The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Description of Site
2.2. Agronomic and Physiological Analysis of Plant
2.2.1. Soil pH and Soil Electrical Conductivity (EC)
2.2.2. Sample Moisture Content Percentage and Field Capacity
2.2.3. Germination Index (GI)
2.2.4. Root–Shoot Ratio (RSR)
2.2.5. Water Use Efficiency (WUE)
2.2.6. Relative Water Content (RWC)
2.2.7. Total Biomass (TB)
2.2.8. Determination of Total Chlorophyll Content (TCC)
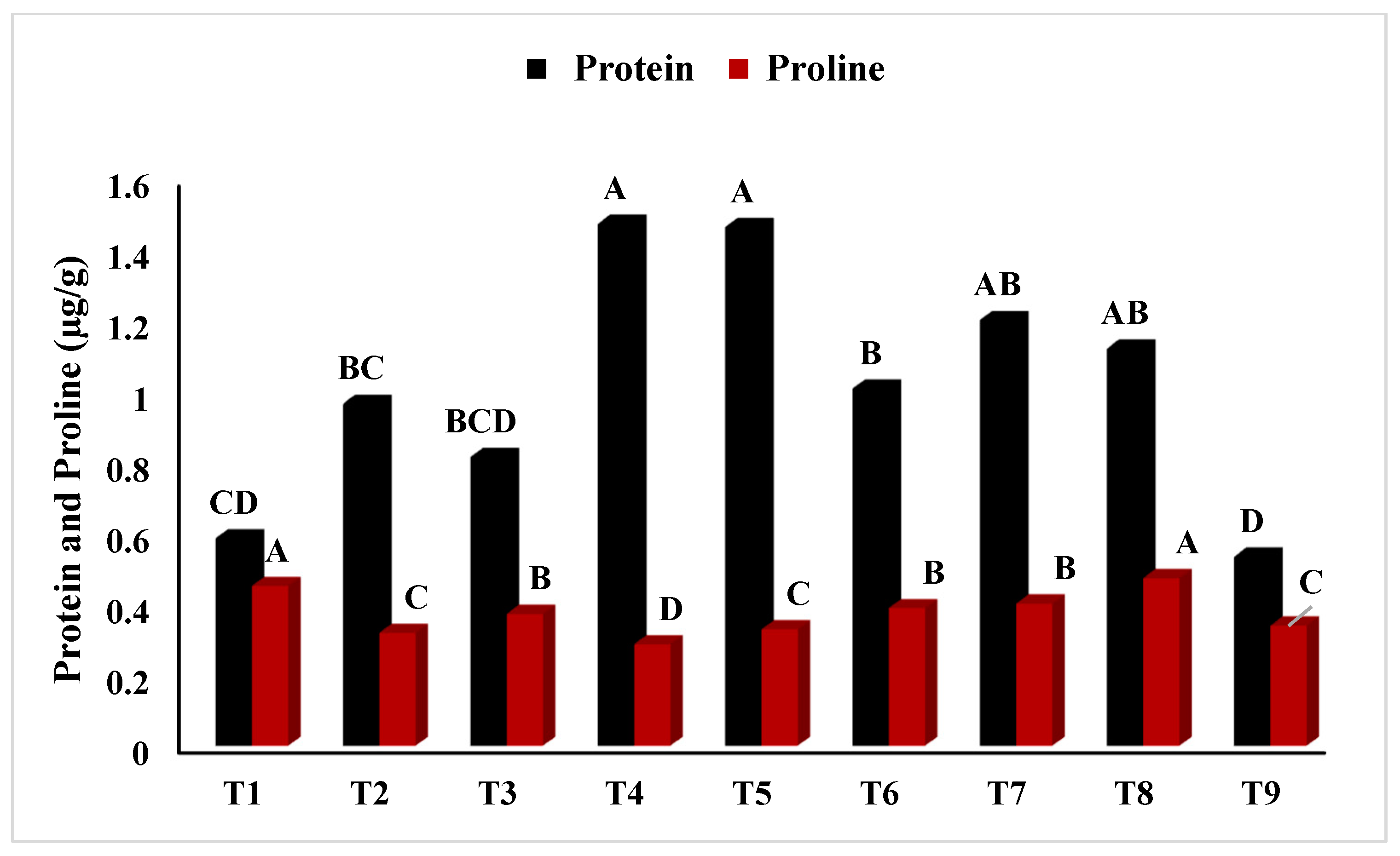
2.2.9. Approximation of Soluble Protein Content (SPC)
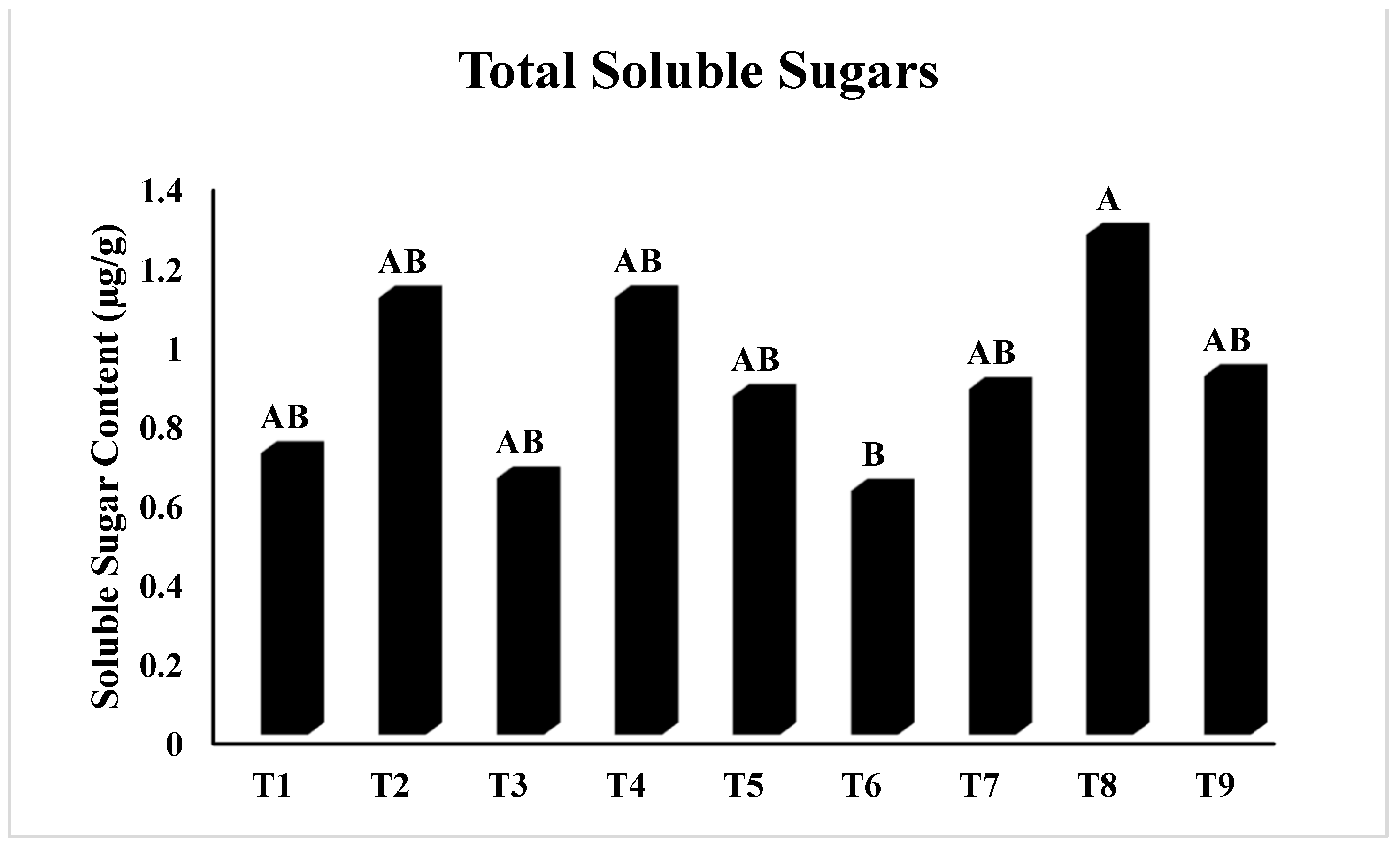
2.2.10. Measurement of Soluble Sugar Content (SSC)
2.2.11. Assay for Antioxidant Enzymes
2.2.12. Determination of Total Proline Content (TPC)
2.3. Statistical Analysis
3. Results
3.1. Agronomic Study
3.2. Physiological Study
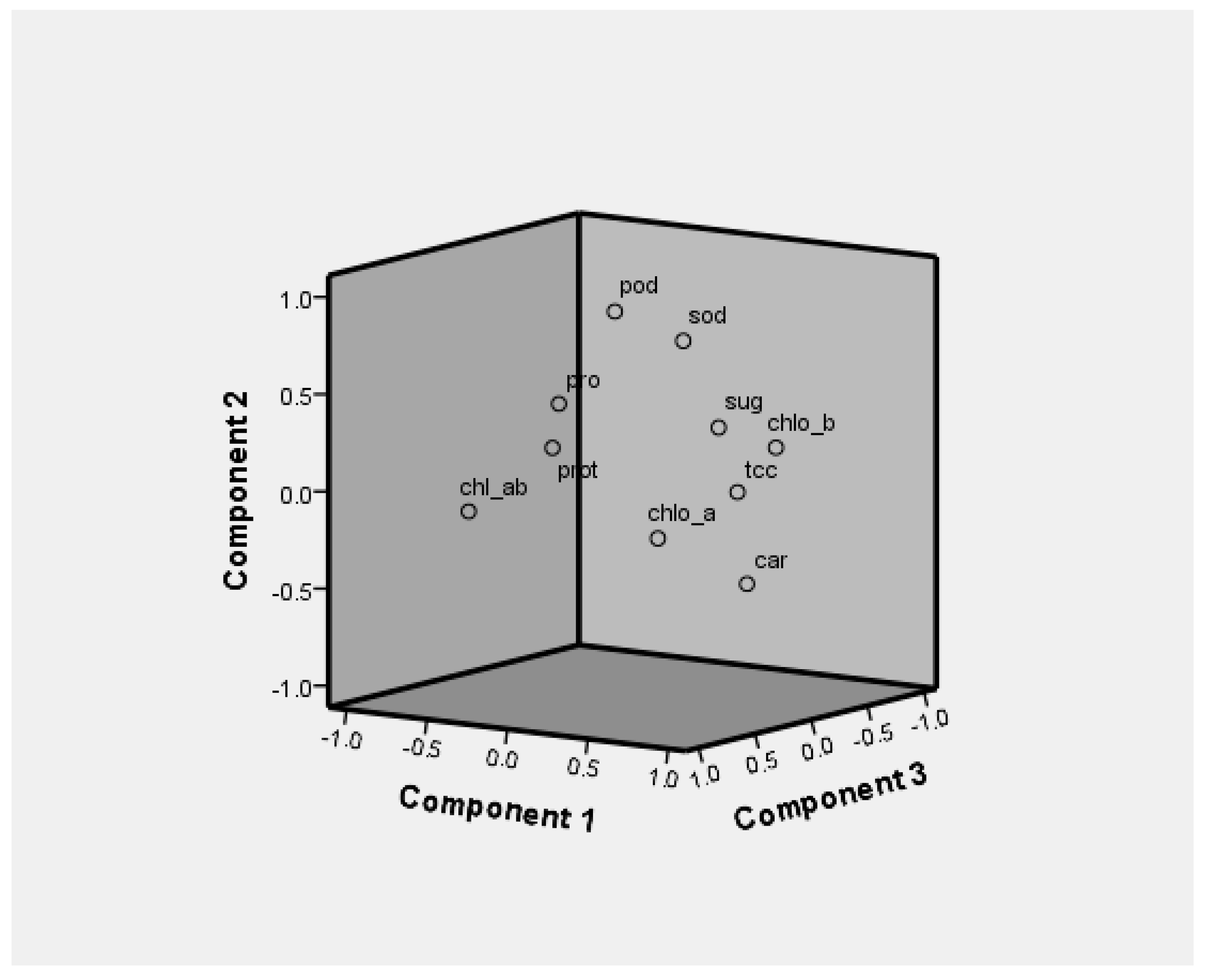
3.3. Principal Component Analysis
3.4. Physiological and Biochemical Component Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; López-Jurado, J.; García-López, J.V.; Mariscal, V.; Molina-Heredia, F.P.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Flowers, T.J.; et al. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve Response of Eight Crops to Soil Salinization and Climate Change Conditions. Agronomy 2021, 11, 1609. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Effects of heat stress on photosynthetic characteristics and chloroplast ultrastructure of a heat-sensitive and heat-tolerant cultivar of wucai (Brassica campestris L.). Acta Physiol. Plant. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Pandolfi, C.; Mancuso, S.; Shabala, S. Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ. Exp. Bot. 2012, 84, 44–51. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards saving freshwater: Halophytes as unconventional feedstuffs in livestock feed: A review. Environ. Sci. Pollut. Res. 2018, 25, 14397–14406. [Google Scholar] [CrossRef] [PubMed]
- Ivushkin, K.; Bartholomeus, H.; Bregt, A.K.; Pulatov, A. Satellite Thermography for Soil Salinity Assessment of Cropped Areas in Uzbekistan. Land Degrad. Dev. 2016, 28, 870–877. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Mohammed, A.H.M.; Zaki, L.M.; Mogazy, A.M. Physiological and Biochemical Effects of γ-Irradiation on Cowpea Plants (Vigna sinensis) under Salt Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 104–114. [Google Scholar] [CrossRef]
- Latef, A.A. Growth and some physiological activities of pepper (Capsicum annuum L.) in response to cadmium stress and mycorrhizal symbiosis. J. Agr. Sci. Tech. 2013, 37, 1437–1448. [Google Scholar]
