Time to Conquer Fungal Infectious Diseases: Employing Nanoparticles as Powerful and Versatile Antifungal Nanosystems against a Wide Variety of Fungal Species
Abstract
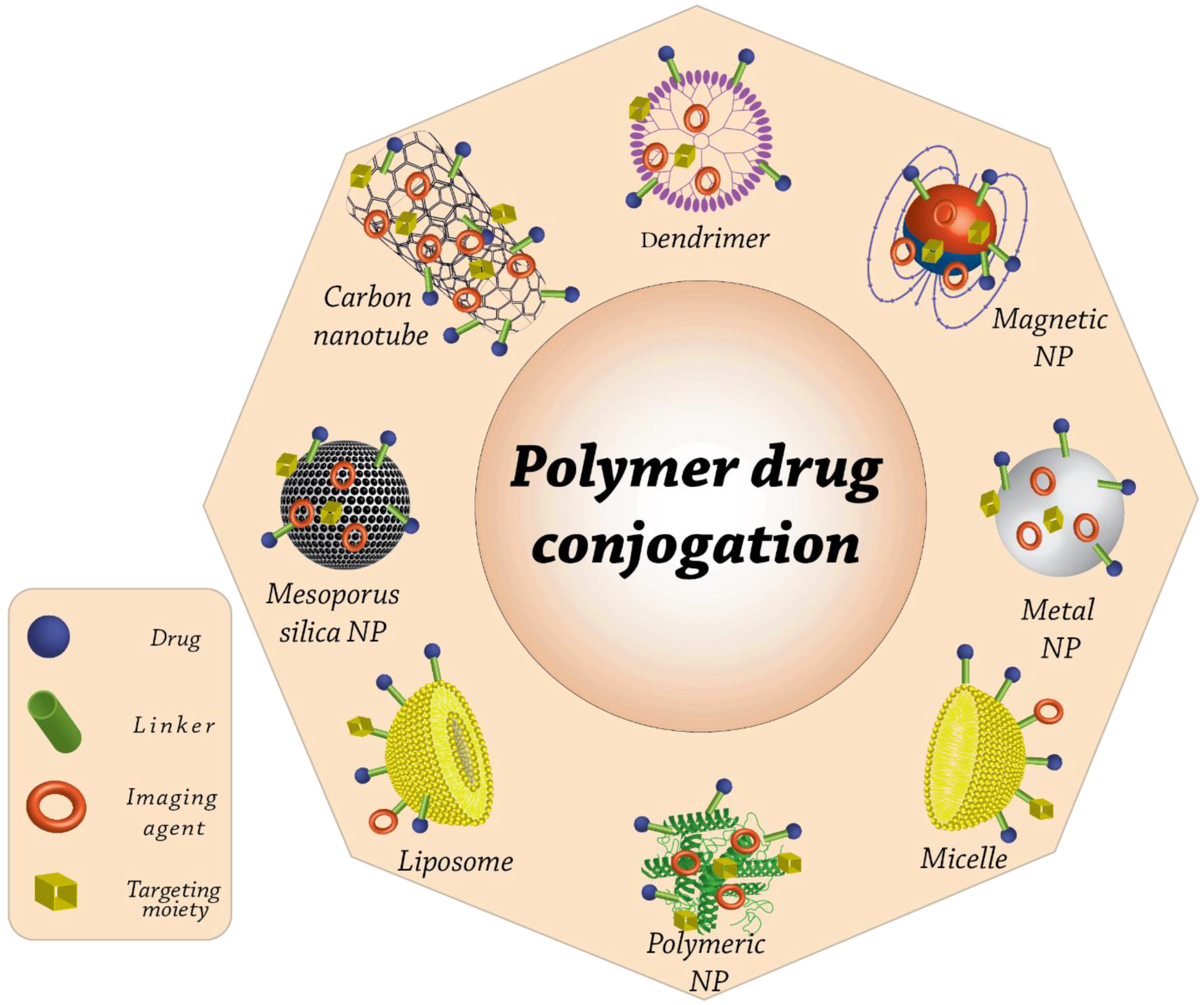
:1. Introduction
2. Fungal Infection and Biofilm Formation
3. Preventive and Therapeutic Application of Antifungal Nanomaterials
3.1. Metal Oxide and Non-Metal Oxide Nanoparticles
3.2. Polymeric Nanoparticles
3.3. Carbon Nanoparticles
3.4. Nanostructured Lipid Carriers
3.5. ZnO Quantum Dots
3.6. Peptides That Are Antimicrobial and Have Efficacy against Fungi
Synthetic Antimicrobial Peptides Having Antifungal Properties
3.7. Nanofibers
3.8. Antifungal Nanocomposites That Have SMOOTH Surfaces, Which Helps Reduce the Attachment of Microorganisms
4. Toxicity Effect of Nanoparticles
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Consent to Participate
Consent to Publish
References
- Vajargah, M.F.; Hashemi, G.; Bibak, M.; Yalsuyi, A.M. The effect of vitamin c-fortified artemia on growth and survival of sepia pharaonis larvae. J. Environ. Treat. Tech. 2021, 9, 815–818. [Google Scholar]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal diseases as neglected pathogens: A wake-up call to public health officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garayemi, S.; Raeisi, F. Graphene Oxide as a Docking Station for Modern Drug Delivery System. by Ulva lactuca species study its antimicrobial, anti-fungal and anti-Blood cancer activity. Adv. Appl. NanoBio-Technol. 2020, 1, 53–62. [Google Scholar]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, rv13–rv165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeny, P.T.; Nessel, T.A.; Zito, P.M. Antifungal Antibiotics; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Ansarifard, E.; Zareshahrabadi, Z.; Sarafraz, N.; Zomorodian, K. Evaluation of antimicrobial and antibiofilm activities of copper oxide nanoparticles within soft denture liners against oral pathogens. Bioinorg. Chem. Appl. 2021, 2021, 9939275. [Google Scholar] [CrossRef]
- Hejazi, M.; Zareshahrabadi, Z.; Ashayeri, S.; Saharkhiz, M.J.; Iraji, A.; Alishahi, M.; Zomorodian, K. Characterization and Physical and Biological Properties of Tissue Conditioner Incorporated with Carum copticum L. BioMed. Res. Int. 2021, 2021, 5577760. [Google Scholar] [CrossRef]
- Tseng, A.; Seet, J.; Phillips, E.J. The evolution of three decades of antiretroviral therapy: Challenges, triumphs and the promise of the future. Br. J. Clin. Pharmacol. 2015, 79, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [Green Version]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that infect humans. Microbiol. Spectr. 2017, 5, a019273. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Meng, G. Pathogenic fungal infection in the lung. Front. Immunol. 2019, 10, 1524. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Marcos, C.M.; de Oliveira, H.C.; de Melo, W.d.C.M.A.; Da Silva, J.D.F.; Assato, P.A.; Scorzoni, L.; Rossi, S.A.; de Paula e Silva, A.C.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Anti-immune strategies of pathogenic fungi. Front. Cell. Infect. Microbiol. 2016, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wolf, A.K.; Thusek, S.; Heinekamp, T.; Bromley, M.; Krappmann, S.; Beilhack, A. Direct Visualization of Fungal Burden in Filamentous Fungus-Infected Silkworms. J. Fungi 2021, 7, 136. [Google Scholar] [CrossRef]
- Góralska, K.; Blaszkowska, J.; Dzikowiec, M. Neuroinfections caused by fungi. Infection 2018, 46, 443–459. [Google Scholar] [CrossRef] [Green Version]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, R.F.; Zaoutis, T.E.; Seo, S.K. Antifungal stewardship considerations for adults and pediatrics. Virulence 2017, 8, 658–672. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez, M. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Cornely, O.A.; Schmidt-Hieber, M.; Alakel, N.; Boell, B.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Heinz, W.J.; Hentrich, M.; et al. Treatment of invasive fungal diseases in cancer patients—Revised 2019 Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Mycoses 2020, 63, 653–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.A.; Roberts, M.S.; Semark, A.; Udy, A.A.; Kirkpatrick, C.M.J.; Paterson, D.L.; Roberts, M.J.; Kruger, P.; Lipman, J. Antibiotic dosing in the ‘at risk’ critically ill patient: Linking pathophysiology with pharmacokinetics/pharmacodynamics in sepsis and trauma patients. BMC Anesthesiol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz Codina, M.; Zeitlinger, M. Biomarkers Predicting Tissue Pharmacokinetics of Antimicrobials in Sepsis: A Review. Clin. Pharmacokinet. 2022, 61, 593–617. [Google Scholar] [CrossRef]
- Dzierba, A.L.; Abrams, D.; Brodie, D. Medicating patients during extracorporeal membrane oxygenation: The evidence is building. Crit. Care 2017, 21, 66. [Google Scholar] [CrossRef] [Green Version]
- Wildschut, E.D.; van Saet, A.; Pokorna, P.; Ahsman, M.J.; van den Anker, J.N.; Tibboel, D. The impact of extracorporeal life support and hypothermia on drug disposition in critically ill infants and children. Pediatric Clin. 2012, 59, 1183–1204. [Google Scholar] [CrossRef] [Green Version]
- Pea, F. Plasma Pharmacokinetics of Antimicrobial Agents in Critically Ill Patients. Curr. Clin. Pharmacol. 2013, 8, 5–12. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1669. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Aberoumandi, S.M.; Mohammadhosseini, M.; Abasi, E.; Saghati, S.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; Davaran, S. An update on applications of nanostructured drug delivery systems in cancer therapy: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1058–1068. [Google Scholar] [CrossRef]
