Red Mud as Adsorbent to Recover Phosphorous from Wastewater Streams
Abstract
:1. Introduction
2. Materials and Methods
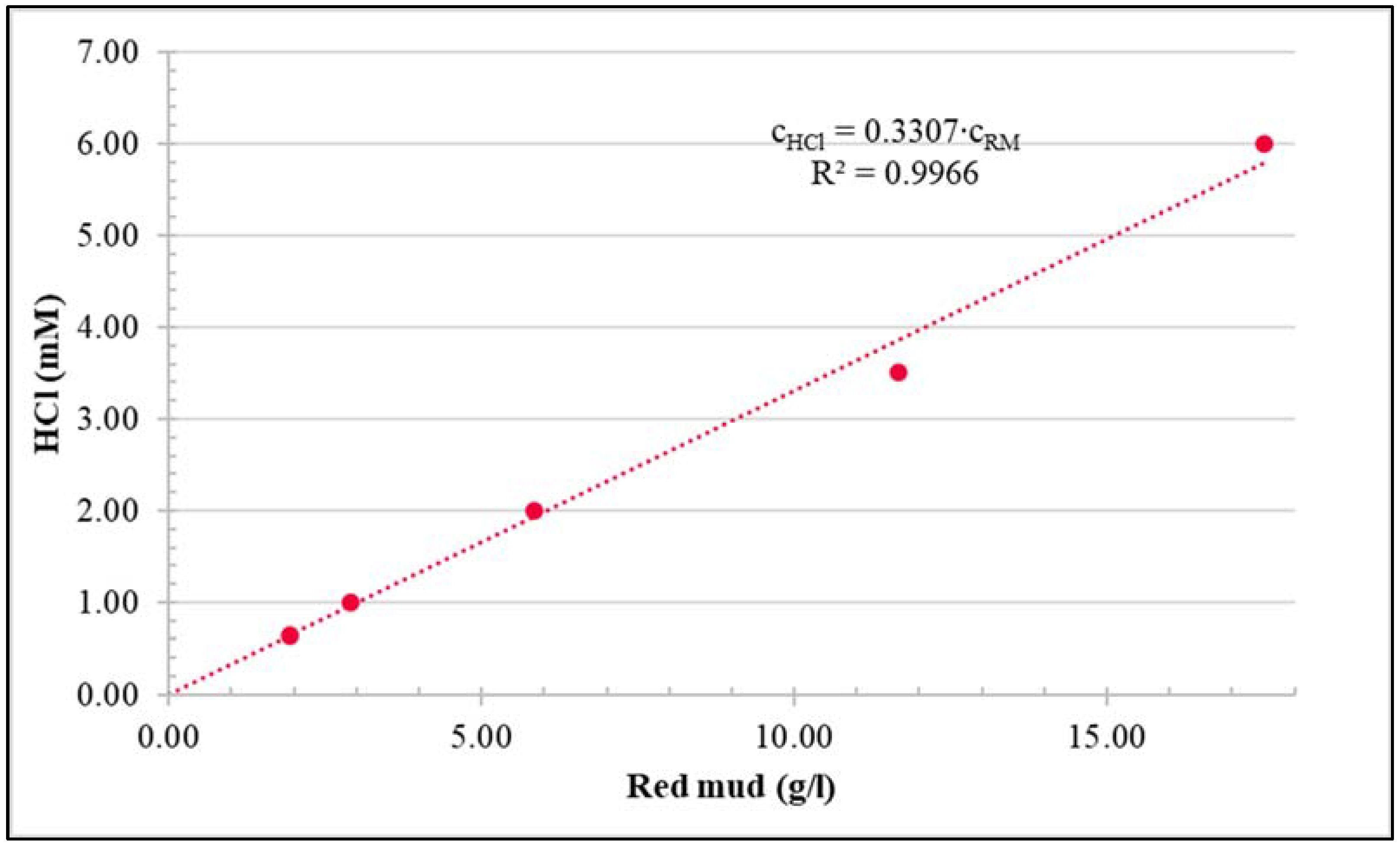
2.1. Red Mud
2.2. Experiments with Model Wastewater
2.3. Experiments with Real Wastewater
3. Results
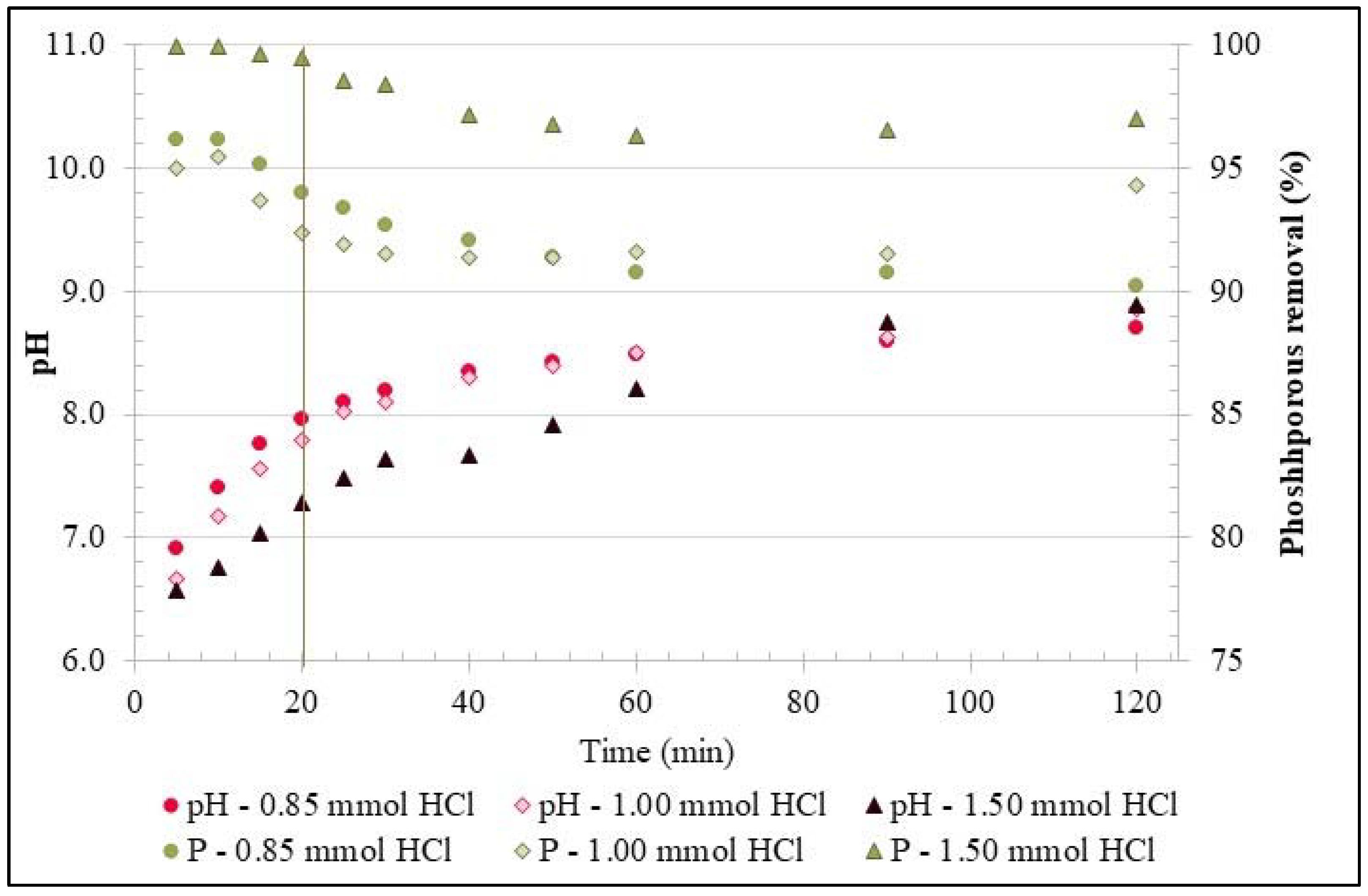
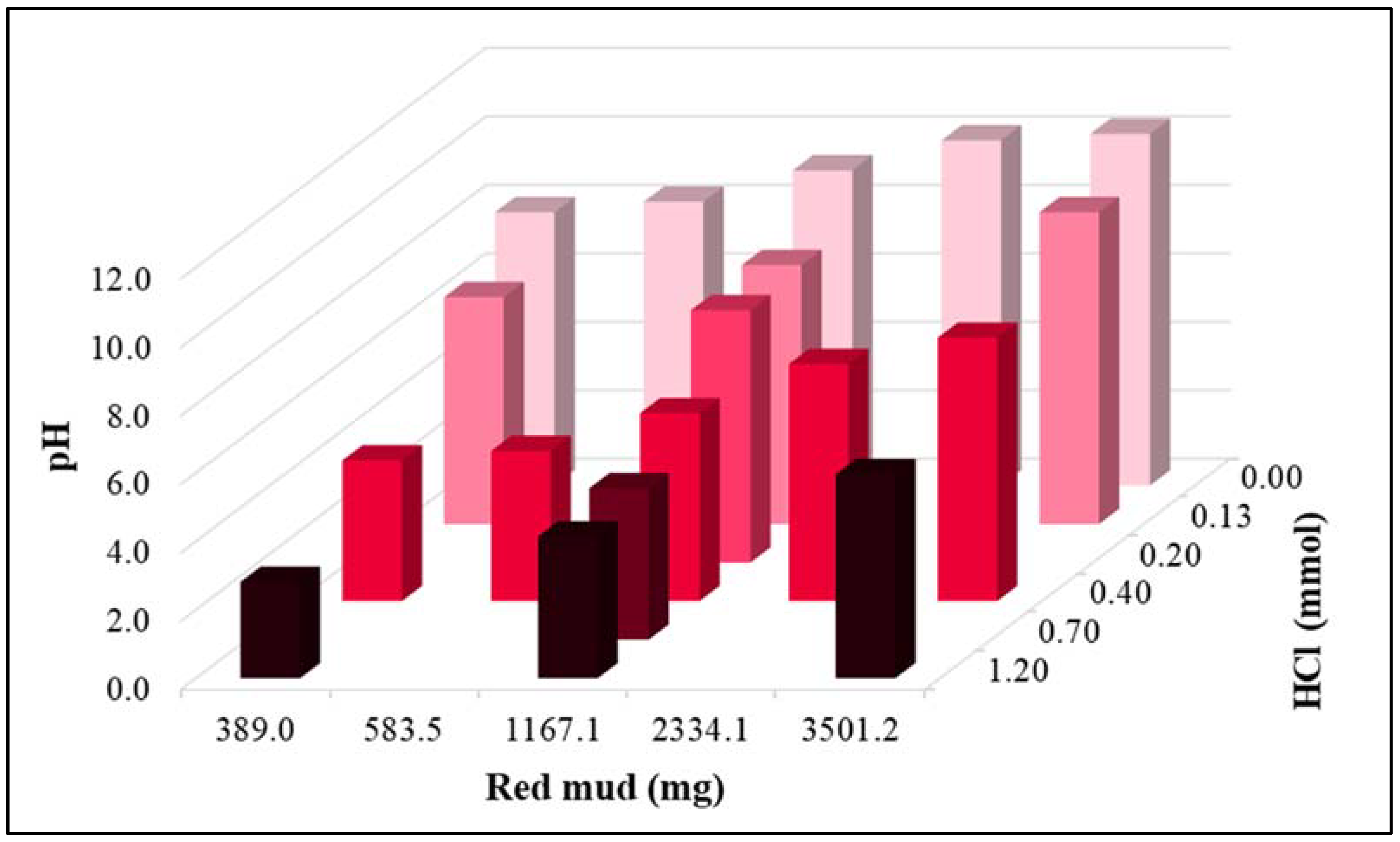
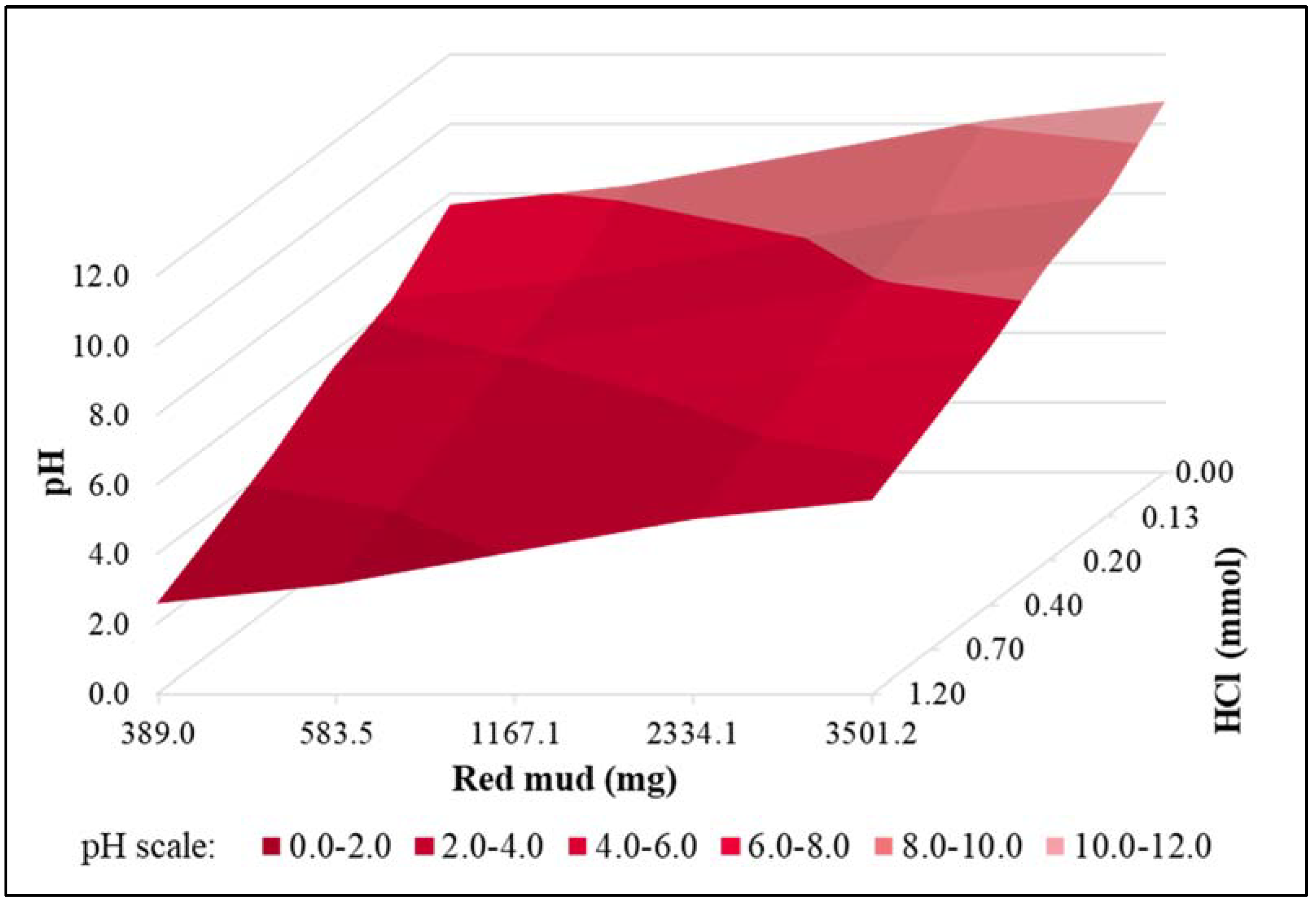
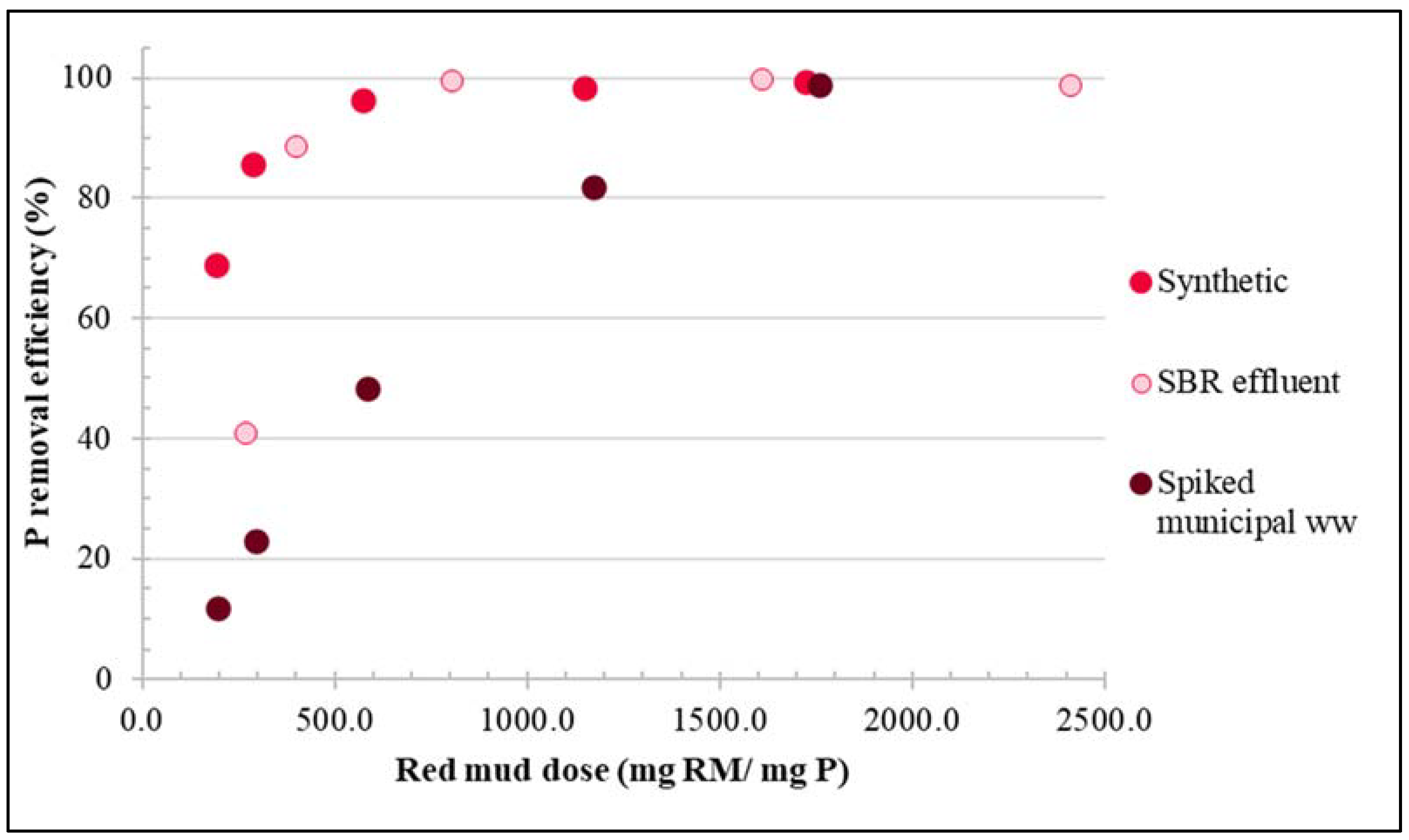
3.1. Experiments with Synthetic Phosphorous Solution
3.2. Experiments with Real Wastewater
