Geochemical and Advanced Electron Microscopical Characterisations of Artisanal Gold Mining Rejects in Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Samples
2.2. Analytical Methods
3. Results
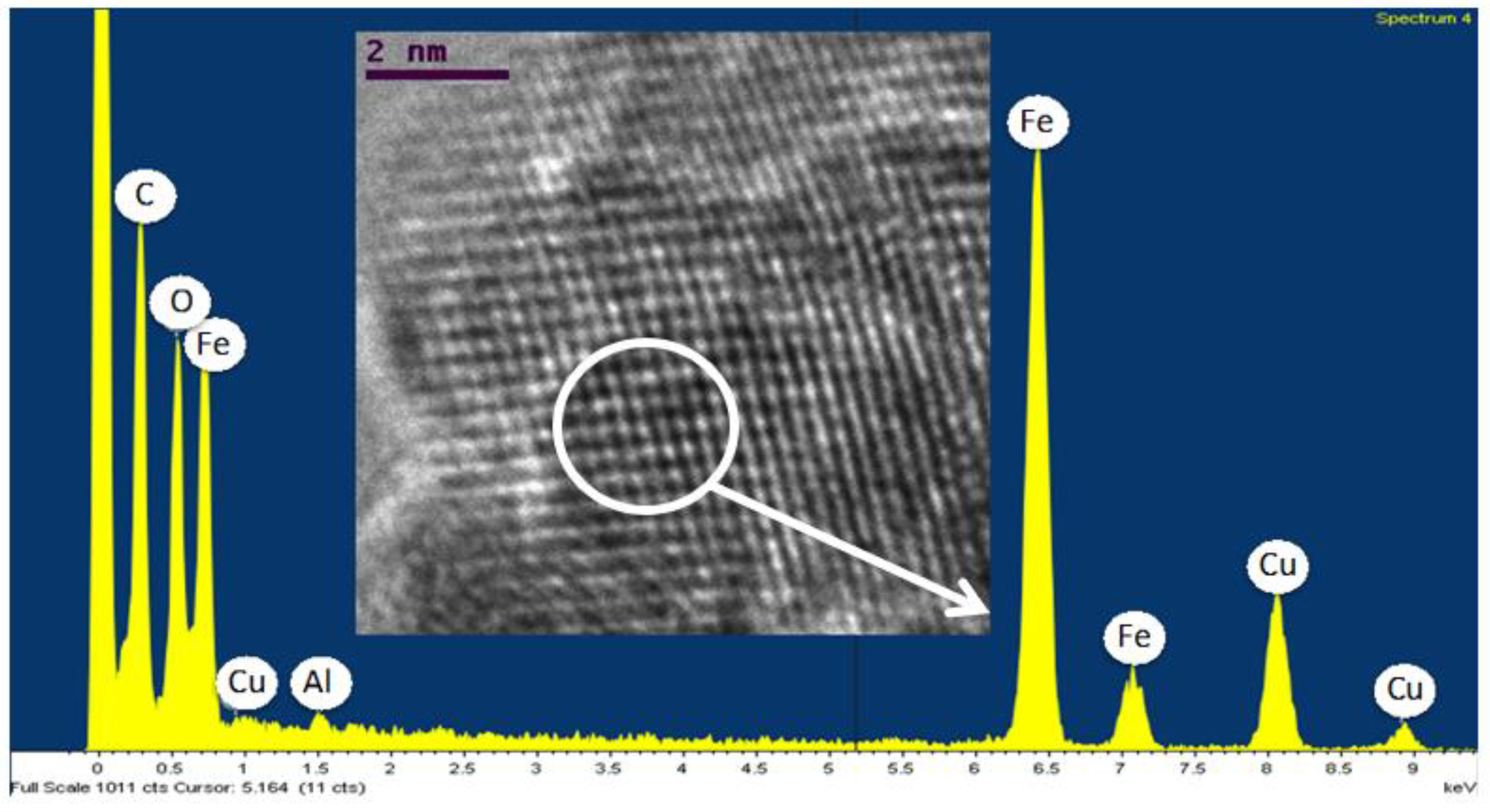
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Peña, N.E.; Narváez-Semanate, J.L.; Pabón-Patiño, D.; Fernández-Mera, J.E.; Marcos LSOliveira, M.L.S.; da Boit, K.; Tutikian, B.F.; Crissien, T.J.; Pinto, D.C.; Serrano, I.D. Chemical and nano-mineralogical study for determining potential uses of legal Colombian gold mine sludge: Experimental evidence. Chemosphere 2018, 191, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Torres, Y.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 2018, 193, 421–430. [Google Scholar] [CrossRef]
- Kessler, R. The Minamata Convention on Mercury: A First Step toward Protecting Future Generations. Natl. Inst. Environ. Health Sci. 2013, 121, A304–A309. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.C.; Keane, S.E.; Basu, N.; Buck, D. Evaluating the effectiveness of the Minamata convention on Mercury: Principles and recommendations for next steps. Sci. Total Environ. 2016, 569–570, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Mackey, T.K.; Contreras, J.T.; Liang, B.A. The Minamata Convention on Mercury: Attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci. Total Environ. 2014, 472, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Selin, H.; Keane, S.E.; Wang, S.; ESelin, N.E.; Davis, K.; Bally, D. Linking science and policy to support the implementation of the Minamata Convention on Mercury. Ambio 2018, 47, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Armah, F.A.; Luginaah, I.N.; Taabazuing, J.; Odoi, J.O. Artisanal gold mining and surface water pollution in Ghana: Have the foreign invaders come to stay? Environ. Justice 2013, 6, 94–102. [Google Scholar] [CrossRef]