- Mohammadian, F.; Eatemadi, A. Drug loading and delivery using nanofibers scaffolds. Artif. Cells Nanomed. Biotechnol. 2017, 45, 881–888. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 381, 587012. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Review nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. Recent Developments in the Application of Polymeric Nanoparticles as Drug Carriers. Adv. Clin. Exp. Med. 2015, 24, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly (lactic acid)/poly (lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Wang, W.; Zhang, X.; Zhang, J. Research progress of biodegradable polymers in repairing Achilles tendon injury. Front. Mater. 2022, 2022, 6. [Google Scholar]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.M.; Mao, H.Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Faustino, C.; Pinheiro, L. Lipid Systems for the Delivery of Amphotericin B in Antifungal Therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Funari, R.; Shen, A.Q. Detection and Characterization of Bacterial Biofilms and Biofilm-Based Sensors. ACS Sens. 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Tits, J.; Cammue, B.P.A.; Thevissen, K. Combination Therapy to Treat Fungal Biofilm-Based Infections. Int. J. Mol. Sci. 2020, 21, 8873. [Google Scholar] [CrossRef]
- Zara, G.; Budroni, M.; Mannazzu, I.; Fancello, F.; Zara, S. Yeast biofilm in food realms: Occurrence and control. World J. Microbiol. Biotechnol. 2020, 36, 134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [Green Version]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talaiekhozani, A.; Rezania, S.; Kim, K.-H.; Sanaye, R.; Amani, A.M. Recent advances in photocatalytic removal of organic and inorganic pollutants in air. J. Clean. Prod. 2021, 278, 123895. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; de Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Pires, R.H.; Martinez, L.R.; Mendes-Giannini, M.J.S.; Stoianoff, M.A.R. Editorial: Pathogenesis of Fungal Biofilms in Different Environmental Conditions and Clinical Outcomes. Front. Cell. Infect. Microbiol. 2021, 11, 778458. [Google Scholar] [CrossRef]
- Harding, M.W.; Marques, L.L.; Howard, R.J.; Olson, M.E. Can filamentous fungi form biofilms? Trends Microbiol. 2009, 17, 475–480. [Google Scholar] [CrossRef]
- Valsecchi, I.; Dupres, V.; Stephen-Victor, E.; Guijarro, J.I.; Gibbons, J.; Beau, R.; Bayry, J.; Coppee, J.-Y.; Lafont, F.; Latgé, J.-P.; et al. Role of Hydrophobins in Aspergillus fumigatus. J. Fungi 2017, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Orlandi, C.B.; Sardi, J.C.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Pires, R.H.; Montanari, L.B.; Martins, C.H.; Zaia, J.E.; Almeida, A.M.; Matsumoto, M.T.; Mendes-Giannini, M.J. Anticandidal efficacy of cinnamon oil against planktonic and biofilm cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 2011, 172, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Fries, B.C. Fungal biofilms: Relevance in the setting of human disease. Curr. Fungal Infect. Rep. 2010, 4, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, E.; Morace, G.; Borgo, F.; Rajendran, R.; Sherry, L.; Nile, C.; Ramage, G. New strategic insights into managing fungal biofilms. Front. Microbiol. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sardi Jde, C.; Pitangui Nde, S.; Voltan, A.R.; Braz, J.D.; Machado, M.P.; Fusco Almeida, A.M.; Mendes Giannini, M.J. In vitro Paracoccidioides brasiliensis biofilm and gene expression of adhesins and hydrolytic enzymes. Virulence 2015, 6, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Abd Elmegeed, A.S.M.; Ouf, S.A.; Moussa, T.A.A.; Eltahlawi, S.M.R. Dermatophytes and other associated fungi in patients attending to some hospitals in Egypt. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2015, 46, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Burkhart, C.N.; Burkhart, C.G.; Gupta, A.K. Dermatophytoma: Recalcitrance to treatment because of existence of fungal biofilm. J. Am. Acad. Derm. 2002, 47, 629–631. [Google Scholar] [CrossRef]
- Pitangui, N.S.; Sardi, J.C.; Silva, J.F.; Benaducci, T.; Moraes da Silva, R.A.; Rodríguez-Arellanes, G.; Taylor, M.L.; Mendes-Giannini, M.J.; Fusco-Almeida, A.M. Adhesion of Histoplasma capsulatum to pneumocytes and biofilm formation on an abiotic surface. Biofouling 2012, 28, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef]
- Sandhu, C.; Qureshi, A.; Emili, A. Panomics for Precision Medicine. Trends Mol. Med. 2018, 24, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, M.; Henriques, M.; Lopez-Ribot, J.L.; Oliveira, R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 2012, 55, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hawser, S.P.; Douglas, L.J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect. Immun. 1994, 62, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, C.H.G.; Pires, R.H.; Cunha, A.O.; Pereira, C.A.M.; Singulani, J.L.; Abrão, F.; Moraes, T.; Mendes-Giannini, M.J.S. Candida/Candida biofilms. First description of dual-species Candida albicans/C. rugosa biofilm. Fungal Biol. 2016, 120, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Řičicová, M.; Kucharíková, S.; Tournu, H.; Hendrix, J.; Bujdáková, H.; van Eldere, J.; Lagrou, K.; van Dijck, P. Candida albicans biofilm formation in a new in vivo rat model. Microbiology 2010, 156 Pt 3, 909–919. [Google Scholar] [CrossRef]