3.2.1. Treatment of SBR Effluent
3.2.2. Treatment of Spiked Municipal Wastewater
3.2.3. Treatment of Landfill Leachate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Van Puijenbroek, P.J.T.M.; Beusen, A.H.W.; Bouwman, A.F. Global nitrogen and phosphorus in urban wastewater based on the Shared Socio-economic pathways. J. Environ. Manag. 2019, 231, 446–456. [Google Scholar] [CrossRef]
- Gómez, M.; Corona, F.; Hidalgo, M.D. Variations in the properties of leachate according to landfill age. Desalin Water Treat 2019, 159, 24–31. [Google Scholar] [CrossRef]
- Bakhshoodeh, R.; Alavi, N.; Oldham, C.; Santos, R.M.; Babaei, A.A.; Vymazal, J.; Paydary, P. Constructed wetlands for landfill leachate treatment: A review. Ecol. Eng. 2020, 146, 105725. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Bashar, R.; Gungor, K.; Karthikeyan, K.G.; Barak, P. Cost-effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef]
- Farley, M. Eutrophication in Fresh Waters: An International Review. In Encyclopedia of Lakes and Reservoirs; Bengtsson, L., Herschy, R.W., Fairbridge, R.W., Eds.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- 28/2004 (XII.25) KvVM Decree on the Limit Values Applicable to Discharges of Water Pollutants and Certain Rules of Application in Respect of Recipients. Available online: https://net.jogtar.hu/jogszabaly?docid=a0400028.kvv (accessed on 10 September 2022).
- Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Wastewater Treatment, OJ L 135, 30/05/1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31991L0271 (accessed on 29 September 2022).
