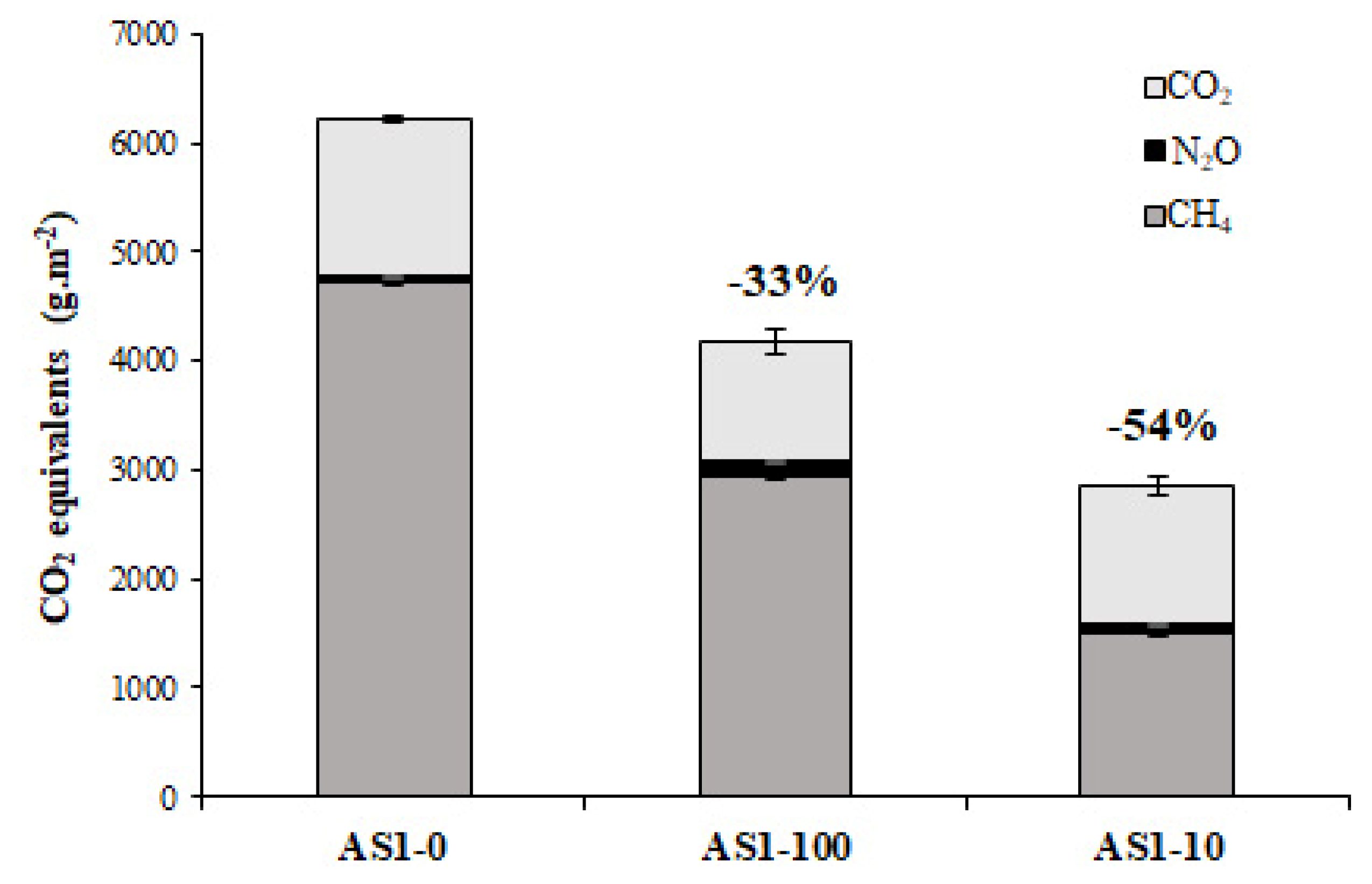
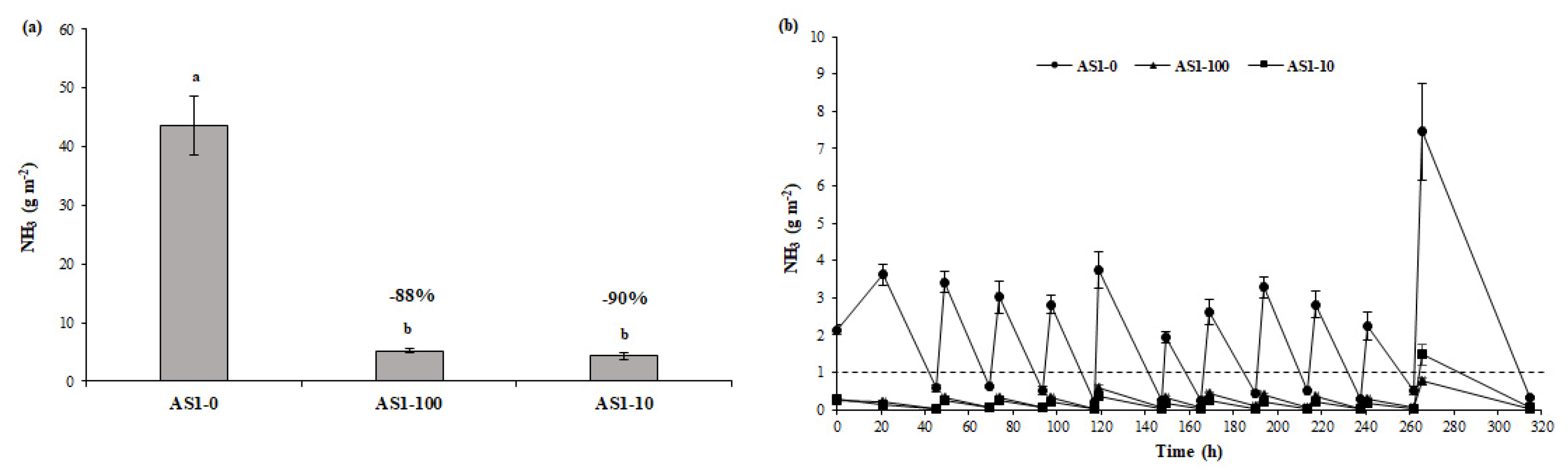
3.2. Ammonia and GHG Emissions from Acidified Slurry
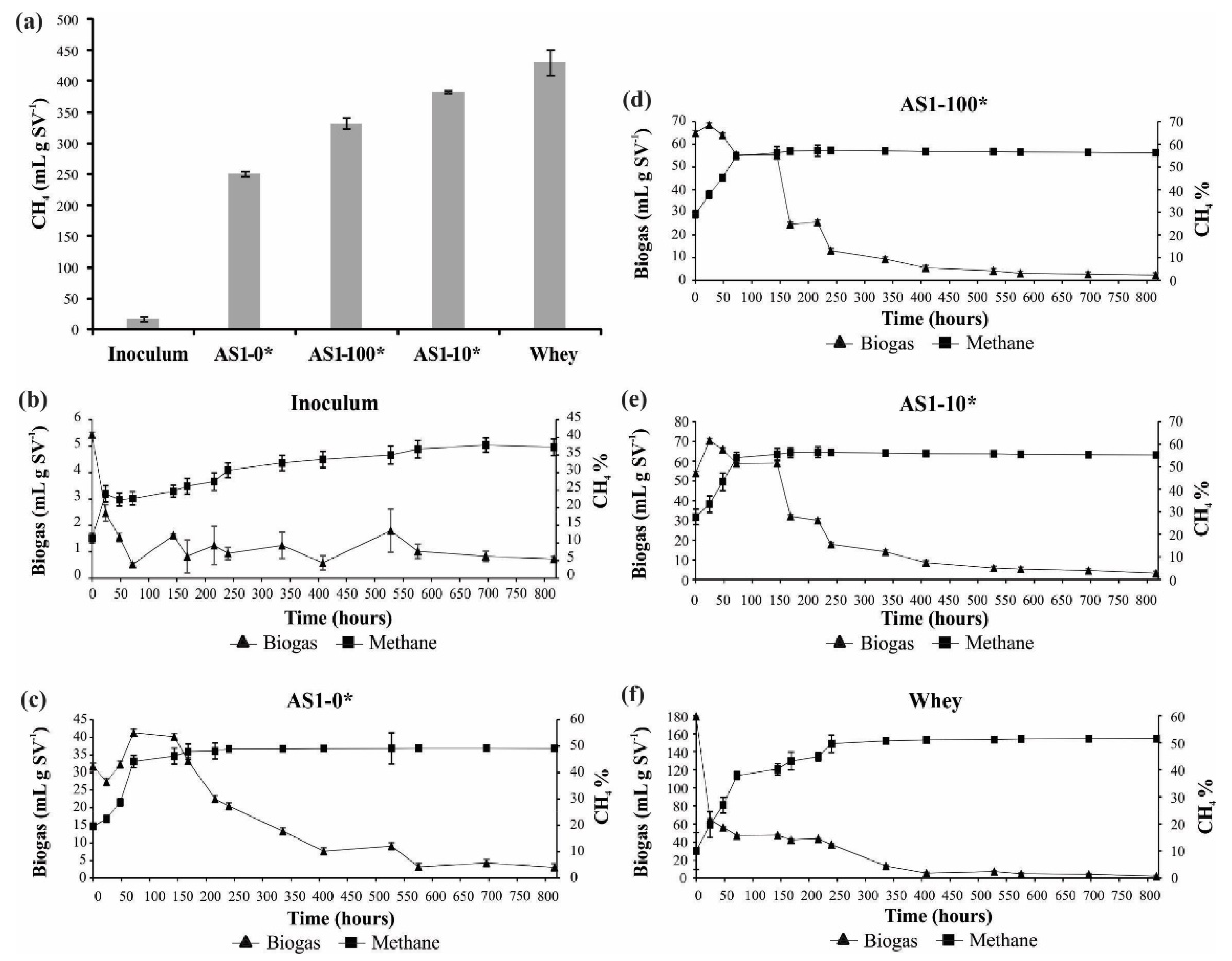
3.3. Biogas Yield of Acidified Slurry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Appendix A
References
- van der Weerden, T.J.; Luo, J.; Di, H.J.; Podolyan, A.; Phillips, R.L.; Saggar, S.; de Klein, C.A.M.; Cox, N.; Ettema, P.; Rys, G. Nitrous oxide emissions from urea fertiliser and effluent with and without inhibitors applied to pasture. Agric. Ecosyst. Environ. 2016, 219, 58–70. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, S.J.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Maguire, R.O. Manure Management. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 402–410. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database; FAO: Rome, Italy, 2019. [Google Scholar]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Westhoek, H. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10, 115004. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014 Mitigation of Climate Change: Working Group III Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, NY, USA, 2015. [Google Scholar]