- Kucharíková, S.; Vande Velde, G.; Himmelreich, U.; van Dijck, P. Candida albicans biofilm development on medically-relevant foreign bodies in a mouse subcutaneous model followed by bioluminescence imaging. J. Vis. Exp. 2015, 95, 52239. [Google Scholar] [CrossRef] [Green Version]
- Pires, R.H.; Santos, J.M.; Zaia, J.E.; Martins, C.H.; Mendes-Giannini, M.J. Candida parapsilosis complex water isolates from a haemodialysis unit: Biofilm production and in vitro evaluation of the use of clinical antifungals. Mem. Inst. Oswaldo Cruz 2011, 106, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Bonhomme, J.; d’Enfert, C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [Google Scholar] [CrossRef]
- Xu, C.; Liang, C.; Wullschleger, S.; Wilson, C.; Mcdowell, N. Nature Reviews. Microbiology. Nat. Reviews Microbiol. 2011, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Paramonova, E.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. Hyphal content determines the compression strength of Candida albicans biofilms. Microbiology 2009, 155 Pt 6, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Henriques, M.; Martins, A.; Oliveira, R.; Williams, D.; Azeredo, J. Biofilms of non-Candida albicans Candida species: Quantification, structure and matrix composition. Med. Mycol. 2009, 47, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Asadian, F.; Zareshahrabadi, Z.; Saharkhiz, M.J.; Ahadian, S.; Zomorodian, K. Antimicrobial core–shell electrospun nanofibers containing Ajwain essential oil for accelerating infected wound healing. Int. J. Pharm. 2021, 603, 120698. [Google Scholar] [CrossRef] [PubMed]
- Giti, R.; Zomorodian, K.; Firouzmandi, M.; Zareshahrabadi, Z.; Rahmannasab, S. Antimicrobial activity of thermocycled polymethyl methacrylate resin reinforced with titanium dioxide and copper oxide nanoparticles. Int. J. Dent. 2021, 2021, 6690806. [Google Scholar] [CrossRef]
- Maliszewska, I.; Lisiak, B.; Popko, K.; Matczyszyn, K. Enhancement of the Efficacy of Photodynamic Inactivation of Candida albicans with the Use of Biogenic Gold Nanoparticles. Photochem. Photobiol. 2017, 93, 1081–1090. [Google Scholar] [CrossRef]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold Nanoparticle-Photosensitizer Conjugate Based Photodynamic Inactivation of Biofilm Producing Cells: Potential for Treatment of C. albicans Infection in BALB/c Mice. PLoS ONE 2015, 10, e0131684. [Google Scholar] [CrossRef]
- Kischkel, B.; Castilho, P.F.; de Oliveira, K.M.; Rezende, P.S.; Bruschi, M.L.; Svidzinski, T.I.; Negri, M. Silver nanoparticles stabilized with propolis show reduced toxicity and potential activity against fungal infections. Future Microbiol. 2020, 15, 521–539. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef] [Green Version]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Han, X.; Lai, L.; Tian, F.; Jiang, F.-L.; Xiao, Q.; Li, Y.; Yu, Q.; Li, D.; Wang, J.; Zhang, Q.; et al. Toxicity of CdTe Quantum Dots on Yeast Saccharomyces Cerevisiae. Small 2012, 8, 2680–2689. [Google Scholar] [CrossRef]
- Du, H.; Lo, T.-M.; Sitompul, J.; Chang, M.W. Systems-level analysis of Escherichia coli response to silver nanoparticles: The roles of anaerobic respiration in microbial resistance. Biochem. Biophys. Res. Commun. 2012, 424, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Yacamán, M.J.; Ascencio, J.A.; Liu, H.B.; Gardea-Torresdey, J. Structure shape and stability of nanometric sized particles. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1091–1103. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.-Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.-K.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L.; Yang, R.-Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Dibrov, P.; Dzioba, J.; Gosink, K.K.; Häse, C.C. Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kwon, S.; Jeong, S. Preparation of biodegradable polymer/silver nanoparticles composite and its antibacterial efficacy. J. Nanosci. Nanotechnol. 2009, 9, 1098–1102. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. Ayu 2018, 39, 182–186. [Google Scholar] [CrossRef]
- Qasim Nasar, M.; Zohra, T.; Khalil, A.T.; Saqib, S.; Ayaz, M.; Ahmad, A.; Shinwari, Z.K. Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chem. Lett. Rev. 2019, 12, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Howayda, M.; Mahmoud, H. Effect of zinc oxide nanoparticles on the growth of mycotoxigenic mould. SCPT 2013, 1, 66–74. [Google Scholar]
- Refai, H.; Badawy, M.; Hassan, A.; Sakr, H.; Baraka, Y. Antimicrobial effect of biologically prepared silver nanoparticles (AgNPs) on two different obturator’s soft linings in maxillectomy patients. Eur. J. Acad. Essays 2017, 4, 15–25. [Google Scholar]
- Atef, A.H.; Mogda, K.M.; Mahmoud, H. Biosynthesis of silver nanoparticles (AgNps)(a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans). N. Y. Sci. J. 2013, 6, 27–43. [Google Scholar]
- Hassan, A.; Abo-Zaid, K.; Oraby, N. Molecular and conventional detection of antimicrobial activity of zinc oxide nanoparticles and cinnamon oil against Escherichia coli and Aspergillus flavus. Adv. Anim. Vet. Sci. 2020, 8, 839–847. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tsai, P.-J.; Chen, Y.-C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef]
- De Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B. Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Seong, M.; Lee, D.G. Reactive oxygen species-independent apoptotic pathway by gold nanoparticles in Candida albicans. Microbiol. Res. 2018, 207, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef] [PubMed]