- Mohammed, A. Physiological aspects of mungbean plant (Vigna radiata L. Wilczek) in response to salt stress and gib-berellic acid treatment. Res. J. Agr. Biol. Sci. 2007, 3, 200–213. [Google Scholar]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Iqbal, M. Identification of physiological and biochemical markers for salt (NaCl) stress in the seedlings of mungbean [Vigna radiata (L.) Wilczek] genotypes. Saudi J. Biol. Sci. 2018, 26, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Ikram-ul-Haq, A.A.K.; Azhar, F.; Ullah, E. Genetic basis of variation for salinity tolerance in okra (Abelmoschus esculentus L.). Pak. J. Bot 2010, 42, 1567–1581. [Google Scholar]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ali, M.A.; Pal, A.K. Effect of seed priming on germination behavior, oxidative stress and antioxidant enzyme activities in groundnut (Arachis hypogaea L.) under salinity stress. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 479–485. [Google Scholar]
- Nasri, N.; Kaddour, R.; Mahmoudi, H.; Baatour, O.; Bouraoui, N.; Lachaâl, M. The effect of osmopriming on germination, seedling growth and phosphatase activities of lettuce under saline condition. Afr. J. Biotechnol. 2011, 10, 14366–14372. [Google Scholar]
- Mouradi, M.; Bouizgaren, A.; Farissi, M.; Makoudi, B.; Kabbadj, A.; Very, A.-A.; Sentenac, H.; Qaddoury, A.; Ghoulam, C. Osmopriming improves seeds germination, growth, antioxidant responses and membrane stability during early stage of Moroccan alfalfa populations under water deficit. Chil. J. Agric. Res. 2016, 76, 265–272. [Google Scholar] [CrossRef][Green Version]
- Siri, B.; Vichitphan, K.; Kaewnaree, P.; Vichitphan, S.; Klanrit, P. Improvement of quality, membrane integrity and anti-oxidant systems in sweet pepper (’Capsicum annuum’Linn.) seeds affected by osmopriming. Aust. J. Crop Sci. 2013, 7, 2068–2073. [Google Scholar]
- Piwowarczyk, B.; Tokarz, B.; Kamińska, I. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell, Tissue Organ Cult. (PCTOC) 2015, 124, 227–240. [Google Scholar] [CrossRef]
- Nascimento, W.M.; Huber, D.J.; Cantliffe, D.J. Carrot seed germination and ethylene production at high temperature in response to seed osmopriming. Hortic. Bras. 2013, 31, 554–558. [Google Scholar] [CrossRef]
- De Souza, M.O.; Pelacani, C.R.; Willems, L.A.; De Castro, R.D.; Hilhorst, H.W.; Ligterink, W. Effect of osmopriming on germination and initial growth of Physalis angulata L. under salt stress and on expression of associated genes. Anais Acad. Bras. Ciênc. 2016, 88, 503–516. [Google Scholar] [CrossRef]