- Mbamba, C.K.; Lindblom, E.; Flores-Alsina, X.; Tait, S.; Anderson, S.; Saagi, R.; Batstone, D.J.; Gernaey, K.V.; Jeppsson, U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. [Google Scholar] [CrossRef]
- Poikane, S.; Kelly, M.G.; Herrero, F.S.; Pitt, J.A.; Jarvie, H.P.; Claussen, U.; Leujak, W.; Solheim, A.L.; Teixeira, H.; Phillips, G. Nutrient criteria for surface waters under the European Water Framework Directive: Current state-of-the-art, challenges and future outlook. Sci. Total Environ. 2019, 695, 133888. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Zhang, X.; Guo, J.; Ni, B.J.; Chang, S.W.; Nguyen, D.D. Insight into biological phosphate recovery from sewage. Bioresour. Tech. 2016, 218, 874–881. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A critical review. Environ. Eng. Sci. 2019, 36, 511–529. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable sewage sludge management: From current practices to emerging nutrient recovery technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef] [Green Version]
- 17. Tomei, M.C.; Stazi, V.; Daneshgar, S.; Capodaglio, A.G. Holistic approach to phosphorus recovery from urban wastewater: Enhanced biological removal combined with precipitation. Sustainability 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- Turp, G.A.; Turp, S.M.; Ozdemir, S.; Yetilmezsoy, K. Vermicomposting of biomass ash with bio-waste for solubilizing nutrients and its effect on nitrogen fixation in common beans. Environ. Technol. Innov. 2021, 23, 101691. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ejerssa, W.W.; Wegener, C.C.; Korving, L.; Dugulan, A.I.; Temmink, H.; Loosdrecht, M.C.; Witkamp, G.J. Understanding and improving the reusability of phosphate adsorbents for wastewater effluent polishing. Water Res. 2018, 145, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Mahdavi, S.; Akhzari, D. The removal of phosphate from aqueous solutions using two nano-structures: Copper oxide and carbon tubes. Clean Tech. Environ. Policy 2016, 18, 817–827. [Google Scholar] [CrossRef]
- Yirong, C.; Vaurs, L.P. Wasted salted duck egg-shells as an alternative adsorbent for phosphorus removal. J. Environ. Chem. Eng. 2019, 7, 103443. [Google Scholar] [CrossRef]
- López, R.; Antelo, J.; Fiol, S.; Macías-García, F. Phosphate adsorption on an industrial residue and subsequent use as an amendment for phosphorous deficient soils. J. Clean Prod. 2019, 230, 844–853. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of red mud in wastewater treatment. Minerals 2019, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, D.; Shaterian, M.; Rashidipour, F.; Nasseri, S.; Mousavi, S.G.A. High-efficiency removal of phosphorous from filtered activated sludge effluent using electrochemical process. J. Clean Prod. 2020, 263, 121444. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Ni, F.; Xi, B.; Xia, X.; Peng, X.; Luan, Z. Evaluation of a novel composite inorganic coagulant prepared by red mud for phosphate removal. Desalination 2011, 273, 414–420. [Google Scholar] [CrossRef]