- Dupee, M.C. Already a Scourge, Illegal Gold Mining in Colombia Is Getting Worse. 2018. Available online: https://www.worldpoliticsreview.com/insights/25266/already-a-scourge-illegal-gold-mining-in-colombia-is-getting-worse (accessed on 27 July 2018).
- Veiga, M.M.; Masson, P.; Perron, D.; Laflamme, A.C.; Gagnon, R.; Jimenez, G.; Marshall, B.G. An affordable solution for micro-miners in Colombia to process gold ores without mercury. J. Clean. Prod. 2018, 205, 995–1005. [Google Scholar] [CrossRef]
- Torrance, K.W.; Redwood, S.D.; Cecchi, A. The impact of artisanal gold mining, ore processing and mineralization on water quality in Marmato, Colombia. Environ. Geochem. Health 2021, 43, 4265–4282. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Carranza-Lopez, L.; Caballero-Gallardo, K.; Ripoll-Arboleda, A.; Muñoz-Sosa, D. Human exposure and risk assessment associated with mercury pollution in the Caqueta River, Colombian Amazon. Environ. Sci. Pollut. Res. 2016, 23, 20761–20771. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.K.; Saikia, J.; Rabha, S.; Silva, L.F.O.; Finkelman, R. Ambient nanoparticles/nanominerals and hazardous elements from coal combustion activity: Implications on energy challenges and health hazards. Geosci. Front. 2018, 9, 863–875. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Da Boit, K.; Schneider, I.L.; Teixeira, E.C.; Borrero, T.J.C.; Silva, L.F.O. Study of coal cleaning rejects by FIB and sample preparation for HR-TEM: Mineral surface chemistry and nanoparticle-aggregation control for health studies. J. Clean. Prod. 2018, 188, 662–669. [Google Scholar] [CrossRef]