- Singh, A.; Dahiru, R.; Musa, M.; Sani Haliru, B. Effect of Osmopriming duration on germination, emergence, and early growth of Cowpea (Vigna unguiculata (L.) Walp.) in the Sudan Savanna of Nigeria. Int. J. Agron. 2014, 2014. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.N.; Ullah, H.; Basit, A.; Razzaq, A.; Ahmad, M.; Ozdemir, F. A comparative assessment of proximate and elemental composition six weedy grasses for their potential use as fodder. Prog. Nutr. 2018, 20, 182–190. [Google Scholar]
- Rehman, I.; Rahim, H.U.; Rafiq, S. Comparative analysis of soil physio-chemical properties of two different districts Peshawar and Swabi, KP, Pakistan. Int. J. Environ. Sci. Nat. Resour. 2017, 7, 58–62. [Google Scholar]
- Warwate, S.; Kandoliya, U.; Bhadja, N.; Golakiya, B. The effect of seed priming with plant growth promoting rhizobacteria (PGPR) on growth of Coriander (Coriandrum sativum L.) seedling. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1926–1934. [Google Scholar]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Sumaira Afridi, M.S.; Dawoud, T.M.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; Alkhalifah, D.H.M.; et al. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- McLean, E. Soil pH and lime requirement. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; Volume 9, pp. 199–224. [Google Scholar]
- Kader, M. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. Royal Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Bina, F.; Bostani, A. Effect of Salinity (NaCl) stress on germination and early seedling growth of three medicinal plant species. Adv. Life Sci. 2017, 4, 77–83. [Google Scholar]
- Chuyong, G.B.; Acidri, T. Light and moisture levels affect growth and physiological parameters differently in Faidherbia albida (Delile) A. Chev. seedlings. Acta Physiol. Plant. 2017, 39, 117. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; et al. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Mehmood, S.; Khatoon, Z.; Amna Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021, 1971654. [Google Scholar] [CrossRef]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Javed, M.A.; Ahmad, S.; Afridi, M.S.; Sumaira Nadeem, M.; Khan, A.U.R.; Malik, A.; Ullah, A.; Alwahibi, M.S.; et al. Bacterial-mediated salt tolerance in maize: Insights into plant growth promotion, antioxidant defense system, oxidative stress, and surfactant production. Front. Plant Sci. 2022, 13, 978291. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; Amaral Júnior, A.T.; et al. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Ali, B.; Rasheed, R.; Ashraf, M.A.; Aziz, H.; Ercisli, S.; Riaz, S.; Elsharkawy, M.M.; Hussain, I.; et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Yasin, G.; Mumtaz, S.; Qureshi, F.F.; Ali, B.; Ercisli, S.; Alhag, S.K.; Ahmed, A.E.; Vodnar, D.C.; et al. Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 2022, 13, 973740. [Google Scholar] [CrossRef]
- Ma, J.; Ali, S.; Saleem, M.H.; Mumtaz, S.; Yasin, G.; Ali, B.; Al-Ghamdi, A.A.; Elshikh, M.S.; Vodnar, D.C.; Marc, R.A.; et al. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 13, 983156. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alhag, S.K.; Dablool, A.S.; Ahmed, A.E.; Alghamdi, S.; Ali, B.; Al-Saeed, F.A.; Saleem, M.H.; Poczai, P. Manufactured Nano-Objects Confer Viral Protection against Cucurbit Chlorotic Yellows Virus (CCYV) Infecting Nicotiana benthamiana. Microorganisms 2022, 10, 1837. [Google Scholar] [CrossRef]
- Solanki, M.K.; Solanki, A.C.; Rai, S.; Srivastava, S.; Kashyap, B.K.; Divvela, P.K.; Kumar, S.; Yandigeri, M.S.; Kashyap, P.L.; Shrivastava, A.K.; et al. Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front. Microbiol. 2022, 13, 990850. [Google Scholar] [CrossRef]
- Naz, R.; Khan, M.K.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y.; et al. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz. J. Biol. 2022, 84, e264473. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, S.; Hafeez, A.; Khan, M.N.; Javed, M.A.; Ali, B.; Din, I.U.; Bangash, S.A.K.; Wahab, S.; Wahid, N.; et al. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84, e264642. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Ali, A.; Saleem, M.H.; Ameer, A.; Hafeez, A.; Alharbi, K.; Ezzat, A.; Khan, A.; Jamil, M.; Farid, G. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Farooq, T.H.; Rafay, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-physiological growth performance and phytoremediation capabilities of selected xerophyte grass species toward Cr and Pb stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Mamun, M.A.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Amna Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Cham, Switzerland, 2021; pp. 345–371. [Google Scholar]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Al-Jouni, S.K.