Activated Carbon and Coconut Coir with the Incorporation of ABR System as Greywater Filter: The Implications for Wastewater Treatment
Abstract
1. Introduction
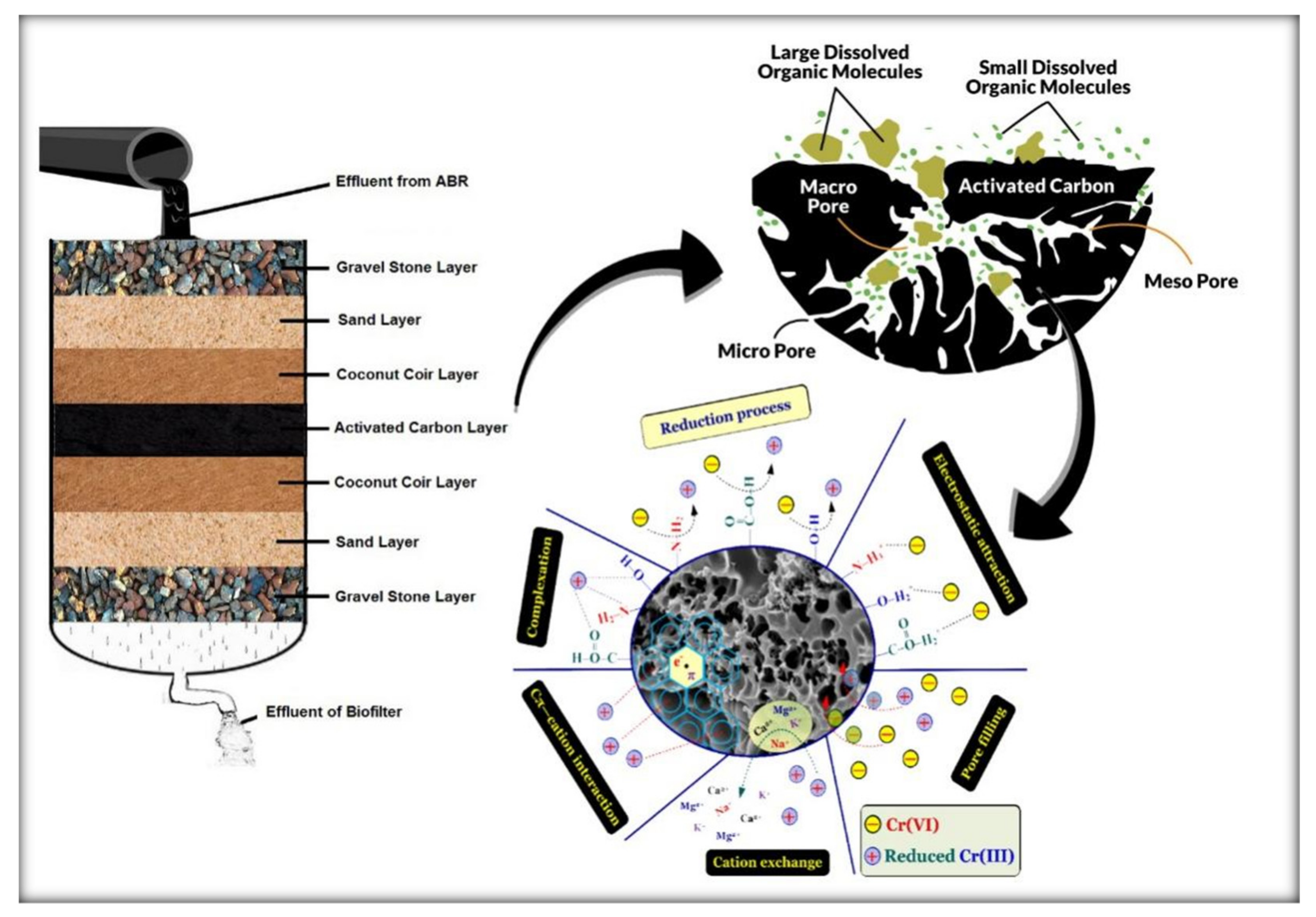
Chemical Interaction Process and Material Properties
2. Materials and Methods