- Sojinrin, T.; Conde, J.; Liu, K.; Curtin, J.; Byrne, H.J.; Cui, D.; Tian, F. Plasmonic gold nanoparticles for detection of fungi and human cutaneous fungal infections. Anal. Bioanal. Chem. 2017, 409, 4647–4658. [Google Scholar] [CrossRef] [Green Version]
- Chow, T.H.; Li, N.; Bai, X.; Zhuo, X.; Shao, L.; Wang, J. Gold Nanobipyramids: An Emerging and Versatile Type of Plasmonic Nanoparticles. Acc. Chem. Res. 2019, 52, 2136–2146. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Hashim, A.F.; Alghuthaymi, M.A.; Said-Galiev, E. Nanobiotechnological strategies for toxigenic fungi and mycotoxin control. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–364. [Google Scholar]
- Joshaghani, H.; Eskandari, M. Antifungal effect of Sodium Dodecil Sulfate and Nano particle ZnO on growth inhibition of standard strain of Candida albicans. J. Gorgan Univ. Med. Sci. 2011, 12, 64–69. [Google Scholar]
- Hernández-Meléndez, D.; Salas-Téllez, E.; Zavala-Franco, A.; Téllez, G.; Méndez-Albores, A.; Vázquez-Durán, A. Inhibitory effect of flower-shaped zinc oxide nanostructures on the growth and aflatoxin production of a highly toxigenic strain of Aspergillus flavus Link. Materials 2018, 11, 1265. [Google Scholar] [CrossRef] [Green Version]
- Rostamizadeh, S.; Shadjou, N.; Amani, A.M.; Balalaie, S. Silica supported sodium hydrogen sulfate (NaHSO4/SiO2): A mild and efficient reusable catalyst for the synthesis of aryl-14-H-dibenzo [a, j] xanthenes under solvent-free conditions. Chin. Chem. Lett. 2008, 19, 1151–1155. [Google Scholar] [CrossRef]
- Nabawy, G.A.; Hassan, A.A.; Sayed El-Ahl, R.; Refai, M.K. Effect of metal nanoparticles in comparison with commercial antifungal feed additives on the growth of Aspergillus flavus and aflatoxin b1 production. J. Glob. Biosci. 2014, 3, 954–971. [Google Scholar]
- Mousavi, M.; Hashemi, A.; Arjmand, O.; Amani, A.M.; Babapoor, A.; Fateh, M.A.; Fateh, H.; Mojoudi, F.; Esmaeili, H.; Jahandideh, S. Erythrosine Adsorption from Aqueous Solution via Decorated Graphene Oxide with Magnetic Iron Oxide Nano Particles: Kinetic and Equilibrium Studies. Acta Chim. Slov. 2018, 65, 882–894. [Google Scholar] [CrossRef] [Green Version]
- Mouhamed, A.E.; Hassan, A.A.; Hassan, M.; El Hariria, M.; Refai, M. Effect of metal nanoparticles on the growth of ochratoxigenic moulds and ochratoxin A production isolated from food and feed. Int. J. Res. Stud. Biosci 2015, 3, 1–14. [Google Scholar]
- Ashraf, A.; Ahmed, M.; Diasty, E.; Fatma, I.; Ahmed Youssef, M. A comparative study on antifungal activity of FE2O3, and FE3O4 nanoparticles. Int. J. Adv. Res. 2018, 6, 189–194. [Google Scholar]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.; El-Matbouli, M.; Saleh, M. In vitro assessment of the antimicrobial efficacy of chitosan nanoparticles against major fish pathogens and their cytotoxicity to fish cell lines. J. Fish Dis. 2020, 43, 1049–1063. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Yu. Vasil’kov, A. Copper-chitosan nanocomposite hydrogels against aflatoxigenic Aspergillus flavus from dairy cattle feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef]
- Anaraki, M.R.; Jangjoo, A.; Alimoradi, F.; Dizaj, S.M.; Lotfipour, F. Comparison of antifungal properties of acrylic resin reinforced with ZnO and Ag nanoparticles. Pharm. Sci. 2017, 23, 207–214. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sayed-Elahl, R.M.; Oraby, N.H.; El-Hamaky, A.M. Metal nanoparticles for management of mycotoxigenic fungi and mycotoxicosis diseases of animals and poultry. In Nanomycotoxicology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–269. [Google Scholar]
- Hassan, A.A.; Mansour, M.K.; El Hamaky, A.M.; El Ahl, R.M.S.; Oraby, N.H. Nanomaterials and nanocomposite applications in veterinary medicine. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 583–638. [Google Scholar]
- Atef, H.A.; Mansour, M.K.; Ibrahim, E.M.; Sayed El-Ahl, R.; Al-Kalamawey, N.M.; El Kattan, Y.; Ali, M. Efficacy of zinc oxide nanoparticles and curcumin in amelioration the toxic effects in aflatoxicated rabbits. Int. J. Curr. Microbiol. Appl. Sci 2016, 5, 795–818. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-J.; Liu, B.-H.; Hsu, Y.-T.; Yu, F.-Y. Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2011, 22, 964–969. [Google Scholar] [CrossRef]
- Osama, E.; El-Sheikh, S.M.; Khairy, M.H.; Galal, A.A. Nanoparticles and their potential applications in veterinary medicine. J. Adv. Vet. Res. 2020, 10, 268–273. [Google Scholar]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Głuszek, K.; Wilczewska, A.Z.; Misztalewska, I.; Mystkowska, J.; Michalak, G.; Sodo, A.; Wątek, M.; et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomedicine 2016, 12, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in mesoporous silica nanoparticles as a multifunctional controlled drug delivery nanoplatform for infectious diseases treatment. Pharm. Res. 2020, 37, 191. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.H.; Rostamizadeh, S.; Amani, A.M.; Sepehrian, H. Fast and efficient method for the synthesis of 2-arylbenzimidazoles using MCM-41-SO3H. Heterocycl. Commun. 2012, 18, 33–37. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Shadjou, N. Silica supported ammonium dihydrogen phosphate (NH4H2PO4/SiO2): A mild, reusable and highly efficient heterogeneous catalyst for the synthesis of 14-aryl-14-H-dibenzo [a, j] xanthenes. Chin. Chem. Lett. 2009, 20, 779–783. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Mesoporous silica nanoparticles: Their projection in nanomedicine. Int. Sch. Res. Not. 2012, 2012, 608548. [Google Scholar] [CrossRef] [Green Version]