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. South Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Hussain, S.Q.; Rasheed, M.; Saleem, M.H.; Ahmed, Z.I.; Hafeez, A.; Jilani, G.; Alamri, S.; Hashem, M.; Ali, S. Salt tolerance in maize with melatonin priming to achieve sustainability in yield on salt affected soils. Pak. J. Bot. 2021, 55, 1. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the Impact of Water Potential and Temperature on Seed Germination of Wheat (Triticum aestivum L.) via Hydrothermal Time Model. Life 2022, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Arif, M.; Ahmad, M.; Shah, W.A.; Romman, M.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ishaq, M.; Shah, W.A.; Adnan, M.; Fahad, S.; Saleem, M.H.; Khan, F.U.; Mussarat, M.; Khan, S.; Ali, B.; et al. Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 2022, 14, 7669. [Google Scholar] [CrossRef]
- Sani, B.; Jodaeian, V. The Role of Thermo Priming on Improving Seedling Production Technology (Ispt) in Soybean [Glycine max (L.) Merrill] Seeds. Int. J. Agric. Biosyst. Eng. 2015, 9, 753–756. [Google Scholar]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, S.; Nafees, M. Effect of osmopriming and thermopriming on amelioration of mercuric chloride stress tolerance in mungbean (Vigna radiata L.). Plant Physiol. Rep. 2020, 25, 516–528. [Google Scholar] [CrossRef]
- Abdolahpour, M.; Lotfi, R. Seed priming affected physiology and grain yield of chickpea under salt stress. J. Biodivers. Environ. Sci 2014, 5, 442–446. [Google Scholar]
- Maroufi, K.; Farahani, H.A.; Moradi, O. Thermo priming influence on seedling production in wheat (Triticum Aestivum L.). Adv. Environ. Biol. 2011, 3664–3668. [Google Scholar]
- Moreira, G.S.; Jillett, J.B.; Vernberg, W.B.; Weinrich, M. The combined effects of temperature and salinity on the survival of Euterpina acutifrons (Dana) (Copepoda, Harpacticoida) from the New Zealand and Brazilian coasts. J. Plankton Res. 1982, 4, 85–91. [Google Scholar] [CrossRef]
- Farahmandfar, E.; Shirvan, M.B.; Sooran, S.A.; Hoseinzadeh, D. Effect of seed priming on morphological and physiological parameters of fenugreek seedlings under salt stress. Int. J. Agric. Crop Sci. 2013, 5, 811. [Google Scholar]
- Mirlotfi, A.; Bakhtiari, S.; Bazrgar, A.B. Effect of seed priming on germination and seedling traits of Marigold (Calendula officinalis) at saline condition. Biol. Forum –Int. J. 2015, 7, 1626–1630. [Google Scholar]
- El-Saifi, S.; Ahmed, H.; Hasan, S.M.; Morsi, M.; El-Shatoury, R.S. Seed priming influences seed germination and seedling growth of tomato under different salinity levels. J. Plant Prod. 2010, 1, 159–170. [Google Scholar] [CrossRef]