- León-Mejía, G.; Machado, M.N.; Okuro, R.T.; Silva, L.F.; Telles, C.; Dias, J.; Niekraszewicz, L.; Da Silva, J.; Henriques, J.A.P.; Zin, W.A. Intratracheal instillation of coal and coal fly ash particles in mice induces DNA damage and translocation of metals to extrapulmonary tissues. Sci. Total Environ. 2018, 625, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.P.; Da Silva, J.; de Souza, C.T.; Niekraszewicz, L.A.B.; Dias, J.F.; da Boit, K.; Oliveira, M.L.S.; Grivicich, I.; Garcia, A.L.H.; Silva, L.F.O. In vitro genotoxic effect of secondary minerals crystallized in rocks from coal mine drainage. J. Hazard. Mater. 2018, 346, 263–272. [Google Scholar] [CrossRef]
- Xia, F.; Qu, L.; Wang, T.; Luo, L.; Chen, H.; Dahlgren, R.A.; Zhang, M.; Mei, K.; Huang, H. Distribution and source analysis of heavy metal pollutants in sediments of a rapid developing urban river system. Chemosphere 2018, 207, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-de-Francisco, J.C.; del Cairo, C.; Ortiz-Gallego, D.; Velez-Triana, J.S.; Vergara-Gutiérrez, T.; Hein, J. Post-conflict transition and REDD+ in Colombia: Challenges to reducing deforestation in the Amazon. For. Policy Econ. 2021, 127, 102450. [Google Scholar] [CrossRef]
- Alvarez-Berríos, N.L.; L’Roe, J.; Naughton-Treves, L. Does formalizing artisanal gold mining mitigate environmental impacts? Deforestation evidence from the Peruvian Amazon. Environ. Res. Lett. 2021, 16, 064052. [Google Scholar] [CrossRef]
- Cerqueira, B.; Vega, F.A.; Silva, L.F.; Andrade, L. Effects of vegetation on chemical and mineralogical characteristics of soils developed on a decantation bank from a copper mine. Sci. Total Environ. 2012, 421–422, 220–229. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Vega, F.A.; Silva, L.F.O.; Andrade, M.L. Copper distribution in surface and subsurface soil horizons. Environ. Sci. Pollut. Res. 2014, 21, 10997–11008. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, S.A.; Gitari, W.M.; Petrik, L.F.; Nyakuma, B.B.; Hower, J.C.; Ward, C.R.; Oliveira, M.L.; Silva, L.F. Environmental evaluation and nano-mineralogical study of fresh and unsaturated weathered coal fly ashes. Sci. Total Environ. 2019, 663, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, S.A.; Nyakuma, B.B.; Jauro, A.; Olanipekun, T.A.; Mudzielwana, R.; Gitari, M.W.; Saikia, B.K.; Dotto, G.L.; Hower, J.C.; Silva, L.F.O. Rare earth elements study of Cretaceous coals from Benue Trough basin, Nigeria: Modes of occurrence for greater sustainability of mining. Fuel 2021, 304, 121468. [Google Scholar] [CrossRef]
- Quispe, D.; Pérez-López, R.; Silva, L.F.O.; Nieto, J.M. Changes in mobility of hazardous elements during coal combustion in Santa Catarina power plant (Brazil). Fuel 2012, 94, 495–503. [Google Scholar] [CrossRef]
- Zaafarani, N.; Raabe, D.; Singh, R.N.; Roters, F.; Zaefferer, S. Three-dimensional investigation of the texture and microstructure below a nanoindent in a Cu single crystal using 3D EBSD and crystal plasticity finite element simulations. Acta Mater. 2006, 54, 1863–1876. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Ward, C.; Izquierdo, M.; Sampaio, C.H.; de Brum, I.A.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F. Chemical composition and minerals in pyrite ash of an abandoned sulphuric acid production plant. Sci. Total Environ. 2012, 430. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; da Boit, K.; Pacheco, F.; Teixeira, E.C.; Schneider, I.L.; Crissien, T.J.; Pinto, D.C.; Oyaga, R.M.; Silva, L.F. Multifaceted processes controlling the distribution of hazardous compounds in the spontaneous combustion of coal and the effect of these compounds on human health. Environ. Res. 2018, 160, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Izquierdo, M.; Querol, X.; Finkelman, R.B.; Oliveira, M.L.S.; Wollenschlager, M.; Towler, M.; Pérez-López, R.; Macias, F. Leaching of potential hazardous elements of coal cleaning rejects. Environ. Monit. Assess. 2011, 175, 109–126. [Google Scholar] [CrossRef]
- Silva, L.; Macias, F.; Oliveira, M.; da Boit, M.K.; Waanders, F.B. Coal cleaning residues and Fe-minerals implications. Environ. Monit. Assess. 2011, 172, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Meischein, M.; Ludwig, A. Rapid Assessment of Sputtered Nanoparticle Ionic Liquid Combinations. ACS Comb. Sci. 2018, 20, 243–250. [Google Scholar] [CrossRef]
- Garzón-Manjón, A.; Meyer, H.; Grochla, D.; Löffler, T.; Schuhmann, W.; Ludwig, A.; Scheu, C. Controlling the Amorphous and Crystalline State of Multinary Alloy Nanoparticles in An Ionic Liquid. Nanomaterials 2018, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Valentim, B.; Ward, C.; Flores, D. Comprehensive characterization of anthracite fly ash from a thermo-electric power plant and its potential environmental impact. Int. J. Coal Geol. 2011, 86, 204–212. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S.; Neace, E.R.; O’Keefe, J.M.K.; Henke, K.R.J.; Hower, J.C. Nanominerals and ultrafine particles in sublimates from the Ruth Mullins coal fire, Perry County, Eastern Kentucky, USA. Int. J. Coal Geol. 2011, 85, 237–245. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Milanes, C.; Diana Pinto, D.; Ramirez, O.; Lima, B.D. Multiple hazardous elements in nanoparticulate matter from a Caribbean industrialized atmosphere. Chemosphere 2020, 239, 124776. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.O.; Teixeira, E.C.; Agudelo-Castañeda, D.M.; Braga, M.; Alabarse, P.G.; Wiegand, F.; Kautzmann, R.M.; Silva, L.F. Assessment of nitro-polycyclic aromatic hydrocarbons in PM1 near an area of heavy-duty traffic. Sci. Total Environ. 2014, 479-480, 57–65. [Google Scholar] [CrossRef]
- Engin, A.B. Combined Toxicity of Metal Nanoparticles: Comparison of Individual and Mixture Particles Effect. In Protein Kinase-Mediated Decisions Between Life and Death; Springer: Cham, Switzerland, 2021; pp. 165–193. [Google Scholar]
- Kim, H.; Bishop, J.K.B.; Dietrich, W.E.; Fung, I.Y. Process dominance shift in solute chemistry as revealed by long-term high-frequency water chemistry observations of groundwater flowing through weathered argillite underlying a steep forested hillslope. Geochim. Cosmochim. Acta 2014, 140, 1–19. [Google Scholar] [CrossRef]
- Civeira, M.; Oliveira, M.; Hower, J.C.; Agudelo-Castañeda, D.M.; Taffarel, S.R.; Ramos, C.G.; Kautzmann, R.M.; Silva, L.F.O. Modification, adsorption, and geochemistry processes on altered minerals and amorphous phases on the nanometer scale: Examples from copper mining refuse, Touro, Spain. Environ. Sci. Pollut. Res. 2015, 23, 6535–6545. [Google Scholar] [CrossRef]
- Civeira, M.S.; Pinheiro, R.N.; Gredilla, A.; de Vallejuelo, S.F.O.; Oliveira, M.L.; Ramos, C.G.; Taffarel, S.R.; Kautzmann, R.M.; Madariaga, J.M.; Silva, L.F. The properties of the nano-minerals and hazardous elements: Potential environmental impacts of Brazilian coal waste fire. Sci. Total Environ. 2016, 544, 892–900. [Google Scholar] [CrossRef]