- Ni, F.; Peng, X.; Zhao, Y.; He, J.; Li, Y.; Luan, Z. Preparation of coagulant from red mud and semi-product of polyaluminum chloride for removal of phosphate from water. Desalination Water Treat 2012, 40, 153–158. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef]
- Poulin, É.; Blais, J.F.; Mercier, G. Transformation of red mud from aluminium industry into a coagulant for wastewater treatment. Hydrometallurgy 2008, 92, 16–25. [Google Scholar] [CrossRef]
- Ujaczki, É.; Feigl, V.; Farkas, É.; Vaszita, E.; Gruiz, K.; Molnár, M. Red mud as acidic sandy soil ameliorant: A microcosm incubation study. J. Chem. Tech. Biotech. 2016, 91, 1596–1606. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kang, S.H.; Hwang, K.Y.; Kim, H.S.; Kim, J.G.; Song, H.; Hwang, I. Evaluation of phosphate fertilizers and red mud in reducing plant availability of Cd, Pb, and Zn in mine tailings. Environ. Earth Sci. 2015, 74, 2659–2668. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Yang, H.; Liu, Q.; Gu, H.; Wang, N.; Yu, W.; Dai, Y. Adsorptive removal of phosphate from aqueous solutions using different types of red mud. Water Sci. Technol. 2018, 2017, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Tangde, V.M.; Prajapati, S.S.; Mandal, B.B.; Kulkarni, N.P. Study of kinetics and thermodynamics of removal of phosphate from aqueous solution using activated red mud. Int. J. Environ. Res. 2017, 11, 39–47. [Google Scholar] [CrossRef]
- Cusack, P.B.; Healy, M.G.; Ryan, P.C.; Burke, I.T.; O′Donoghue, L.M.; Ujaczki, É.; Courtney, R. Enhancement of bauxite residue as a low-cost adsorbent for phosphorus in aqueous solution, using seawater and gypsum treatments. J. Clean Prod. 2018, 179, 217–224. [Google Scholar] [CrossRef]
- Lin, J.Y.; Kim, M.; Li, D.; Kim, H.; Huang, C.P. The removal of phosphate by thermally treated red mud from water: The effect of surface chemistry on phosphate immobilization. Chemosphere 2020, 247, 125867. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, G.; Xu, Y.; Guo, M.; Xiao, Y.; Ma, T.; Liu, C. Adsorption of inorganic and organic phosphorus onto polypyrrole modified red mud: Evidence from batch and column experiments. Chemosphere 2022, 286, 131862. [Google Scholar] [CrossRef] [PubMed]
- Despland, L.M.; Clark, M.W.; Vancov, T.; Erler, D.; Aragno, M. Nutrient and trace-metal removal by Bauxsol pellets in wastewater treatment. Environ. Sci. Technol. 2011, 45, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, Y.; Lv, F.; Tong, W.; Xin, H.; Meng, Z.; Wang, X.; Chu, P.K. Preparation of layered double hydroxides using boron mud and red mud industrial wastes and adsorption mechanism to phosphate. Water Environ. J. 2016, 31, 145–157. [Google Scholar] [CrossRef]