- Aloui, H.; Souguir, M.; Latique, S.; Hannachi, C. Germination and growth in control and primed seeds of pepper as affected by salt stress. Cercet. Agron. În Mold. 2014, 47, 83–95. [Google Scholar] [CrossRef]
- Mohamed, I.A.; Shalby, N.; El-Badri, A.M.A.; Saleem, M.H.; Khan, M.N.; Nawaz, M.A.; Qin, M.; Agami, R.A.; Kuai, J.; Wang, B. Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants. Agronomy 2020, 10, 1186. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Tanaka, K.; Eneji, A.E.; Eltayeb, A.E.; Siddig, K.E. Salt-induced stress effects on biomass, photosynthetic rate, and reactive oxygen species-scavenging enzyme accumulation in common bean. J. Plant Nutr. 2009, 32, 837–854. [Google Scholar] [CrossRef]
- Esfandiari, E.; Shekari, F.; Shekari, F.; Esfandiari, M. The effect of salt stress on antioxidant Enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Horti Agrobot. Cluj-Napoca 2007, 35, 48. [Google Scholar]
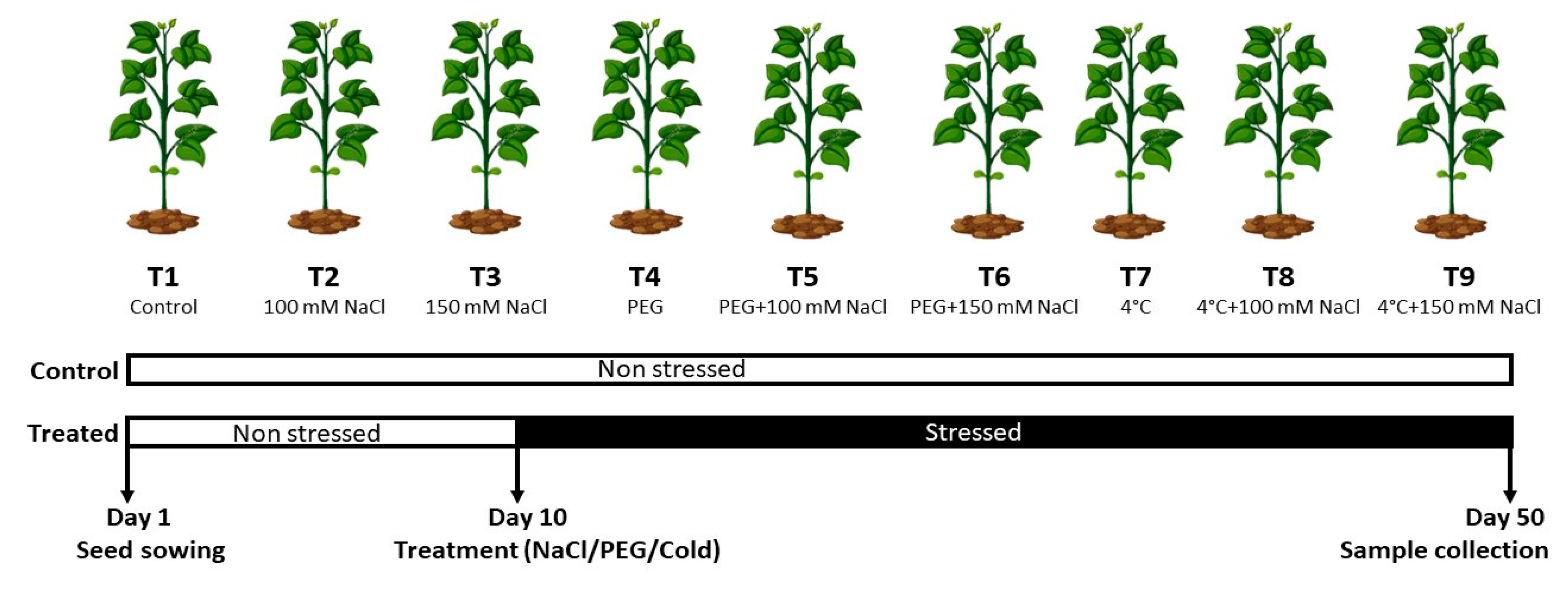
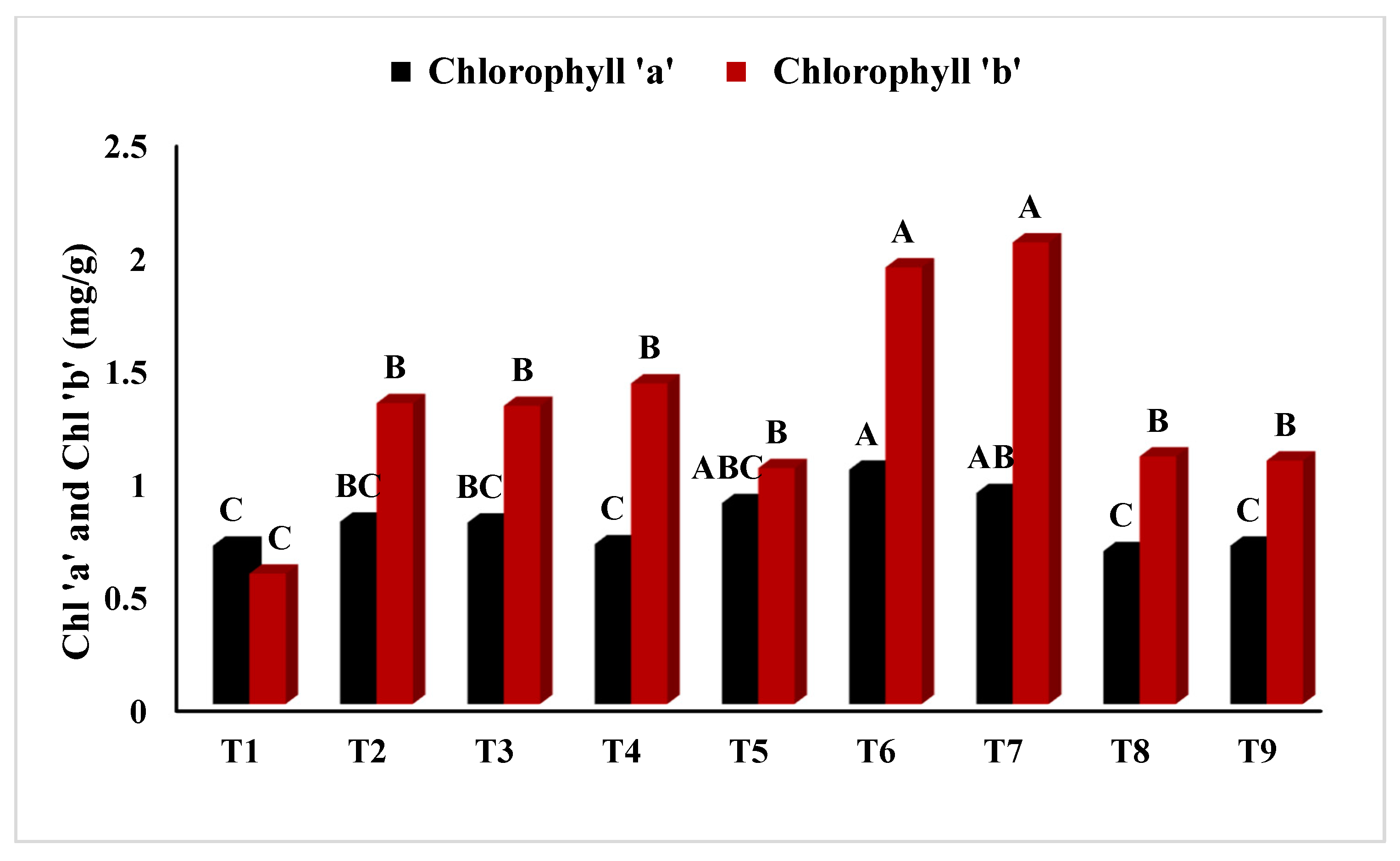
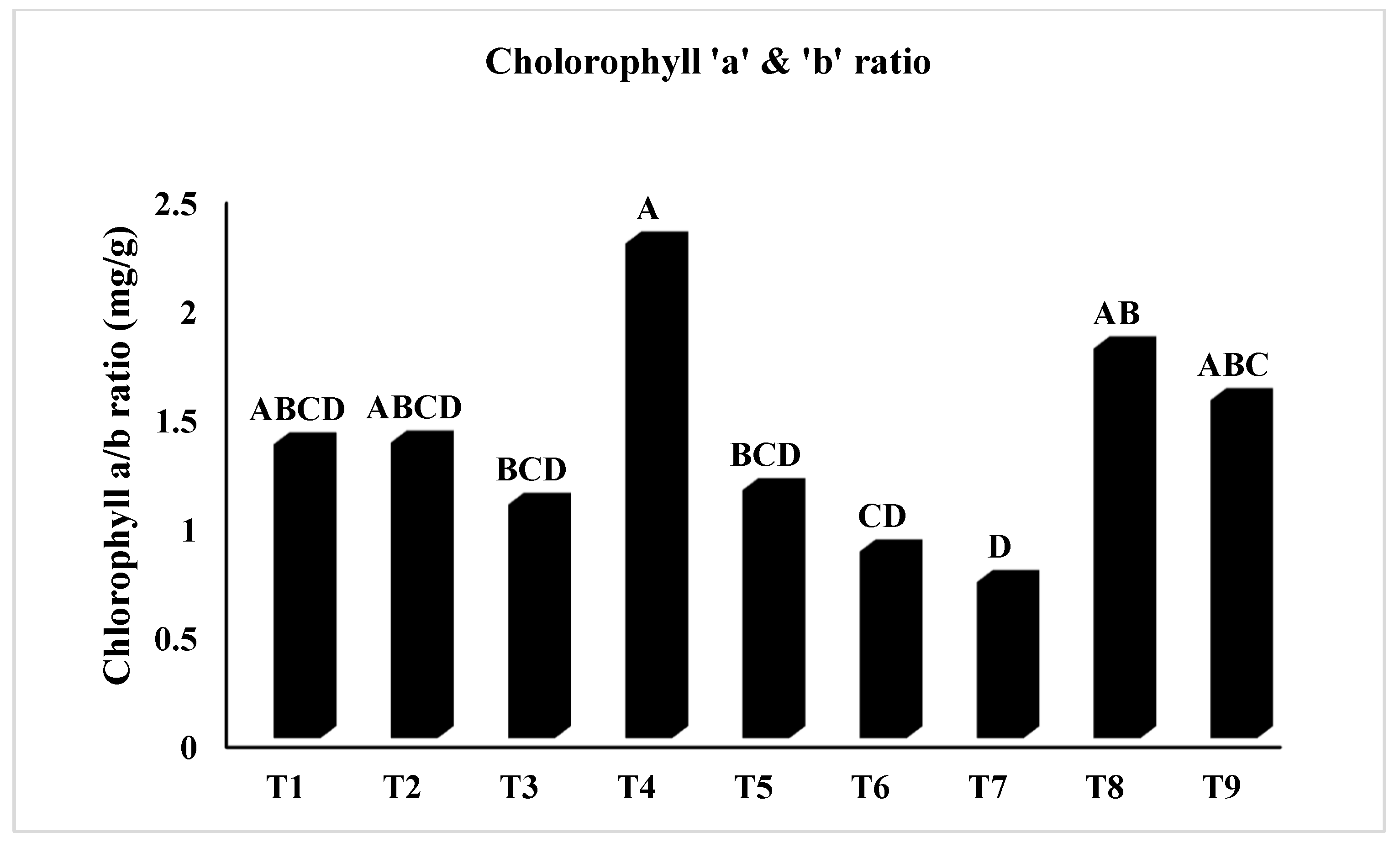
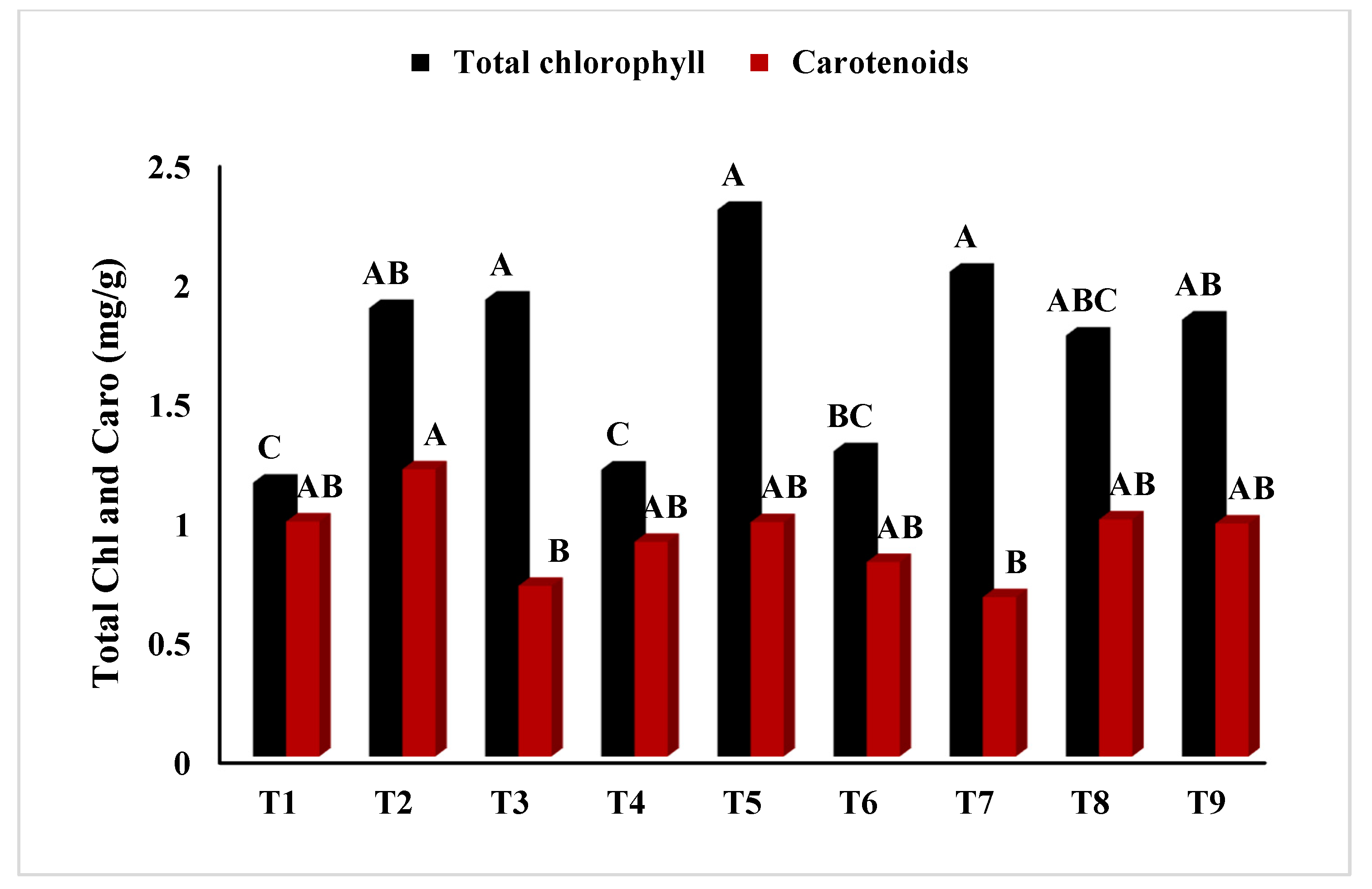

| Treatment | Shoot Fresh Weight | Shoot Dry Weight | Shoot Moisture Content | Root Fresh Weight | Root Dry Weight | Root Moisture Content |
|---|---|---|---|---|---|---|
| T1 | 0.45 ± 0.1 b | 0.07 ± 0.02 c | 83.7 ± 3.0 ab | 0.04 ± 0.01 b | 0.013 ± 0 ab | 64.4 ± 17 bc |
| T2 | 0.46 ± 0.1 b | 0.06 ± 0.01 d | 86.9 ± 0.5 ab | 0.06 ± 0.04 ab | 0.012 ± 0 ab | 75.4 ± 13 ab |
| T3 | 0.47 ± 0.1 b | 0.07 ± 0.03 c | 85.7 ± 2.6 ab | 0.07 ± 0.04 ab | 0.019 ± 0 ab | 69.7 ± 17 abc |
| T4 | 0.61 ± 0.2 ab | 0.09 ± 0.06 b | 86.5 ± 4.8 ab | 0.10 ± 0.03 a | 0.019 ± 0 a | 78.0 ± 16 ab |
| T5 | 0.81 ± 0.0 a | 0.08 ± 0.04 c | 89.9 ± 5.8 a | 0.05 ± 0.02 ab | 0.017 ± 0 ab | 72.1 ± 13 abc |
| T6 | 0.47 ± 0.2 b | 0.06 ± 0.04 d | 87.0 ± 5.8 ab | 0.05 ± 0.02 b | 0.017 ± 0 b | 82.6 ± 10 a |
| T7 | 0.51 ± 0.1 ab | 0.08 ± 0.02 b | 82.2 ± 0.7 b | 0.08 ± 0.02 ab | 0.015 ± 0 ab | 81.0 ± 5 a |
| T8 | 0.67 ± 0.1 ab | 0.10 ± 0.02 b | 84.9 ± 1.1 ab | 0.09 ± 0.03 ab | 0.016 ± 0 ab | 77.1 ± 18 ab |
| T9 | 0.72 ± 0.1 ab | 0.12 ± 0.05 a | 82.9 ± 4.6 b | 0.05 ± 0.01 b | 0.014 ± 0 ab | 72.4 ± 8 abc |
| Treatment | Leaf Fresh Weight | Leaf Dry Weight | Leaf Moisture Content | Soil Dry Weight | Soil Moisture Content | Root Shoot Ratio |
|---|---|---|---|---|---|---|
| T1 | 0.12 ± 0.06 ab | 0.017 ± 0 b | 84.6 ± 4 a | 8.10 ± 0.88 b | 19.0 ± 8.8 ab | 0.36 ± 0.2 bc |