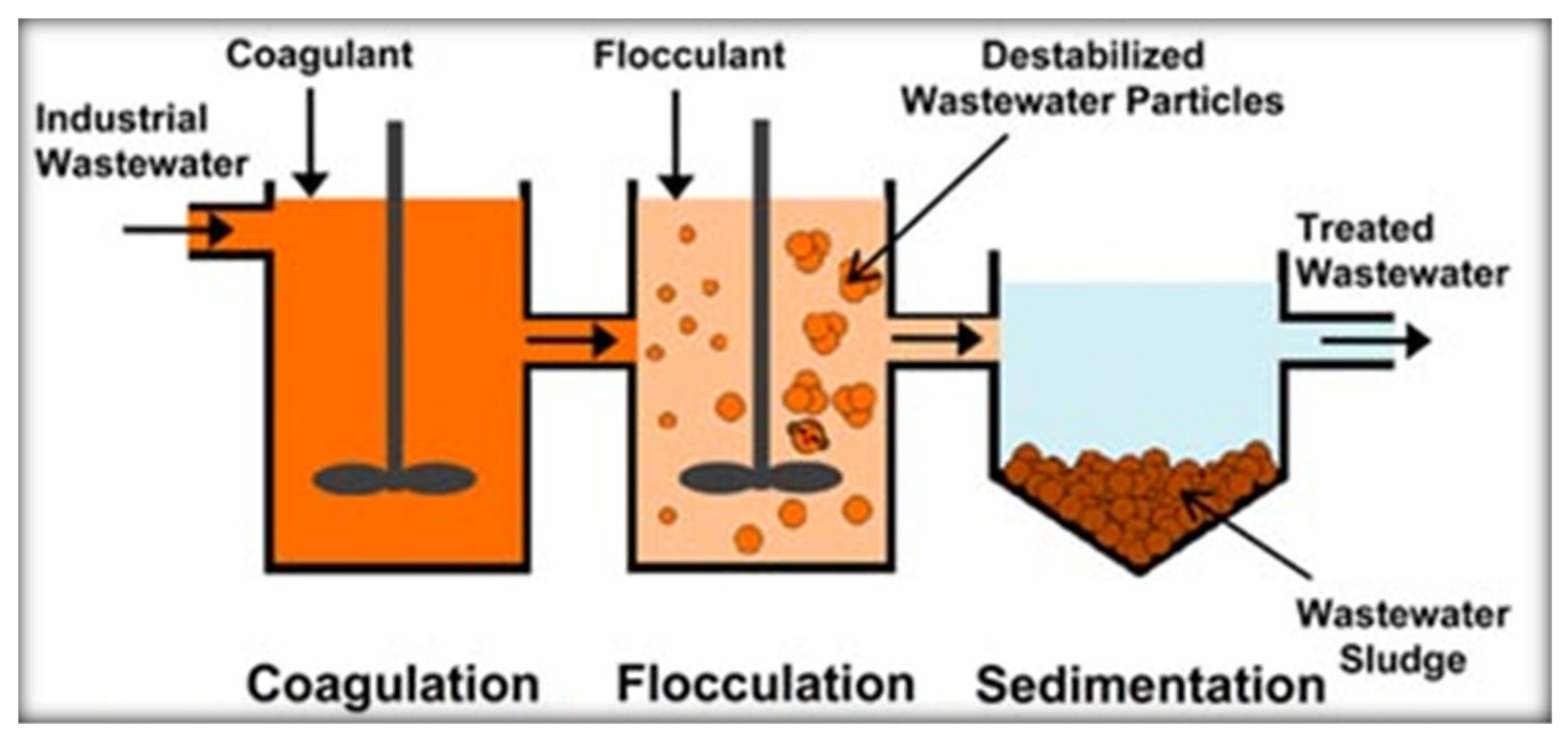
2.1. Coagulation and Flocculation Processes
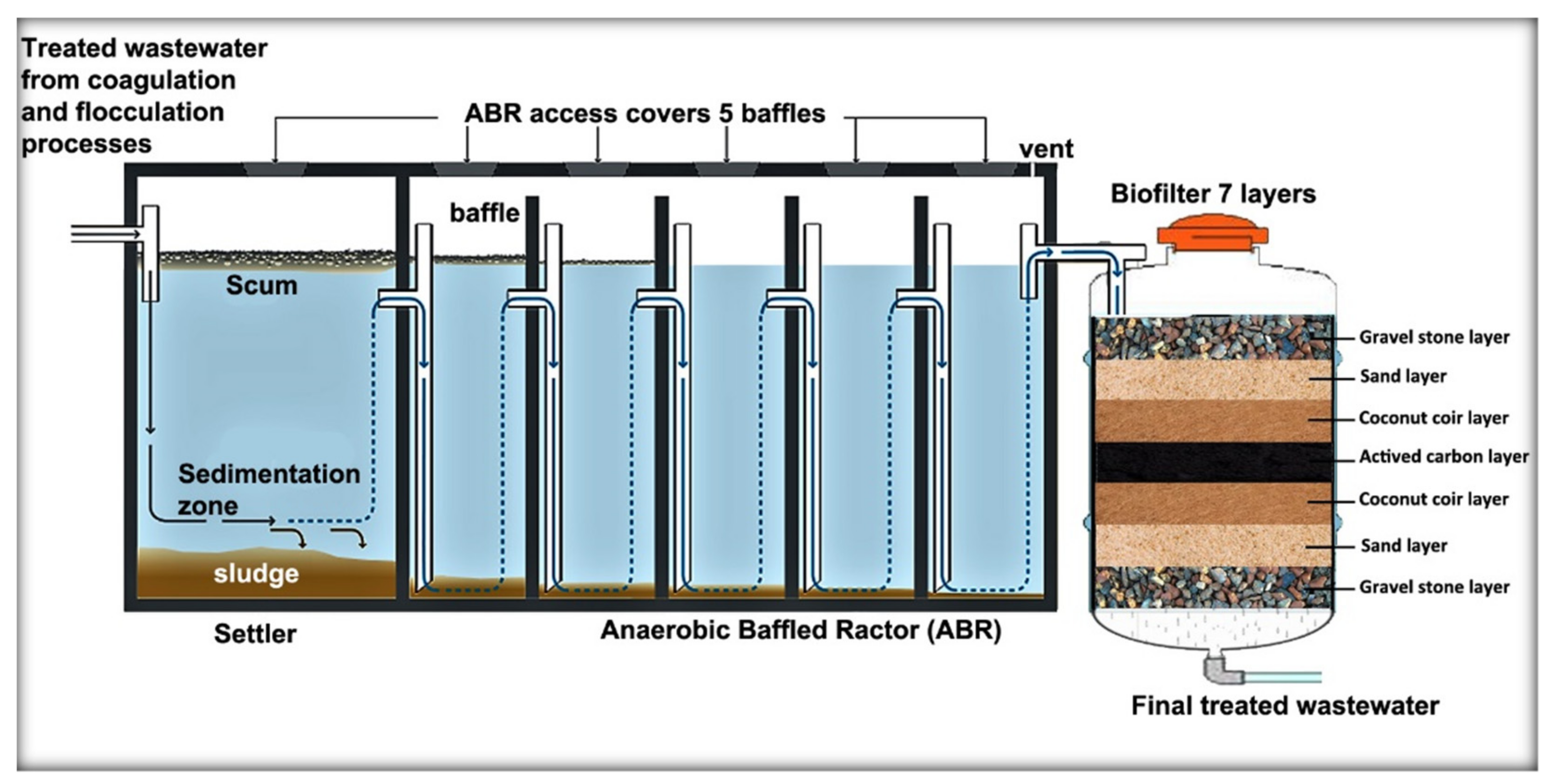
2.2. Anaerobic Baffled Reactor (ABR) Process
2.3. Membrane Filter (MF) Process with Activated Carbon (AC) and Coconut Coir (CC)
3. Results