- Berta, K.M.; Kurdi, R.; Lukács, P.; Penk, M.; Somogyi, V. Red mud with other waste materials as artificial soil substitute and its effect on Sinapis alba. J. Environ. Manag. 2021, 287, 112311. [Google Scholar] [CrossRef] [PubMed]
- Makó, A.; Anton, A.; Csitári, G.; Rékási, M.; Uzinger, N.; Barna, G.; Széplábi, G.; Hernádi, H. Model experiments on the physical properties of soil contaminated with red mud. (Vörösiszappal szennyezett talaj fizikai tulajdonságainak vizsgálata modell oszlopkísérletben, In Hungarian). Agrochem. Soil Sci. 2014, 63, 203–222. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, Á.; Hoffer, A.; Imre, K.; Nyirő-Kósa, I.; Csákberényi-Malasics, D.; Tóth, A.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effects of fugitive dust. Environ. Sci. Tech. 2011, 45, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Garcia-Cabellos, G.; Ryan, D.; Enright, D.; Enright, A.M. Low-cost physicochemical treatment for removal of ammonia, phosphate and nitrate contaminants from landfill leachate. J. Environ. Sci. Health Part A 2019, 54, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.J.; Akashi, K. Phosphate removal from aqueous solution from activated red mud. J. Water. Pollut. Cont. Fed. 1977, 49, 280–285. [Google Scholar]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. Options for residue utilisation. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Luan, Z.; Peng, X.; Zhu, C.; Chen, Z.; Zhang, Z.; Fan, J.; Jia, Z. Phosphate removal from aqueous solutions using raw and activated red mud and fly ash. J. Hazard Mater. 2006, 137, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.M.; Rodgers, M.; Clifford, E. Studying the adsorption properties of modified red mud towards phosphate removal from its solutions. Int. J. Environ. Sci. 2012, 3, 354–356. [Google Scholar] [CrossRef] [Green Version]
- Akhurst, D.J.; Jones, G.B.; Clark, M.; McConchie, D. Phosphate removal from aqueous solutions using neutralized bauxite refinery residues (Bauxsol™). Environ. Chem. 2006, 3, 65–74. [Google Scholar] [CrossRef]
- Cabral, L.L.; Pereira, I.C.; Perretto, F.; Nagalli, A.; Rizzo-Domingues, R.C.P.; Passig, F.H.; de Carvalho, K.Q. Adsorption and desorption of phosphate onto chemically and thermochemically pre-activated red ceramic waste: Characteristics, batch studies, and mechanisms. J. Environ. Chem. Eng. 2021, 9, 106695. [Google Scholar] [CrossRef]
- Ghosh, S.; Prabhakar, R.; Samadder, S.R. Performance of γ-aluminium oxide nanoparticles for arsenic removal from groundwater. Clean Tech. Environ. Policy 2019, 21, 121–138. [Google Scholar] [CrossRef]

| Parameter | pH | COD | TN | NH4+-N | NO3−-N | NO2−-N | OP | TP |
|---|---|---|---|---|---|---|---|---|
| - | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| SBR effluent | 7.20 | 33 | 25 | 0.10 | 22.22 | 0.01 | 7.23 | 7.62 |