- Kanugala, S.; Jinka, S.; Puvvada, N.; Banerjee, R.; Kumar, C.G. Phenazine-1-carboxamide functionalized mesoporous silica nanoparticles as antimicrobial coatings on silicone urethral catheters. Sci. Rep. 2019, 9, 6198. [Google Scholar] [CrossRef] [Green Version]
- Montazeri, M.; Razzaghi-Abyaneh, M.; Nasrollahi, S.A.; Maibach, H.; Nafisi, S. Enhanced topical econazole antifungal efficacy by amine-functionalized silica nanoparticles. Bull. Mater. Sci. 2019, 43, 13. [Google Scholar] [CrossRef]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly (ethylene imine) s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Dodou Lima, H.V.; de Paula Cavalcante, C.S.; Rádis-Baptista, G. Antifungal in vitro activity of pilosulin-and ponericin-like peptides from the giant ant Dinoponera quadriceps and synergistic effects with antimycotic drugs. Antibiotics 2020, 9, 354. [Google Scholar] [CrossRef]
- Ramamourthy, G.; Park, J.; Seo, C.J.; Vogel, H.; Park, Y. Antifungal and antibiofilm activities and the mechanism of action of repeating lysine-tryptophan peptides against candida albicans. Microorganisms 2020, 8, 758. [Google Scholar] [CrossRef] [PubMed]
- Rank, L.A.; Walsh, N.M.; Lim, F.Y.; Gellman, S.H.; Keller, N.P.; Hull, C.M. Peptide-like nylon-3 polymers with activity against phylogenetically diverse, intrinsically drug-resistant pathogenic fungi. Msphere 2018, 3, e00223-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codling, C.E.; Maillard, J.-Y.; Russell, A.D. Aspects of the antimicrobial mechanisms of action of a polyquaternium and an amidoamine. J. Antimicrob. Chemother. 2003, 51, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Jiang, Z.; Zhang, N.; Yang, Z.; Zhou, Z. Interactions of biocidal polyhexamethylene guanidine hydrochloride and its analogs with POPC model membranes. Polymers 2017, 9, 517. [Google Scholar] [CrossRef]
- Azevedo, M.; Ramalho, P.; Silva, A.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Jung, J.; Bae, Y.; Cho, Y.K.; Ren, X.; Sun, Y. Structural insights into conformation of amphiphilic quaternary ammonium chitosans to control fungicidal and anti-biofilm functions. Carbohydr. Polym. 2020, 228, 115391. [Google Scholar] [CrossRef]
- Hoque, J.; Adhikary, U.; Yadav, V.; Samaddar, S.; Konai, M.M.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Sanyal, K.; Haldar, J. Chitosan derivatives active against multidrug-resistant bacteria and pathogenic fungi: In vivo evaluation as topical antimicrobials. Mol. Pharm. 2016, 13, 3578–3589. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Lopez-Jimenez, J.; Pérez-Berná, A.; Huang, I.C.; Jansson, H.B.; Salinas, J.; Villalaín, J.; Read, N.; Lopez-Llorca, L. Membrane fluidity determines sensitivity of filamentous fungi to chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; Sepulveda, M.; Ramallo, J.; Rapisarda, V.A.; Volentini, S.I. Polyhexamethylene guanidine as a fungicide, disinfectant and wound protector in lemons challenged with Penicillium digitatum. Food Microbiol. 2018, 76, 128–134. [Google Scholar] [CrossRef]
- Spadari, C.d.C.; Wirth, F.; Lopes, L.B.; Ishida, K. New Approaches for Cryptococcosis Treatment. Microorganisms 2020, 8, 613. [Google Scholar] [CrossRef]
- Pedroso, L.S.; Khalil, N.M.; Mainardes, R.M. Preparation and In vitro Evaluation of Efficacy and Toxicity of Polysorbate 80-coated Bovine Serum Albumin Nanoparticles containing Amphotericin B. Curr. Drug Deliv. 2018, 15, 1055–1063. [Google Scholar] [CrossRef]
- Xu, N.; Gu, J.; Zhu, Y.; Wen, H.; Ren, Q.; Chen, J. Efficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in mice. Int. J. Nanomed. 2011, 6, 905–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramge, P.; Unger, R.E.; Oltrogge, J.B.; Zenker, D.; Begley, D.; Kreuter, J.; Von Briesen, H. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur. J. Neurosci. 2000, 12, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Xu, N.; Cao, C.; Yuan, W.; Yu, X.; Chen, J.; Ren, J. Preparation and therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J. Biomater. Sci. Polym. Ed. 2009, 20, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.A.; AlQuadeib, B.T.; Šiller, L.; Wright, M.C.; Horrocks, B. Oral administration of amphotericin B nanoparticles: Antifungal activity, bioavailability and toxicity in rats. Drug Deliv. 2017, 24, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2016, 27, 1880–1898. [Google Scholar] [CrossRef]
- Yang, C.; Xue, B.; Song, W.; Kan, B.; Zhang, D.; Yu, H.; Shen, N.; Li, X.; Tang, Z.; Chen, X. Reducing the toxicity of amphotericin B by encapsulation using methoxy poly(ethylene glycol)-b-poly(l-glutamic acid-co-l-phenylalanine). Biomater. Sci. 2018, 6, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Italia, J.L.; Sharp, A.; Carter, K.C.; Warn, P.; Kumar, M.N. Peroral amphotericin B polymer nanoparticles lead to comparable or superior in vivo antifungal activity to that of intravenous Ambisome® or Fungizone™. PLoS ONE 2011, 6, e25744. [Google Scholar]
- Van de Ven, H.; Paulussen, C.; Feijens, P.B.; Matheeussen, A.; Rombaut, P.; Kayaert, P.; van den Mooter, G.; Weyenberg, W.; Cos, P.; Maes, L.; et al. PLGA nanoparticles and nanosuspensions with amphotericin B: Potent in vitro and in vivo alternatives to Fungizone and AmBisome. J. Control. Release 2012, 161, 795–803. [Google Scholar] [CrossRef]
- Deaguero, I.G.; Huda, M.N.; Rodriguez, V.; Zicari, J.; Al-Hilal, T.A.; Badruddoza, A.Z.M.; Nurunnabi, M. Nano-vesicle based anti-fungal formulation shows higher stability, skin diffusion, biosafety and anti-fungal efficacy in vitro. Pharmaceutics 2020, 12, 516. [Google Scholar] [CrossRef]