4. Discussion
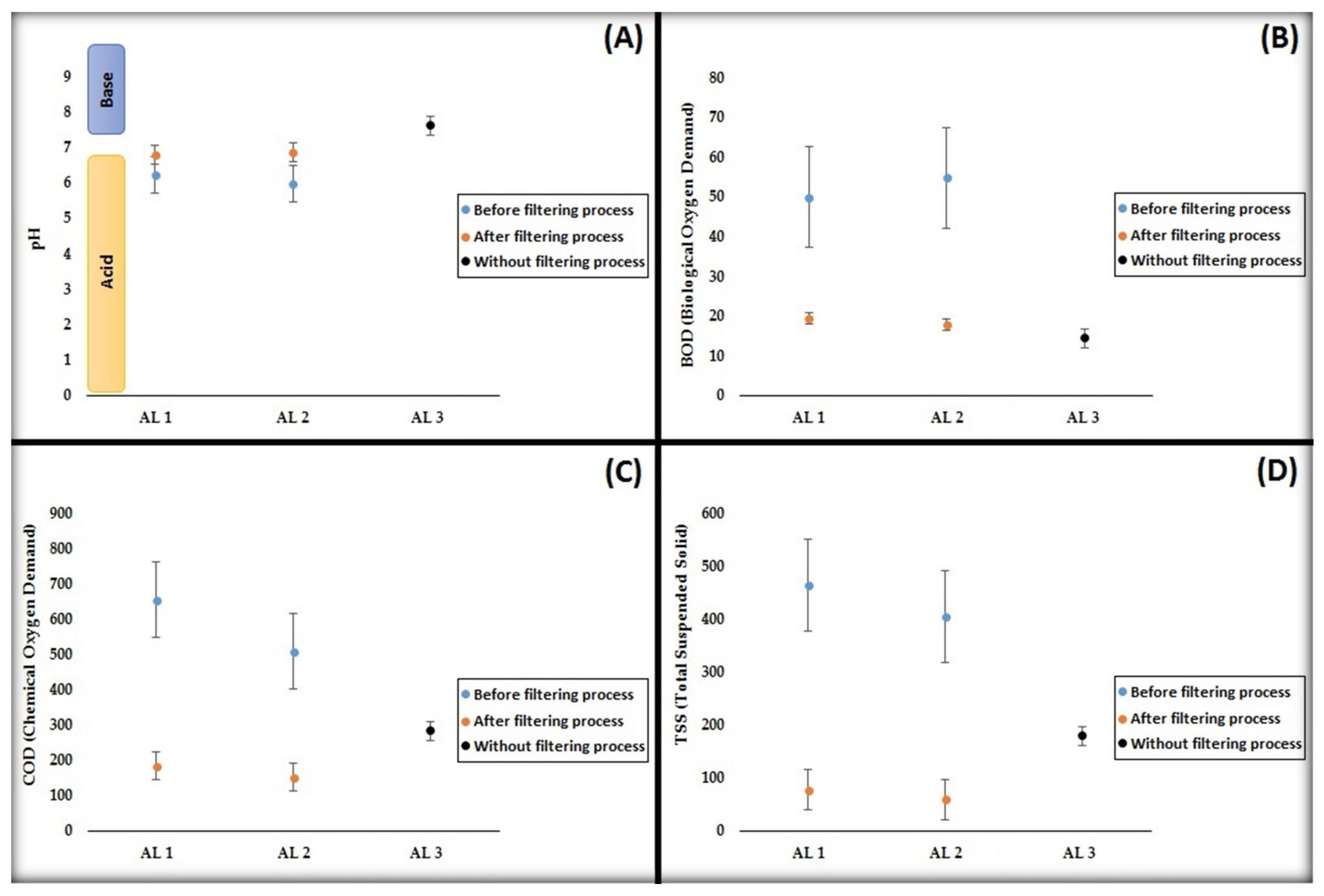
4.1. pH (Degree of Acidity)
4.2. BOD (Biological Oxygen Demand)
4.3. COD (Chemical Oxygen Demand)
4.4. TSS (Total Suspended Solid)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Easton, Z.M.; Bock, E. Hydrology Basics and the Hydrologic Cycle; Virginia Cooperative Extension: Blacksburg, VA, USA, 2015; pp. 1–9. Available online: https://ext.vt.edu/content/dam/ext_vt_edu/topics/agriculture/water/documents/Hydrology-Basics-and-the-Hydrologic-Cycle.pdf (accessed on 10 October 2021).