| T2 | 0.06 ± 0.03 b | 0.018 ± 0 ab | 66.7 ± 5 b | 8.33 ± 0.58 a | 16.6 ± 5.8 abc | 0.24 ± 0.1 c |
| T3 | 0.09 ± 0.05 ab | 0.021 ± 0 ab | 69.7 ± 22 ab | 8.13 ± 0.66 b | 18.6 ± 6.6 ab | 0.39 ± 0.1 bc |
| T4 | 0.14 ± 0.06 ab | 0.027 ± 0 a | 79.8 ± 4 ab | 8.10 ± 0.95 b | 19.0 ± 9.5 ab | 0.52 ± 0.1 ab |
| T5 | 0.17 ± 0.02 a | 0.026 ± 0 ab | 85.3 ± 2 a | 7.63 ± 1.01 c | 23.6 ± 9.9 a | 0.41 ± 0.1 bc |
| T6 | 0.13 ± 0.07 ab | 0.021 ± 0 ab | 82.2 ± 6 ab | 8.33 ± 0.49 a | 16.6 ± 4.9 abc | 0.41 ± 0.1 bc |
| T7 | 0.12 ± 0.03 ab | 0.024 ± 0 ab | 78.7 ± 3 ab | 8.40 ± 0.78 a | 16.0 ± 7.8 abc | 0.57 ± 0.1 ab |
| T8 | 0.12 ± 0.03 ab | 0.023 ± 0 ab | 79.8 ± 10 ab | 8.66 ± 0.41 a | 13.3 ± 4.1 bc | 0.38 ± 0.1 bc |
| T9 | 0.10 ± 0.02 ab | 0.023 ± 0 ab | 77.0 ± 3 ab | 8.36 ± 0.90 a | 16.3 ± 9.0 abc | 0.67 ± 0.1 a |
| Treatment | Leaf Area Index | Seed Vigor Index | Germination Index | Leaf Area | Relative Water Content | Water Use Efficiency | Total Biomass |
|---|---|---|---|---|---|---|---|
| T1 | 0.84 ± 0.07 ab | 2057 ± 410 cd | 149.6 ± 5.20 b | 4.75 ab | 75 ± 9.2 a | 91 ± 21 ab | 0.10 ± 0.02 d |
| T2 | 0.69 ± 0.35 b | 1641 ± 200 d | 150.0 ± 4.35 c | 3.91 b | 53 ± 16 b | 98 ± 29 ab | 0.09 ± 0.02 d |
| T3 | 0.75 ± 0.44 ab | 2053 ± 300 cd | 144.0 ± 2.51 c | 4.25 ab | 63 ± 23 ab | 88 ± 47 abc | 0.11 ± 0.04 c |
| T4 | 1.23 ± 0.30 ab | 2435 ± 260 bc | 148.3 ± 4.17 c | 7.11 ab | 77 ± 7.4 a | 74 ± 32 bc | 0.13 ± 0.06 b |
| T5 | 1.00 ± 0.35 ab | 3126 ± 580 a | 150.0 ± 6.00 b | 5.66 ab | 83 ± 1.8 a | 86 ± 56 abc | 0.12 ± 0.06 c |
| T6 | 1.01 ± 0.20 ab | 2360 ± 400 bc | 156.3 ± 6.98 b | 5.75 ab | 76 ± 11 a | 99 ± 67 a | 0.09 ± 0.04 d |
| T7 | 0.92 ± 0.49 ab | 2292 ± 270 bcd | 154.6 ± 1.76 a | 5.25ab | 75 ± 5.6 a | 71 ± 16 bc | 0.13 ± 0.02 c |
| T8 | 0.64 ± 0.25 b | 2677 ± 400 abc | 190.6 ± 8.76 a | 3.66 b | 75 ± 7.9 a | 61 ± 10 c | 0.14 ± 0.02 b |
| T9 | 1.47 ± 0.83 a | 2846 ± 530 ab | 211.3 ± 0.88 a | 8.33 a | 72 ± 5.1 ab | 56 ± 25 c | 0.16 ± 0.05 a |
| Correlation Analysis between Salinity and Vigna | |||||||||
| Chlo “a” | Chlo “b” | TCC | Chlo a/b ratio | CC | SSC | SPC | TPC | POD | SOD |
| r = 0.365 | r = 0.077 | r = 0.255 | r = 0.136 | r = 0.053 | r = 0.127 | r = 0.265 | r = 0.033 | r = 0.500 *** | r = 0.242 |
| R2 = 0.133 | R2 = 0.066 | R2 = 0.065 | R2 = 0.018 | R2 = 0.003 | R2 = 0.016 | R2 = 0.070 | R2 = 0.001 | R2 = 0.250 | R2 = 0.059 |
| Correlation Analysis between Priming and Vigna | |||||||||
| Chlo “a” | Chlo “b” | TCC | Chlo a/b ratio | CC | SSC | SPC | TPC | POD | SOD |