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; van Cleemput, O. Closing the global N2O budget: Nitrous oxide emissions through the agricultural nitrogen cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Webb, J.; Sørensen, P.; Velthof, G.; Amon, B.; Pinto, M.; Rodhe, L.; Salomon, E.; Hutchings, N.; Burczyk, P.; Reid, J. An assessment of the variation of manure nitrogen efficiency throughout Europe and an appraisal of means to increase manure-N efficiency. Adv. Agron. 2013, 119, 371–442. [Google Scholar] [CrossRef]
- Bagdoniene, I.; Bleizgys, R. Ammonia emissions from dairy cattle manure under variable ventilation rates. Ann. Anim. Sci. 2014, 14, 141–151. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, G.; Li, B.; Strøm, S.; Dahl, P.J. Pig House: Effects of Ventilation Rate, Floor Slat Opening, and Headspace Height in a Manure Storage Pit. Trans. ASABE 2008, 51, 2113–2122. [Google Scholar] [CrossRef]
- Sommer, S.G.; Zhang, G.Q.; Bannink, A.; Chadwick, D.; Misselbrook, T.; Harrison, R.; Hutchings, N.J.; Menzi, H.; Monteny, G.J.; Ni, J.Q.; et al. Algorithms Determining Ammonia Emission from Buildings Housing Cattle and Pigs and from Manure Stores. Adv. Agron. 2006, 89, 261–335. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Dinuccio, E.; Gioelli, F.; Balsari, P.; Dorno, N. Agriculture, Ecosystems and Environment Ammonia losses from the storage and application of raw and chemo-mechanically separated slurry. Agric. Ecosyst. Environ. 2012, 153, 16–23. [Google Scholar] [CrossRef]