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; E & FN Spon, an Imprint of Routledge: London, UK, 1999; Volume 95, ISBN 0419239308. [Google Scholar]
- Gordon, B.; Callan, P.; Vickers, C. WHO guidelines for drinking-water quality. WHO Chron. 2008, 38, 564. [Google Scholar] [CrossRef]
- Custodio, E. Trends in Groundwater Pollution: Loss of Groundwater Quality & Related Services; Technical University of Catalonia: Barcelona, Spain, 2015. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jung, H.; Koh, D.C.; Kim, Y.S.; Jeen, S.W.; Lee, J. Stable isotopes of water and nitrate for the identification of groundwater flowpaths: A review. Water 2020, 12, 138. [Google Scholar] [CrossRef]
- Khalekuzzaman, M.; Alamgir, M.; Hasan, M.; Hasan, M.N. Performance comparison of uninsulated and insulated hybrid anaerobic baffled reactor (HABR) operating at warm temperature. Water Sci. Technol. 2018, 78, 1879–1892. [Google Scholar] [CrossRef]
- Nakagawa, K.; Amano, H.; Berndtsson, R. Spatial characteristics of groundwater chemistry in Unzen, Nagasaki, Japan. Water 2021, 13, 426. [Google Scholar] [CrossRef]
- Teixeira, C.A.; Ghisi, E. Comparative analysis of granular and membrane filters for rainwater treatment. Water 2019, 11, 1004. [Google Scholar] [CrossRef]
- Asian Development Bank. Country Water Assessment Indonesia Country Water Assessment; Asian Development Bank: Mandaluyong, Philippines, 2016; ISBN 978-92-9257-360-7. [Google Scholar]
- World Health Organization. A Compendium of Standards for Wastewater Reuse in the Eastern Mediterranean Region World Health Organization Regional Office for the Eastern Mediterranean Regional Centre for Environmental Health Activities CEHA; World Health Organization: Geneva, Switzerland, 2006; p. 19. Available online: http://applications.emro.who.int/dsaf/dsa1184.pdf (accessed on 10 October 2021).