| r = 0.266 | r = 0.050 | r = 0.182 | r = 0.153 | r = 0.026 | r = 0.196 | r = 0.330 | r = 0.034 | r = 0.483 *** | r = 0.214 |
| R2 = 0.071 | R2 = 0.02 | R2 = 0.033 | R2 = 0.024 | R2 = 0.001 | R2 = 0.038 | R2 = 0.109 | R2 = 0.001 | R2 = 0.234 | R2 = 0.046 |
| Traits | Eigen Values | Variance (%) | Components | |||
|---|---|---|---|---|---|---|
| Individual | Cumulative | PC1 | PC2 | PC3 | ||
| Chlo “a” | 2.867 | 28.674 | 28.674 | 0.633 | 0.128 | 0.679 |
| Chlo ”b” | 1.922 | 19.225 | 47.899 | 0.877 | 0.263 | −0.021 |
| TCC | 1.585 | 15.853 | 63.752 | 0.912 | 0.094 | 0.37 |
| Chlo a/b ratio | 1.138 | 11.376 | 75.129 | −0.632 | −0.135 | 0.552 |
| CC | 0.989 | 9.895 | 85.023 | 0.533 | 0.507 | 0.255 |
| SSC | 0.64 | 6.402 | 91.425 | 0.34 | 0.274 | 0.279 |
| SPC | 0.443 | 4.431 | 95.856 | −0.196 | 0.218 | 0.431 |
| TPC | 0.28 | 2.798 | 98.654 | −0.155 | 0.449 | 0.432 |
| POD | 0.135 | 1.346 | 100 | −0.061 | 0.881 | 0.07 |
| SOD | 3.868 | 3.868 | 100 | 0.034 | 0.672 | 0.402 |
| Traits | Chlo “a” | Chlo “b” | TCC | Chlo a/b Ratio | CC | SSC | SPC | TPC | POD | SOD |
|---|---|---|---|---|---|---|---|---|---|---|
| Chlo “a” | 1 | |||||||||
| Chlo “b” | 0.394 * | 1 | ||||||||
| TCC | 0.814 ** | 0.855 ** | 1 | |||||||
| Chlo a/b ratio | 0.067 | 0.645 ** | 0.369 | 1 | ||||||
| CC | 0.253 | 0.224 | 0.284 | 0.288 | 1 | |||||
| SSC | 0.086 | 0.173 | 0.158 | 0.288 | 0.257 | 1 | ||||
| SPC | 0.025 | 0.036 | 0.009 | 0.322 | 0.23 | 0.044 | 1 | |||
| TPC | 0.15 | 0.126 | 0.005 | 0.132 | 0.344 | 0.031 * | 0.11 | 1 | ||
| POD | 0.124 | 0.148 | 0.023 | 0.01 | 0.332 | 0.24 | 0.265 | 0.385 * | 1 | |
| SOD | 0.22 | 0.188 | 0.006 | 0.172 | 0.198 | 0.171 | 0.045 | 0.004 | 0.431 * | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. https://doi.org/10.3390/su141912924
Ali S, Ullah S, Khan MN, Khan WM, Razak SA, Wahab S, Hafeez A, Khan Bangash SA, Poczai P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability. 2022; 14(19):12924. https://doi.org/10.3390/su141912924
Chicago/Turabian StyleAli, Saqib, Sami Ullah, Muhammad Nauman Khan, Wisal Muhammad Khan, Sarah Abdul Razak, Sana Wahab, Aqsa Hafeez, Sajid Ali Khan Bangash, and Peter Poczai. 2022. "The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L." Sustainability 14, no. 19: 12924. https://doi.org/10.3390/su141912924
APA StyleAli, S., Ullah, S., Khan, M. N., Khan, W. M., Razak, S. A., Wahab, S., Hafeez, A., Khan Bangash, S. A., & Poczai, P. (2022). The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability, 14(19), 12924. https://doi.org/10.3390/su141912924