| Municipal effluent | 7.71 | 44 | 7 | 0.02 | 5.87 | 0.48 | 0.04/4.98 * | 0.52 |
| Landfill leachate | 9.08 | 5 420 | 212 | 17.66 | 16.03 | 0.71 | 11.89 | 17.58 |
| Dose № | Multiplier | Calculated Amount of RM (mg) | |
|---|---|---|---|
| Factor of 2 | Factor of 60 | ||
| 1 | 1/3 | 13.0 | 389.0 |
| 2 | 1/2 | 19.5 | 583.5 |
| 3 | 1 | 38.9 | 1167.1 |
| 4 | 2 | 77.8 | 2334.1 |
| 5 | 3 | 116.7 | 3501.2 |
| 20 min | 24 h | ||
|---|---|---|---|
| Parameters | a | 3.1075 | 3.3592 |
| b | −0.3617 | 0.0458 | |
| c | −3.7672 | −4.3354 | |
| d | −4.8007 | −4.7560 | |
| SSR | 1.72 | 11.50 | |
| Doses | Results after 20 min | |||
|---|---|---|---|---|
| RM | HCl | pH | OP | Conductivity |
| mg | mmol | - | mg/L | µS/cm |
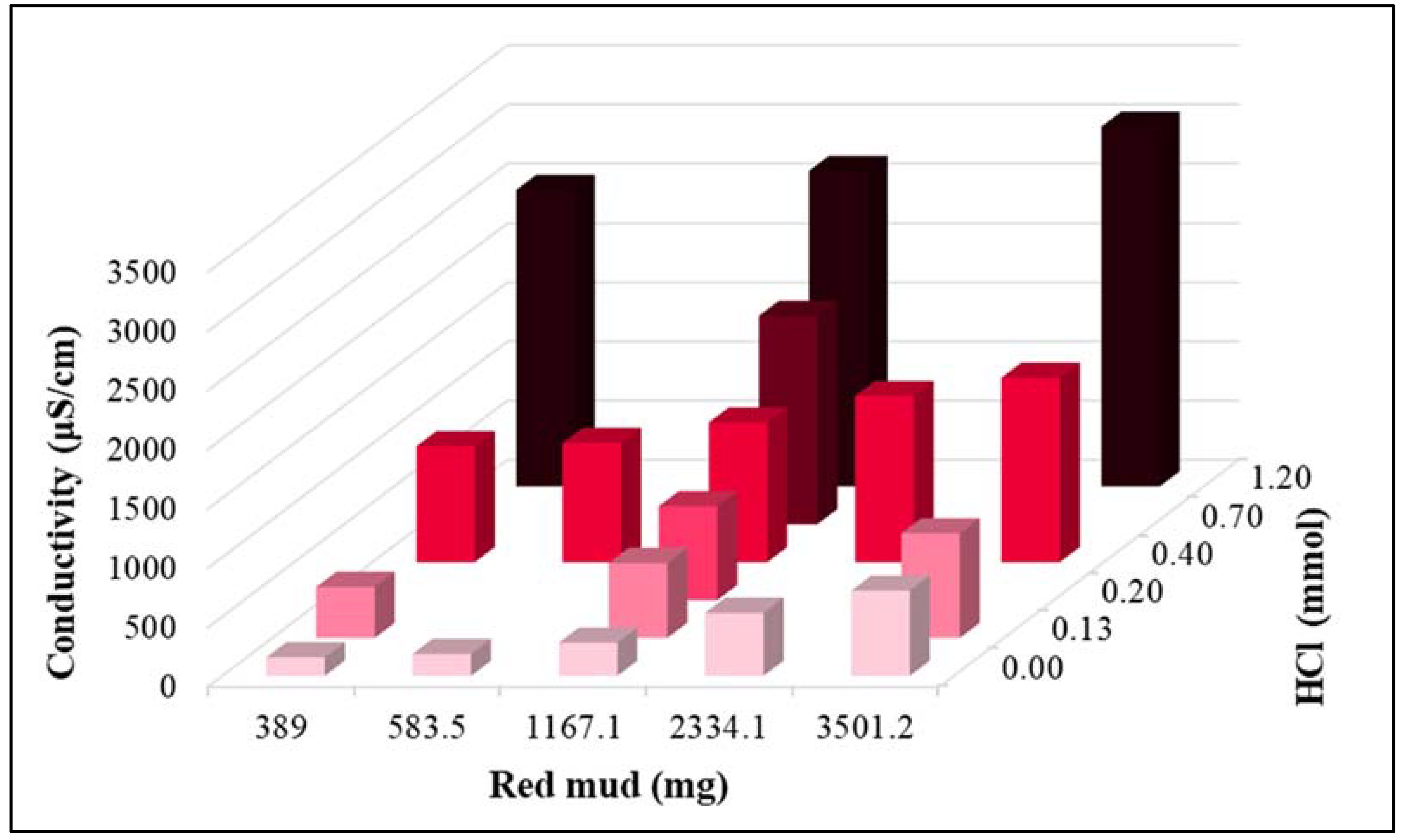
| 0.0 | 0.00 | 7.54 | 7.26 | 1597 |
| 389.5 | 0.13 | 7.12 | 4.30 | 1853 |
| 583.9 | 0.20 | 7.15 | 0.82 | 2035 |
| 1167.4 | 0.40 | 7.15 | 0.05 | 2530 |
| 2334.4 | 0.70 | 7.24 | 0.02 | 3355 |
| 3501.6 | 1.20 | 7.41 | 0.10 | 4325 |
| Doses | Results after 20 min | |||
|---|---|---|---|---|
| RM | HCl | pH | OP | Conductivity |
| mg | mmol | - | mg/L | µS/cm |
| 0 | 0 | 7.79 | 4.98 | 1405 |
| 195.6 | 0.065 | 7.61 | 4.40 | 1485 |
| 293.3 | 0.100 | 7.52 | 3.84 | 1525 |
| 582.9 | 0.200 | 7.41 | 2.57 | 1706 |
| 1168.2 | 0.350 | 7.53 | 0.91 | 2075 |
| 1751.7 | 0.600 | 7.01 | 0.05 | 2635 |
| Doses | After 20 min Mixing | |||
|---|---|---|---|---|
| RM | HCl | pH | OP | Conductivity |
| mg | mmol | - | mg/L | mS/cm |
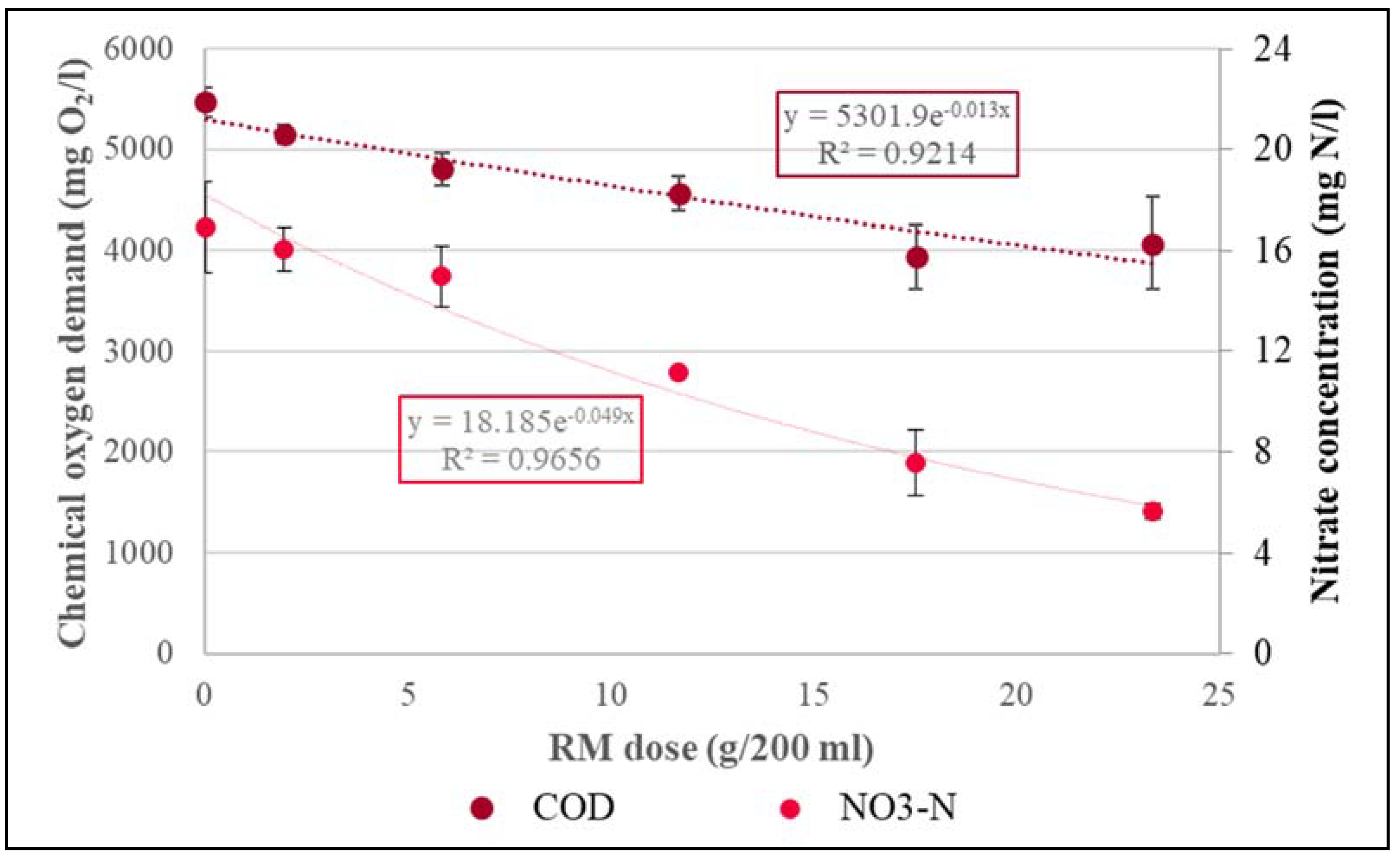
| 0 | 0 | 9.08 | 11.89 | 17.83 |
| 389.02 | 18 | 8.20 | 10.41 | 24.33 |
| 1167.06 | 18 | 8.09 | 4.36 | 25.20 |
| 2334.13 | 18 | 8.14 | 1.64 | 26.33 |
| 3501.19 | 18 | 8.12 | 0.57 | 27.50 |
| 4668.24 | 18 | 8.00 | 0.46 | 28.13 |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| R2 | qs mg/g | KL L/mg | α | R2 | n | KF | α | |
| Synthetic P solution | 0.995 | 4.155 | 1.931 | 0.0002 | 0.977 | 2.070 | 2.307 | 0.0015 |
| Spiked municipal ww * | 0.997 | 0.800 | 21.639 | 0.0017 | 0.938 | 11.794 | 0.720 | 0.0316 |
| SBR effluent | 0.990 | 1.546 | 35.383 | 0.0004 | 0.399 | 5.275 | 1.385 | 0.2530 |
| Landfill leachate * | 0.988 | 0.313 | 0.992 | 0.0060 | 0.964 | 2.544 | 0.145 | 0.0184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somogyi, V.; Pitás, V.; Berta, K.M.; Kurdi, R. Red Mud as Adsorbent to Recover Phosphorous from Wastewater Streams. Sustainability 2022, 14, 13202. https://doi.org/10.3390/su142013202
Somogyi V, Pitás V, Berta KM, Kurdi R. Red Mud as Adsorbent to Recover Phosphorous from Wastewater Streams. Sustainability. 2022; 14(20):13202. https://doi.org/10.3390/su142013202
Chicago/Turabian StyleSomogyi, Viola, Viktória Pitás, Kinga M. Berta, and Róbert Kurdi. 2022. "Red Mud as Adsorbent to Recover Phosphorous from Wastewater Streams" Sustainability 14, no. 20: 13202. https://doi.org/10.3390/su142013202