- Liu, L.; Li, Y.; Fan, S. Preparation of KOH and H3PO4 modified biochar and its application in methylene blue removal from aqueous solution. Processes 2019, 7, 891. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products. Sci. Total Environ. 2018, 635, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Chen, W.; Wang, L. Particle properties in granular activated carbon filter during drinking water treatment. J. Environ. Sci. 2010, 22, 681–688. [Google Scholar] [CrossRef]
- Siwila, S.; Brink, I.C. Drinking water treatment using indigenous wood filters combined with granular activated carbon. J. Water Sanit. Hyg. Dev. 2019, 9, 477–491. [Google Scholar] [CrossRef]
- Telgote, A.R.; Patil, S.S. Study and Application of Various Activated Carbons and Ash used in Water Purification Techniques: A Review. Curr. World Environ. 2020, 15, 384–397. [Google Scholar] [CrossRef]
- Mason, A.; Korostynska, O.; Wylie, S.; Al-Shamma’a, A.I. Non-destructive evaluation of an activated carbon using microwaves to determine residual life. Carbon 2014, 67, 1–9. [Google Scholar] [CrossRef]
- Mopoung, S.; Moonsri, P.; Palas, W.; Khumpai, S. Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(III) Adsorption from Aqueous Solution. Sci. World J. 2015, 2015, 415961. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.M.; Kim, K.H.; Hwang, G.B.; Seo, S.C.; Bae, G.N.; Jung, J.H. Development and evaluation of antimicrobial activated carbon fiber filters using Sophora flavescens nanoparticles. Sci. Total Environ. 2014, 493, 291–297. [Google Scholar] [CrossRef]
- Reungoat, J.; Escher, B.I.; Macova, M.; Argaud, F.X.; Gernjak, W.; Keller, J. Ozonation and biological activated carbon filtration of wastewater treatment plant effluents. Water Res. 2012, 46, 863–872. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondal, A.; Gangopadhyay, S.; Mandal, S. Cadmium bioaccumulation in Lamellidens marginalis and human health risk assessment: A case study in India. Hum. Ecol. Risk Assess. 2020, 26, 713–725. [Google Scholar] [CrossRef]
- Sweetman, M.; May, S.; Mebberson, N.; Pendleton, P.; Vasilev, K.; Plush, S.; Hayball, J. Activated Carbon, Carbon Nanotubes and Graphene: Materials and Composites for Advanced Water Purification. J. Carbon Res. C 2017, 3, 18. [Google Scholar] [CrossRef]
- Dvorak, B.I. Drinking Water Treatment: Activated Carbon Filtration; Institute of Agriculture and Natural Resources, University of Nebraska–Lincoln Extension: Lincoln, NE, USA, 2013; pp. 1–4. [Google Scholar]
- Arshad, M.; Guillaume, J.H.A.; Ross, A. Assessing the feasibility of managed aquifer recharge for irrigation under uncertainty. Water 2014, 6, 2748–2769. [Google Scholar] [CrossRef]
- Stoquart, C.; Servais, P.; Bérubé, P.R.; Barbeau, B. Hybrid Membrane Processes using activated carbon treatment for drinking water: A review. J. Membr. Sci. 2012, 411–412, 1–12. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, H.; Jang, T. Irrigation water quality standards for indirect wastewater reuse in agriculture: A contribution toward sustainablewastewater reuse in South korea. Water 2016, 8, 169. [Google Scholar] [CrossRef]
- Mosbah, M.B.; Mechi, L.; Khiari, R.; Moussaoui, Y. Current state of porous carbon for wastewater treatment. Processes 2020, 8, 1651. [Google Scholar] [CrossRef]
- Vo, A.T.; Nguyen, V.P.; Ouakouak, A.; Nieva, A.; Doma, B.T.; Tran, H.N.; Chao, H.P. Water Efficient Removal of Cr(VI) from Water by Biochar and Activated Carbon Prepared through Hydrothermal Carbonization and Pyrolysis: Adsorption-Coupled Reduction Mechanism. Water 2019, 11, 1164. [Google Scholar] [CrossRef]