- Siopi, M.; Mouton, J.W.; Pournaras, S.; Meletiadis, J. In vitro and in vivo exposure-effect relationship of liposomal amphotericin b against aspergillus fumigatus. Antimicrob. Agents Chemother. 2019, 63, e02673-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadari, C.C.; de Bastiani, F.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.J.; Cha, Y.J.; Kim, H.; Choi, S.S. Antifungal activity of nano and micro charcoal particle polymers against Paecilomyces variotii, Trichoderma virens and Chaetomium globosum. New Biotechnol. 2016, 33, 55–60. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mansour, M.K.; El Ahl, R.M.S.; El Hamaky, A.M.; Oraby, N.H. Toxic and beneficial effects of carbon nanomaterials on human and animal health. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 535–555. [Google Scholar]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatnagar, I.; Kim, S.K. Antimicrobial Activity of Chitosan-Carbon Nanotube Hydrogels. Materials 2014, 7, 3946–3955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbari, S.; Abdi, Y.; Haghighi, F.; Mohajerzadeh, S.; Haghighi, N. Investigating the antifungal activity of TiO2 nanoparticles deposited on branched carbon nanotube arrays. J. Phys. D Appl. Phys. 2011, 44, 245401. [Google Scholar] [CrossRef]
- Vafa, E.; Bazargan-Lari, R. Bovine serum albumin protected gold nanozymes as a novel anti-cancer nanodrug for acute T-type lymphoblastic leukemia treatment via effect on the expression of anti-apoptotic genes. Appl. Biol. Chem. 2021, 64, 86. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Kim, Y.H.; Lee, S.-G.; Han, S. Preparation and characterization of B, S, and N-doped glucose carbon dots: Antibacterial, antifungal, and antioxidant activity. Sustain. Mater. Technol. 2022, 32, e00397. [Google Scholar] [CrossRef]
- Leudjo Taka, A.; Doyle, B.P.; Carleschi, E.; Youmbi Fonkui, T.; Erasmus, R.; Fosso-Kankeu, E.; Pillay, K.; Mbianda, X.Y. Spectroscopic characterization and antimicrobial activity of nanoparticle doped cyclodextrin polyurethane bionanosponge. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111092. [Google Scholar] [CrossRef]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182–191. [Google Scholar]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- El-Sheridy, N.A.; Ramadan, A.A.; Eid, A.A.; El-Khordagui, L.K. Itraconazole lipid nanocapsules gel for dermatological applications: In vitro characteristics and treatment of induced cutaneous candidiasis. Colloids Surf. B Biointerfaces 2019, 181, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.A.; Delgado, A.; Evora, C.; del Pozo-Rodríguez, A.; Rodríguez-Gascón, A. Biodistribution of Nanostructured Lipid Carriers (NLCs) after intravenous administration to rats: Influence of technological factors. Eur. J. Pharm. Biopharm. 2013, 84, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Pichayakorn, W.; Ritthidej, G.C. Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): Effect of drug loading and biopharmaceutical characterizations. Drug Dev. Ind. Pharm. 2018, 44, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Moazeni, M.; Babaei, R.; Saeedi, M.; Akbari, J.; Parkoohi, P.I.; Nabili, M.; Gohar, A.A.; Morteza-Semnani, K.; Nokhodchi, A. Improved yeast delivery of fluconazole with a nanostructured lipid carrier system. Biomed. Pharm. 2017, 89, 83–88. [Google Scholar] [CrossRef]
- Moazeni, M.; Kelidari, H.R.; Saeedi, M.; Morteza-Semnani, K.; Nabili, M.; Gohar, A.A.; Akbari, J.; Lotfali, E.; Nokhodchi, A. Time to overcome fluconazole resistant Candida isolates: Solid lipid nanoparticles as a novel antifungal drug delivery system. Colloids Surf. B. Biointerfaces 2016, 142, 400–407. [Google Scholar] [CrossRef]
- Domingues Bianchin, M.; Borowicz, S.M.; da Rosa Monte Machado, G.; Pippi, B.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Meneghello Fuentefria, A.; Clemes Külkamp-Guerreiro, I. Lipid core nanoparticles as a broad strategy to reverse fluconazole resistance in multiple Candida species. Colloids Surf. B Biointerfaces 2019, 175, 523–529. [Google Scholar] [CrossRef]
- Gupta, M.; Tiwari, S.; Vyas, S.P. Influence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasis. Pharm. Dev. Technol. 2013, 18, 550–559. [Google Scholar] [CrossRef]
- Gupta, M.; Vyas, S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids 2012, 165, 454–461. [Google Scholar] [CrossRef]
- Kim, J.-K.; Park, J.-S.; Kim, C.-K. Development of a binary lipid nanoparticles formulation of itraconazole for parenteral administration and controlled release. Int. J. Pharm. 2010, 383, 209–215. [Google Scholar] [CrossRef]
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Majumdar, D.K.; Mishra, S.K.; Panda, A.K.; Patnaik, S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 2015, 20, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.M.; Rajinikanth, P.S.; Mallikarjun, C.; Kang, Y.B. Formulation and delivery of itraconazole to the brain using a nanolipid carrier system. Int. J. Nanomed. 2014, 9, 2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardeike, J.; Weber, S.; Zarfl, H.P.; Pagitz, M.; Zimmer, A. Itraconazole-loaded nanostructured lipid carriers (NLC) for pulmonary treatment of aspergillosis in falcons. Eur. J. Pharm. Biopharm. 2016, 108, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Ferrarelli, T.; Mauro, M.V.; Cavalcanti, P.; Picci, N.; Trombino, S. Preparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal delivery. Drug Deliv. 2016, 23, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Ng, W.K.; Tan, R.B. Sucrose ester stabilized solid lipid nanoparticles and nanostructured lipid carriers: I. Effect of formulation variables on the physicochemical properties, drug release and stability of clotrimazole-loaded nanoparticles. Nanotechnology 2014, 25, 105101. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Contado, C.; Costenaro, A.; Drechsler, M.; Rossi, D.; Menegatti, E.; Grandini, A.; Cortesi, R. Clotrimazole nanoparticle gel for mucosal administration. Mater. Sci. Eng. C 2013, 33, 411–418. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Kumar, R.; Sinha, V.R. Solid lipid nanoparticle: An efficient carrier for improved ocular permeation of voriconazole. Drug Dev. Ind. Pharm. 2016, 42, 1956–1967. [Google Scholar] [CrossRef]