- Silva, A.A.; Fangueiro, D.; Carvalho, M. Slurry acidification as a solution to minimize ammonia emissions from the combined application of animal manure and synthetic fertilizer in no-tillage. Agronomy 2022, 12, 265. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry–A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- McCrory, D.F.; Hobbs, P.J. Additives to Reduce Ammonia and Odor Emissions from Livestock Wastes. J. Environ. Qual. 2001, 30, 345. [Google Scholar] [CrossRef]
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing ammonia loss from cattle slurry by the use of acidifying additives: The role of the buffer system. J. Sci. Food Agric. 1991, 57, 335–349. [Google Scholar] [CrossRef]
- Stevens, R.J.; Laughlin, R.J.; Frost, J.P. Effect of acidification with sulphuric acid on the volatilization of ammonia from cow and pig slurries. J. Agric. Sci. 1989, 113, 389. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Rotz, C.A. Management to reduce nitrogen losses in animal production. J. Anim. Sci. 2004, 82, E119–E137. [Google Scholar] [CrossRef]
- Hjorth, M. Acidifications effect on transformations in and composition of animal slurry. In Proceedings of the 15th Ramiran International Conference, Versailles, France, 3–5 June 2013. [Google Scholar]
- Fangueiro, D.; Ribeiro, H.; Vasconcelos, E.; Coutinho, J.; Cabral, F. Treatment by acidification followed by solid–liquid separation affects slurry and slurry fractions composition and their potential of N mineralization. Bioresour. Technol. 2009, 100, 4914–4917. [Google Scholar] [CrossRef]
- Sutaryo, S.; Ward, A.J.; Møller, H.B. Anaerobic digestion of acidified slurry fractions derived from different solid–liquid separation methods. Bioresour. Technol. 2013, 130, 495–501. [Google Scholar] [CrossRef]
- Moset, V.; Cambra-López, M.; Møller, H.B. The Inhibiting Effect of Sulfate on Thermophilic Anaerobic Digestion of Cattle and Pig Waste Slurry. Trans. ASABE 2012, 55, 2309–2317. [Google Scholar] [CrossRef]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88. [Google Scholar] [CrossRef]
- European Biogas Association. Eba Statistical Report 2021. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2021/11/EBA-STATISTICAL-REPORT-2021-SHORT-VERSION.pdf (accessed on 23 July 2022).
- Dell’Orefice. Biogas, Italia Leader in Europa con 1600 Impianti Attivi. Available online: https://www.ilsole24ore.com/art/biogas-italia-leader-europa-1600-impianti-attivi-AD0JRQ0 (accessed on 18 July 2022).