- Cescon, A.; Jiang, J.Q. Filtration process and alternative filter media material in water treatment. Water 2020, 12, 3377. [Google Scholar] [CrossRef]
- Furuya, K.; Harada, K. An automated precise Winkler titration for determining dissolved oxygen on board ship. J. Oceanogr. 1995, 51, 375–383. [Google Scholar] [CrossRef][Green Version]
- Benramache, S.; Aoun, Y. Spin Coating Method Fabricated of In2O3 Thin Films. Ann. West Univ. Timisoara Phys. 2019, 61, 56–63. [Google Scholar] [CrossRef]
- Hayase, N.; Kitaoka, Y.; Yakou, Y.; Yoshida, T.; Ishimaru, K.; Yasuno, K. Characteristics of Biological Actived Carbon Filtration and Biological Filtration in Drinking Water Treatment. J. Jpn. Soc. Water Environ. 1998, 21, 507–512. [Google Scholar] [CrossRef][Green Version]
- Sabara, Z.; Junaidi, R.; Umam, R. Robust Decision Making(RD) Investiation in Water Resources Planning and Disaster Mitigation in Makassar City, Indonesia. J. Pertahanan 2018, 4, 61–75. [Google Scholar]


| Parameter | Sand | Gravel | Activated Carbon |
|---|---|---|---|
| pH | 6.8 ± 0.1 | 8.8 ± 0.1 | 6.7 ± 0.1 |
| Volatile matter content (%) | 1.0 ± 0.1 | 2.9 ± 0.1 | 50.8 ± 0.1 |
| Moisture content (%) | 2.38 ± 0.01 | 0.05 ± 0.01 | 48.73 ± 0.01 |
| Ash content (%) | 1.11 ± 0.01 | 6.96 ± 0.01 | 6.25 ± 0.01 |
| Specific mass (g/cm3) | 2.61 ± 0.01 | 2.69 ± 0.01 | 1.27 ± 0.01 |
| Bulk density (g/cm3) | 1.47 ± 0.02 | 1.38 ± 0.02 | 0.63 ± 0.02 |
| Void index (%) | 43.8 ± 0.1 | 48.8 ± 0.1 | 32.9 ± 0.1 |
| Iodine number (mg/g) | - | - | 665.85 ± 0.01 |
| No | Parameter | Method | Unit | Sample Value | Maximum Limit (WHO) | Information | ||
|---|---|---|---|---|---|---|---|---|
| AL 1 | AL 2 | AL 3 (Control) | ||||||
| Before | Turbidity | Turbidimetry | NTU | 313 | 257 | 108 | - | - |
| pH | Potentiometry | - | 6.23 | 5.96 | 7.62 | 6−9 | E | |
| BOD | Winkler Titration | mg/L | 49.98 | 54.88 | 14.7 | 30 | NE | |
| COD | Spectrophotometry | mg/L | 655 | 509 | 284 | 100 | NE | |
| TSS | Gravimetry | mg/L | 464.65 | 404.40 | 181.63 | 30 | NE | |
| After | Turbidity | Turbidimetry | NTU | 48.73 | 16.25 | 108 | - | - |
| pH | Potentiometry | - | 6.78 | 6.86 | 7.62 | 6−9 | E | |
| BOD | Winkler Titration | mg/L | 19.40 | 17.80 | 14.7 | 30 | E | |
| COD | Spectrophotometry | mg/L | 184 | 152 | 284 | 100 | NE | |
| TSS | Gravimetry | mg/L | 77.42 | 59.68 | 181.63 | 30 | NE | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabara, Z.; Anwar, A.; Yani, S.; Prianto, K.; Junaidi, R.; Umam, R.; Prastowo, R. Activated Carbon and Coconut Coir with the Incorporation of ABR System as Greywater Filter: The Implications for Wastewater Treatment. Sustainability 2022, 14, 1026. https://doi.org/10.3390/su14021026
Sabara Z, Anwar A, Yani S, Prianto K, Junaidi R, Umam R, Prastowo R. Activated Carbon and Coconut Coir with the Incorporation of ABR System as Greywater Filter: The Implications for Wastewater Treatment. Sustainability. 2022; 14(2):1026. https://doi.org/10.3390/su14021026
Chicago/Turabian StyleSabara, Zakir, Aswariani Anwar, Setyawati Yani, Kusnul Prianto, Rahmad Junaidi, Rofiqul Umam, and Rizqi Prastowo. 2022. "Activated Carbon and Coconut Coir with the Incorporation of ABR System as Greywater Filter: The Implications for Wastewater Treatment" Sustainability 14, no. 2: 1026. https://doi.org/10.3390/su14021026
APA StyleSabara, Z., Anwar, A., Yani, S., Prianto, K., Junaidi, R., Umam, R., & Prastowo, R. (2022). Activated Carbon and Coconut Coir with the Incorporation of ABR System as Greywater Filter: The Implications for Wastewater Treatment. Sustainability, 14(2), 1026. https://doi.org/10.3390/su14021026