- Khare, A.; Singh, I.; Pawar, P.; Grover, K. Design and evaluation of voriconazole loaded solid lipid nanoparticles for ophthalmic application. J. Drug Deliv. 2016, 2016, 6590361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.H.; Lee, K.M.; Kang, J.B.; Lee, S.G.; Kang, M.J.; Choi, Y.W. Improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. Chem. Pharm. Bull. 2014, 62, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalekar, M.R.; Pokharkar, V.; Madgulkar, A.; Patil, N.; Patil, N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharmscitech 2009, 10, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Jain, S.; Khare, P.; Gulbake, A.; Bansal, D.; Jain, S.K. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv. 2010, 17, 443–451. [Google Scholar] [CrossRef]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharmacol. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Samanta, P.K. Strong and weak quantum confinement and size dependent optoelectronic properties of zinc oxide. Ann. Univ. Craiova Phys. 2018, 28, 17–23. [Google Scholar]
- Siddiqi, K.S.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Manikandan, V.; Balasubramanian, B.; Bharti, B.; Velmurugan, P.; Elango, D.; Baskaran, R.; Jayanthi, P. Development of ZnO/MOGAC nanocomposites for enhanced photocatalytic removal of PO43− and NO3-ions from wastewater under various light irradiations. Biomass Convers. Biorefin. 2022, 1–18. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Asok, A.; Ghosh, S.; More, P.A.; Chopade, B.A.; Gandhi, M.N.; Kulkarni, A.R. Surface defect rich ZnO quantum dots as antioxidants inhibiting α-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J. Mater. Chem. B 2015, 3, 4597–4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chand, P.; Kumari, S.; Mondal, N.; Singh, S.P.; Prasad, T. Synergism of Zinc Oxide Quantum Dots with Antifungal Drugs: Potential Approach for Combination Therapy against Drug Resistant Candida albicans. Front. Nanotechnol. 2021, 3, 624564. [Google Scholar] [CrossRef]
- Fakhroueian, Z.; Dehshiri, A.M.; Katouzian, F.; Esmaeilzadeh, P. In vitro cytotoxic effects of modified zinc oxide quantum dots on breast cancer cell lines (MCF7), colon cancer cell lines (HT29) and various fungi. J. Nanopart. Res. 2014, 16, 2483. [Google Scholar] [CrossRef]
- Chand, P.; Singh, S.P.; Prasad, T. Effect of Antioxidant on ROS Mediated Antifungal Action of ZnO Quantum Dots in Candida albicans. ECS Trans. 2022, 107, 6621. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal peptides as therapeutic agents. Front. Cell Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.; Haagsman, H.P.; Coorens, M. Cathelicidins modulate TLR-activation and inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Łoboda, D.; Kozłowski, H.; Rowińska-Żyrek, M. Antimicrobial peptide–metal ion interactions—A potential way of activity enhancement. New J. Chem. 2018, 42, 7560–7568. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, R.S.; Phoenix, A.D. Anionic Antimicrobial Peptides from Eukaryotic Organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kodedová, M.; Valachovič, M.; Csáky, Z.; Sychrová, H. Variations in yeast plasma-membrane lipid composition affect killing activity of three families of insect antifungal peptides. Cell Microbiol. 2019, 21, e13093. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Zhou, W. The preparation, characterization, anti-ultraviolet and antimicrobial activity of gelatin film incorporated with berberine-HP-β-CD. Colloids Surf. Physicochem. Eng. Asp. 2020, 586, 124273. [Google Scholar] [CrossRef]
- Firmino, H.C.T.; Nascimento, E.P.; Bonan, R.F.; Maciel, P.P.; Castellano, L.R.C.; Santana, L.N.L.; Neves, G.A.; Menezes, R.R. Antifungal activity of TiO2-CeO2 nanofibers against Candida fungi. Mater. Lett. 2021, 283, 128709. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Fernandes, S.C.M.; Diaz, R.H.; Labidi, J. Processing of α-chitin nanofibers by dynamic high pressure homogenization: Characterization and antifungal activity against A. niger. Carbohydr. Polym. 2015, 116, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Tsukiyama, Y.; Yukawa, T.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Facile preparation of silver nanoparticles immobilized on chitin nanofiber surfaces to endow antifungal activities. Carbohydr. Polym. 2015, 117, 813–817. [Google Scholar] [CrossRef]
- Semnani, K.; Shams-Ghahfarokhi, M.; Afrashi, M.; Fakhrali, A.; Semnani, D. Antifungal activity of eugenol loaded electrospun PAN nanofiber mats against Candida albicans. Curr. Drug Del. 2018, 15, 860–866. [Google Scholar] [CrossRef]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al-Thobity, A.M.; Al-Harbi, F.A.; Virtanen, J.I.; Raustia, A. The effect of nanodiamonds on candida albicans adhesion and surface characteristics of PMMA denture base material-an in vitro study. J. Appl. Oral Sci. 2019, 27, 1–10. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; de Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver nanoparticles addition in poly (methyl methacrylate) dental matrix: Topographic and antimycotic studies. Int. J. Mol. Sci. 2019, 20, 4691. [Google Scholar] [CrossRef] [Green Version]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet. Med. Int. 2020, 2020, 5346483. [Google Scholar] [CrossRef] [PubMed]