- Fuchs, A.; Dalby, F.R.; Liu, D.; Kai, P.; Feilberg, A. Improved effect of manure acidification technology for gas emission mitigation by substituting sulfuric acid with acetic acid. Clean. Eng. Technol. 2021, 4, 100263. [Google Scholar] [CrossRef]
- Berg, W.; Brunsch, R.; Pazsiczki, I. Greenhouse gas emissions from covered slurry compared with uncovered during storage. Agric. Ecosyst. Environ. 2006, 112, 129–134. [Google Scholar] [CrossRef]
- Regueiro, I.; Gómez-Muñoz, B.; Lübeck, M.; Hjorth, M.; Jensen, L.S. Bio-acidification of animal slurry: Efficiency, stability and the mechanisms involved. Bioresour. Technol. Rep. 2022, 19, 101135. [Google Scholar] [CrossRef]
- Clemens, J.; Bergmann, S.; Vandré, R. Reduced Ammonia Emissions from Slurry after Self-Acidification with Organic Supplements. Environ. Technol. 2002, 23, 429–435. [Google Scholar] [CrossRef]
- Prado, J.; Chieppe, J.; Raymundo, A.; Fangueiro, D. Bio-acidification and enhanced crusting as an alternative to sulphuric acid addition to slurry to mitigate ammonia and greenhouse gases emissions during short term storage. J. Clean. Prod. 2020, 263, 1–9. [Google Scholar] [CrossRef]
- Bastami, M.S.B.; Jones, D.L.; Chadwick, D.R. Reduction of methane emission during slurry storage by the addition of effective microorganisms and excessive carbon source from brewing sugar. J. Environ. Qual. 2016, 45, 2016–2022. [Google Scholar] [CrossRef]
- Hassan, A.N.; Nelson, B.K. Invited review: Anaerobic fermentation of dairy food wastewater. J. Dairy Sci. 2012, 95, 6188–6203. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 5th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2020. [Google Scholar]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, NY, USA, 2013. [Google Scholar]
- VDI 4630: Fermentation of organic materials-characterisation of the substrate, sampling, collection of material data, fermentation tests. In VDI Handbuch Energietechnik; Verlag des Vereins Deutscher Ingenieure: Düsseldorf, Germany, 2006.
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Pereira, J.; Bichana, A.; Surgy, S.; Cabral, F.; Coutinho, J. Effects of cattle-slurry treatment by acidification and separation on nitrogen dynamics and global warming potential after surface application to an acidic soil. J. Environ. Manag. 2015, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A whole-farm assessment of the efficacy of slurry acidification in reducing ammonia emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Paul, J.W.; Beauchamp, E.G. Relationship between volatile fatty acids, total ammonia, and pH in manure slurries. Biol. Wastes 1989, 29, 313–318. [Google Scholar] [CrossRef]
- Calero, R.; Iglesias-Iglesias, R.; Kennes, C.; Veiga, M.C. Organic loading rate effect on the acidogenesis of cheese whey: A comparison between UASB and SBR reactors. Environ. Technol. 2018, 39, 3046–3054. [Google Scholar] [CrossRef]
- Pind, P.F.; Angelidaki, I.; Ahring, B.K. Dynamics of the anaerobic process: Effects of volatile fatty acids. Biotechnol. Bioeng. 2003, 82, 791–801. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Loyon, L.; Guiziou, F.; Beline, F.; Peu, P. Gaseous Emissions (NH3, N2O, CH4 and CO2) from the aerobic treatment of piggery slurry—Comparison with a conventional storage system. Biosyst. Eng. 2007, 97, 472–480. [Google Scholar] [CrossRef]