- Accomasso, L.; Cristallini, C.; Giachino, C. Risk Assessment and Risk Minimization in Nanomedicine: A Need for Predictive, Alternative, and 3Rs Strategies. Front. Pharmacol. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badman, R.P.; Moore, S.L.; Killian, J.L.; Feng, T.; Cleland, T.A.; Hu, F.; Wang, M.D. Dextran-coated iron oxide nanoparticle-induced nanotoxicity in neuron cultures. Sci. Rep. 2020, 10, 11239. [Google Scholar] [CrossRef]
- Wiesner, M.R.; Lowry, G.V.; Jones, K.L.; Hochella, J.; Michael, F.; Di Giulio, R.T.; Casman, E.; Bernhardt, E.S. Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials. ACS Publ. 2009, 2009, 6458–6462. [Google Scholar] [CrossRef] [Green Version]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [Green Version]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef]


| Polymeric Materials with Antifungal Activity | Cell Wall | Membrane | Intracellular Targets | Toxic Effects | Ref |
|---|---|---|---|---|---|
| Synthetic peptides with antimicrobial properties | - | Permeability to membrane | Likely | Reduced haemolytic activity | [154] |
| Synthetic peptides with antimicrobial properties | - | Does not appear to show permeability for fungal cell membranes | Capable of binding to nucleic acids | Related to length of hydrophobic region | [155] |
| (Nylon-3 copolymers) | - | Permeability to membrane | - | Reduced toxic effects, based on length of hydrophobic region | [156] |
| Polyquaternium-1(PQ 1) | Inhibits germination of conidia species | Permeability to membrane | Likely | - | [157] |
| Polyoctamethylene guanidine hydrochloride (POGH) | - | Permeability to membrane | Likely | Slightly | [158] |
| Polyethylenimine (PEI) | - | Permeability to membrane | Likely depolarization of cellular membrane, capable of binding to nucleic acids | Slightly, related to the length of hydrophobic region | [159] |
| Quaternary ammonium chloride derivatives of chitosan | - | Permeability to membrane | Likely | Reduced toxic effects | [160] |
| (N-(2-hydroxypropyl)-3trimethylammonium chitosan chlorides) HTCC | - | Permeability to membrane | Likely | Reduced toxic effects | [161] |
| Chitosan | - | Permeability to membrane | Nucleus, capable of binding to RNA and/or DNA | Reduced toxic effects | [162] |
| Polyhexamethylene guanidine hydrochloride (PHMGH) derivatives | Capable of targeting cell wall | Permeability to membrane | Likely | Reduced toxic effects but after inhalation showing serve toxicity | [163] |
| Lipid-Based Formulation | Drug | Size (nm) | The Drug Loading Efficiency | Ref |
|---|---|---|---|---|
| SLNs | Fluconazole | 179–279 | 50–75% | [193] |
| SLNs | Fluconazole | 178 | 75.7% | [194] |
| NLCs | Fluconazole | 134 | 81.4% | [194] |
| SLNs | Fluconazole | 85 | 89.6% | [185] |
| NLCs | Itraconazole | 192–240 | 83–95% | [195] |
| SLNs | Itraconazole | 250–545 | 81–88% | [196] |
| SLNs | Itraconazole | 126–199 | 68–94% | [197] |
| NLCs | Itraconazole | 314 | 70.5 ± 0.6% | [198] |
| NLCs | Itraconazole | 102–106 | 99.98% | [199] |
| SLNs | Clotrimazole | 202–460 | 41–43% | [200] |
| SLNs | Clotrimazole | 120 | 87% | [201] |
| NLCs | Clotrimazole | 160 | 88% | [201] |
| NLCs | Clotrimazole | 50–150 | 85–89% | [202] |
| NLCs | Clotrimazole | 202 | 97% | [203] |
| SLNs | Terbinafine | 300 | 300 | [204] |
| SLNs | Voriconazole | 139–334 | 139–334 | [205] |
| SLNs | Voriconazole | 234–343 | 62–84% | [206] |
| NLCs | Voriconazole | 210 | 86% | [207] |
| SLNs | Miconazole | 244–766 | 80–100% | [208] |
| SLNs | Miconazole | 23 | 90.2% | [209] |
| SLNs | Miconazole | 206 | 90.8% | [210] |
| SLNs | Econazole | 150 | 100% | [211] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jangjou, A.; Zareshahrabadi, Z.; Abbasi, M.; Talaiekhozani, A.; Kamyab, H.; Chelliapan, S.; Vaez, A.; Golchin, A.; Tayebi, L.; Vafa, E.; et al. Time to Conquer Fungal Infectious Diseases: Employing Nanoparticles as Powerful and Versatile Antifungal Nanosystems against a Wide Variety of Fungal Species. Sustainability 2022, 14, 12942. https://doi.org/10.3390/su141912942
Jangjou A, Zareshahrabadi Z, Abbasi M, Talaiekhozani A, Kamyab H, Chelliapan S, Vaez A, Golchin A, Tayebi L, Vafa E, et al. Time to Conquer Fungal Infectious Diseases: Employing Nanoparticles as Powerful and Versatile Antifungal Nanosystems against a Wide Variety of Fungal Species. Sustainability. 2022; 14(19):12942. https://doi.org/10.3390/su141912942
Chicago/Turabian StyleJangjou, Ali, Zahra Zareshahrabadi, Milad Abbasi, Amirreza Talaiekhozani, Hesam Kamyab, Shreeshivadasan Chelliapan, Ahmad Vaez, Ali Golchin, Lobat Tayebi, Ehsan Vafa, and et al. 2022. "Time to Conquer Fungal Infectious Diseases: Employing Nanoparticles as Powerful and Versatile Antifungal Nanosystems against a Wide Variety of Fungal Species" Sustainability 14, no. 19: 12942. https://doi.org/10.3390/su141912942
APA StyleJangjou, A., Zareshahrabadi, Z., Abbasi, M., Talaiekhozani, A., Kamyab, H., Chelliapan, S., Vaez, A., Golchin, A., Tayebi, L., Vafa, E., Amani, A. M., & Faramarzi, H. (2022). Time to Conquer Fungal Infectious Diseases: Employing Nanoparticles as Powerful and Versatile Antifungal Nanosystems against a Wide Variety of Fungal Species. Sustainability, 14(19), 12942. https://doi.org/10.3390/su141912942