- Zhu, X.; Burger, M.; Doane, T.A.; Horwath, W.R. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc. Natl. Acad. Sci. USA 2013, 110, 6328–6333. [Google Scholar] [CrossRef]
- Pain, B.F.; Thompson, R.B.; De La Lande Cremer, L.C.N.; Ten Holte, L. The use of additives in livestock slurries to improve their flow properties, conserve nitrogen and reduce odours. In Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1987; pp. 229–246. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ben-Hassan, R.M. Continuous production of biogas from dairy manure using an innovative no-mix reactor. Appl. Biochem. Biotechnol. 1989, 20–21, 541–559. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Dinuccio, E.; Gioelli, F.; Cuk, D.; Rollè, L.; Balsari, P. The use of co-digested solid fraction as feedstock for biogas plants. J. Agric. Eng. 2013, 44, 153–159. [Google Scholar] [CrossRef]
- Valentinuzzi, F.; Cavani, L.; Porfido, C.; Terzano, R.; Pii, Y.; Cesco, S.; Marzadori, C.; Mimmo, T. The fertilising potential of manure-based biogas fermentation residues: Pelleted vs. liquid digestate. Heliyon 2020, 6, e03325. [Google Scholar] [CrossRef]
| Phase 1 | Phase 2 | |||
|---|---|---|---|---|
| Cattle Slurry | Whey | Cattle Slurry | Whey | |
| TS (%) | 6.18 | 7.77 | 8.36 | 7.69 |
| VS (%TS) | 84.59 | 78.52 | 84.10 | 78.42 |
| pH | 7.10 | 4.70 | 6.90 | 4.60 |
| Sample | CH4 (g m−2) | CO2 (g m−2) | N2O (g m−2) |
|---|---|---|---|
| AS1-0 | 168.20(1.84)a | 1448.80(27.63)a | 0.23(0.03)a |
| AS1-100 | 105.06(17.19)b | 1115.61(110.39)a | 0.48(0.09)a |
| AS1-10 | 53.68(5.86)c | 1245.67(90.93)a | 0.37(0.06)a |
| Degrees of Freedom | 2 | 2 | 2 |
| Mean Square | 9870.9 | 84,597 | 0.04887 |
| Pr (>F) | 0.00078 | 0.07916 | 0.08665 |
| F | 29.611 | 3.987 | 3.7796 |
| BMP Test | ||||||
|---|---|---|---|---|---|---|
| Day 0 | Day 35 | |||||
| Sample | TS (%) | VS (% TS) | pH | TS (%) | VS (% TS) | pH |
| AS1-0* | 7.91(0.11)a | 83.87(0.20)a | 7.21(0.02)a | 3.59(0.06)a | 69.95(0.08)a | 7.27(0.05)b |
| AS1-100* | 6.32(0.30)b | 84.13(0.67)a | 4.88(0.14)c | 3.20(0.02)a | 68.72(0.12)b | 7.42(0.02)a |
| AS1-10* | 6.15(0.05)b | 83.53(0.08)a | 5.81(0.03)b | 3.26(0.23)a | 69.42(0.41)ab | 7.38(0.02)ab |
| Whey | 7.80(0.05)a | 78.59(0.16)b | 4.60(0.03)d | 1.58(0.01)b | 61.78(0.14)c | 7.12(0.01)c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioelli, F.; Grella, M.; Scarpeci, T.E.; Rollè, L.; Pierre, F.D.; Dinuccio, E. Bio-Acidification of Cattle Slurry with Whey Reduces Gaseous Emission during Storage with Positive Effects on Biogas Production. Sustainability 2022, 14, 12331. https://doi.org/10.3390/su141912331
Gioelli F, Grella M, Scarpeci TE, Rollè L, Pierre FD, Dinuccio E. Bio-Acidification of Cattle Slurry with Whey Reduces Gaseous Emission during Storage with Positive Effects on Biogas Production. Sustainability. 2022; 14(19):12331. https://doi.org/10.3390/su141912331
Chicago/Turabian StyleGioelli, Fabrizio, Marco Grella, Telma E. Scarpeci, Luca Rollè, Flavia Dela Pierre, and Elio Dinuccio. 2022. "Bio-Acidification of Cattle Slurry with Whey Reduces Gaseous Emission during Storage with Positive Effects on Biogas Production" Sustainability 14, no. 19: 12331. https://doi.org/10.3390/su141912331
APA StyleGioelli, F., Grella, M., Scarpeci, T. E., Rollè, L., Pierre, F. D., & Dinuccio, E. (2022). Bio-Acidification of Cattle Slurry with Whey Reduces Gaseous Emission during Storage with Positive Effects on Biogas Production. Sustainability, 14(19), 12331. https://doi.org/10.3390/su141912331