- Hower, J.C.; O’Keefe, J.M.; Henke, K.R.; Wagner, N.J.; Copley, G.; Blake, D.R.; Garrison, T.; Oliveira, M.L.; Kautzmann, R.M.; Silva, L.F. Gaseous emissions and sublimates from the Truman Shepherd coal fire, Floyd County, Kentucky: A re-investigation following attempted mitigation of the fire. Int. J. Coal Geol. 2013, 116–117, 63–74. [Google Scholar] [CrossRef]
- Martinello, K.; Oliveira, M.L.; Molossi, F.A.; Ramos, C.G.; Teixeira, E.C.; Kautzmann, R.M.; Silva, L.F. Direct identification of hazardous elements in ultra-fine and nanominerals from coal fly ash produced during diesel co-firing. Sci. Total Environ. 2014, 470–471, 444–452. [Google Scholar] [CrossRef]
- Schindler, M.; Hochella, M.F., Jr. Soil memory in mineral surface coatings: Environmental processes recorded at the nanoscale. Geology 2015, 43, 415–418. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, 6434. [Google Scholar] [CrossRef] [PubMed]
- Hower, J.C.; Campbell, J.; Teesdale, W.J.; Nejedly, Z.; Robertson, J.D. Scanning proton microprobe analysis of mercury and other trace elements in Fe-sulfides from a Kentucky coal. Int. J. Coal Geol. 2008, 75, 88–92. [Google Scholar] [CrossRef]
- Slater, E.T.; Kontak, D.J.; Mcdonald, A.M.; Fayek, M. Origin of a multi-stage epithermal Ag-Zn-Pb-Sn deposit: The Miocene Cortaderas breccia body, Pirquitas mine, NW Argentina. Miner. Depos. 2021, 56, 381–406. [Google Scholar] [CrossRef]
- Ramos, V.A. Las provincias geológicas del noroeste argentino. Cienc. De La Tierra Y Recur. Nat. Del NOA. Relat. Del XX Congr. Geológico Argent. 2017, 1–15. [Google Scholar]
- Silva, L.F.O.; Oliveira, M.L.S.; Serra, C.; Hower, J.C. Zinc speciation in power plant burning mixtures of coal and tires. Coal Combust. Gasif. Prod. 2011, 3, 41–50. [Google Scholar] [CrossRef]

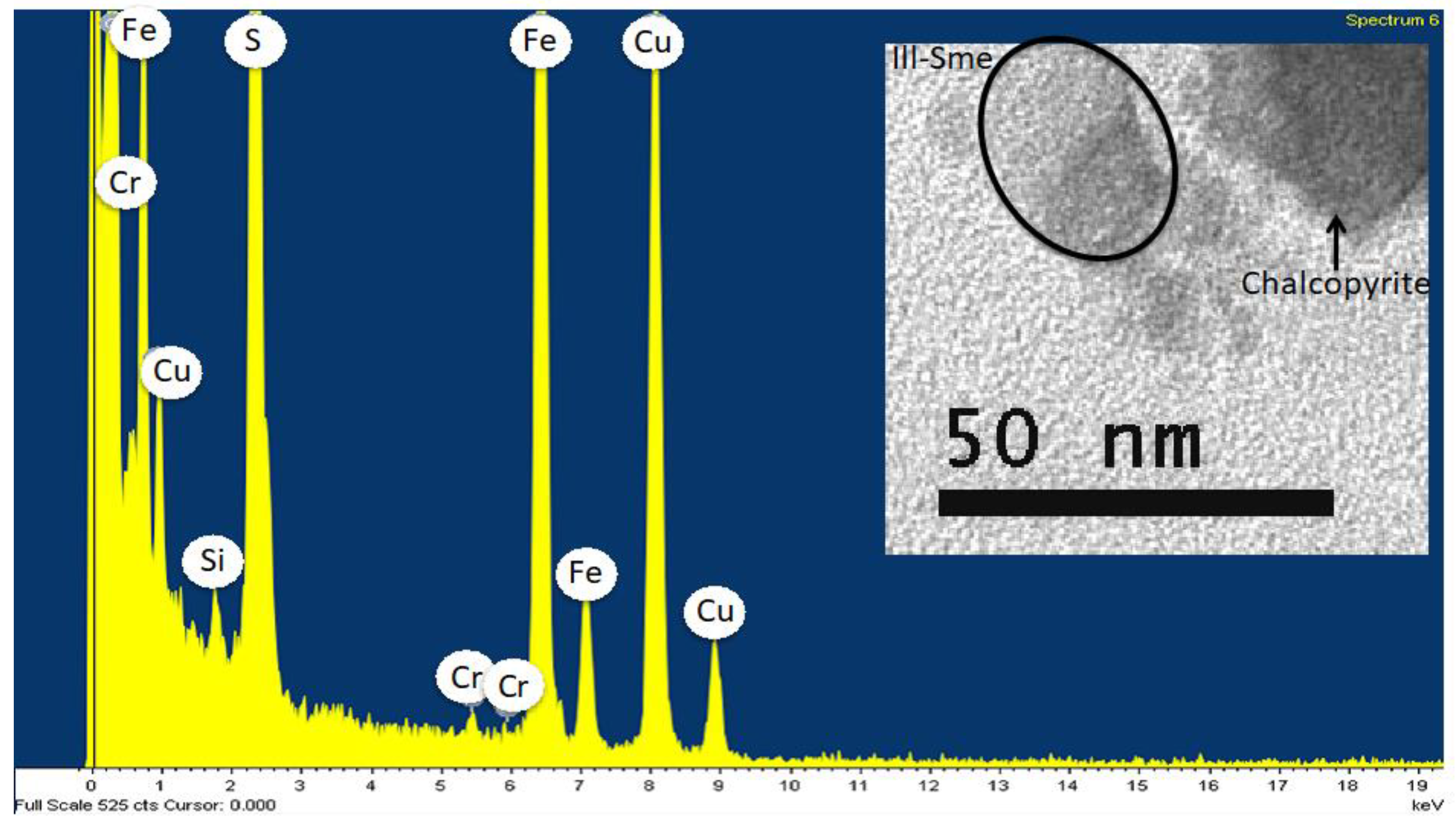
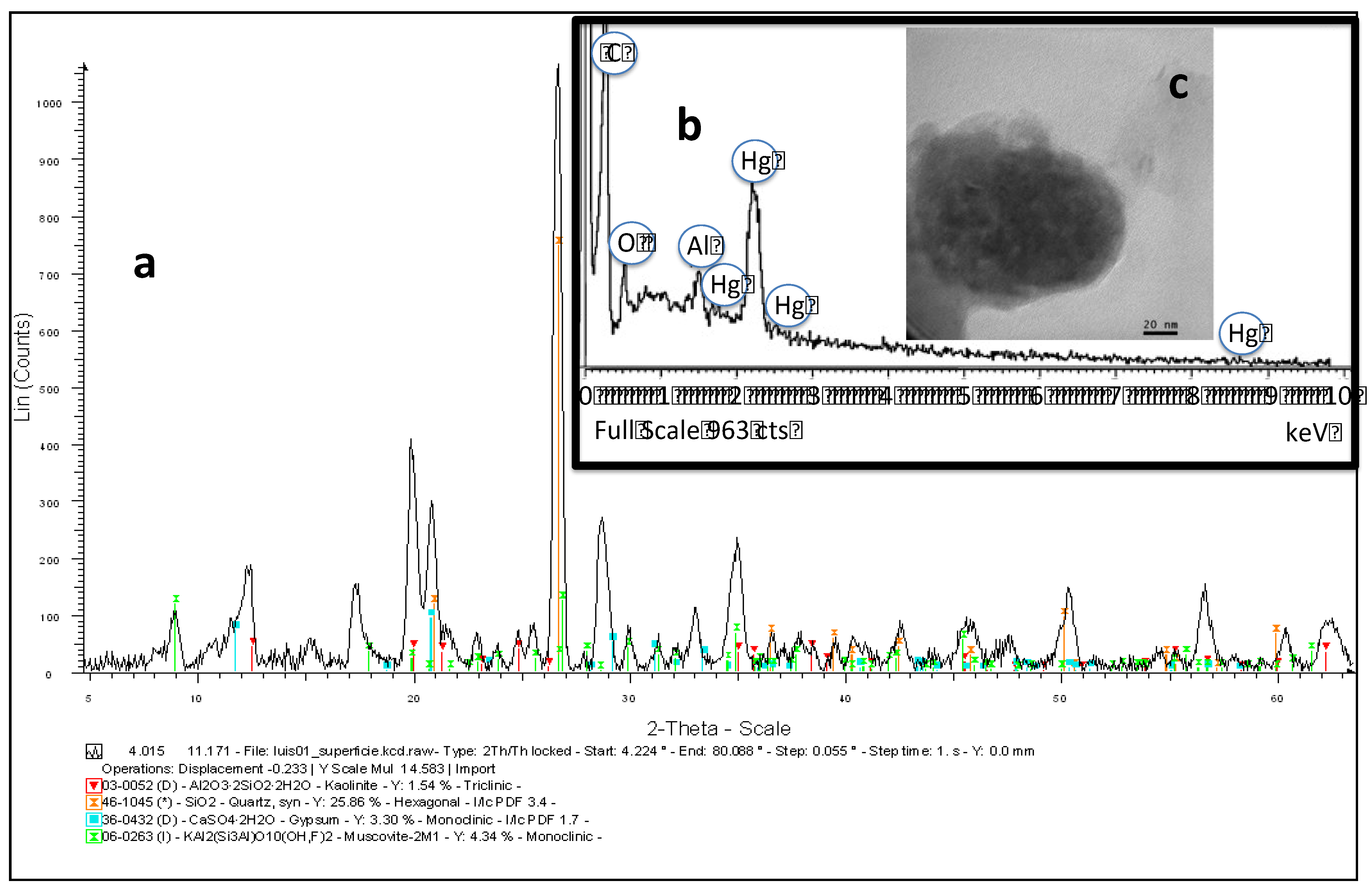
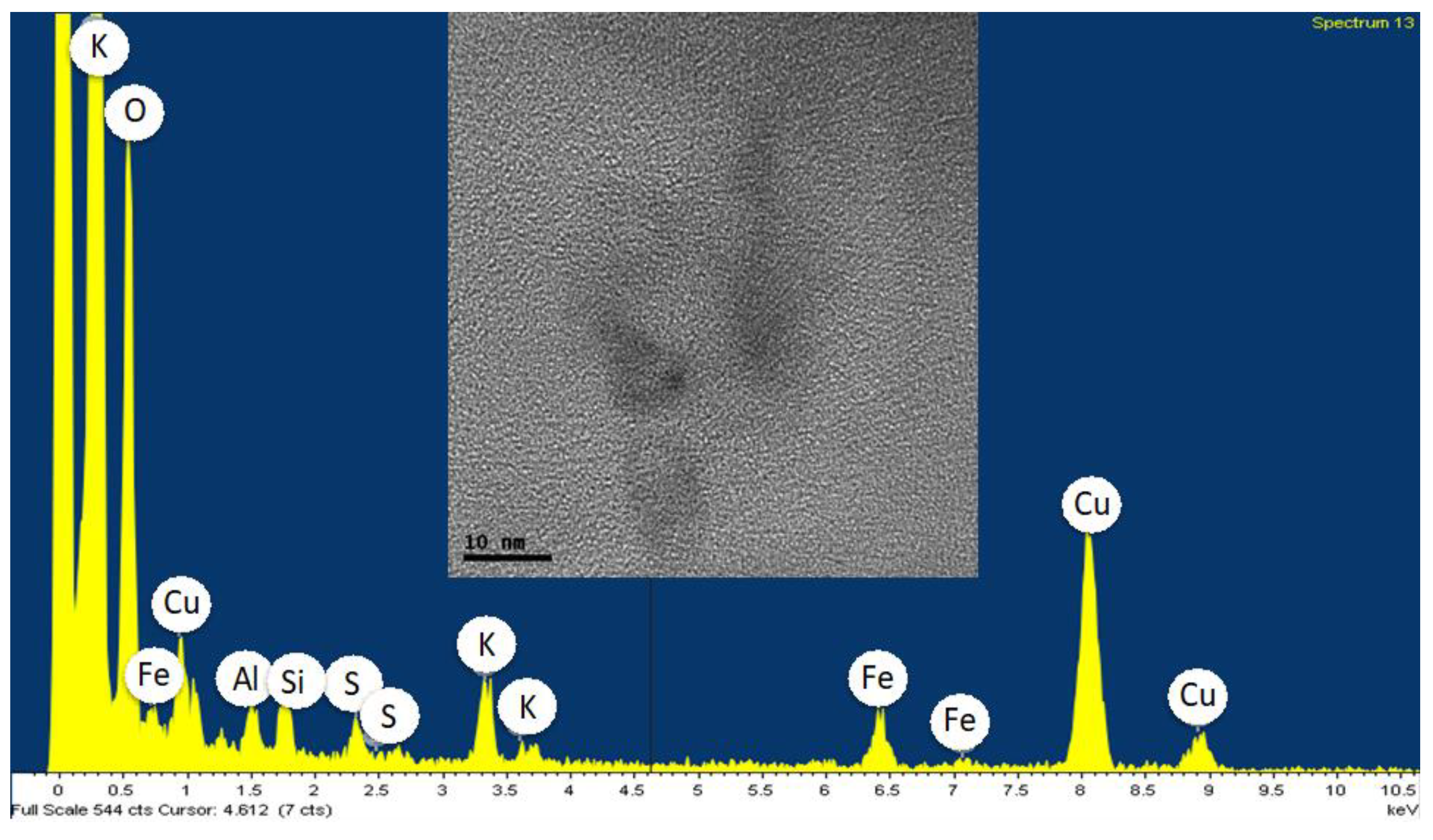
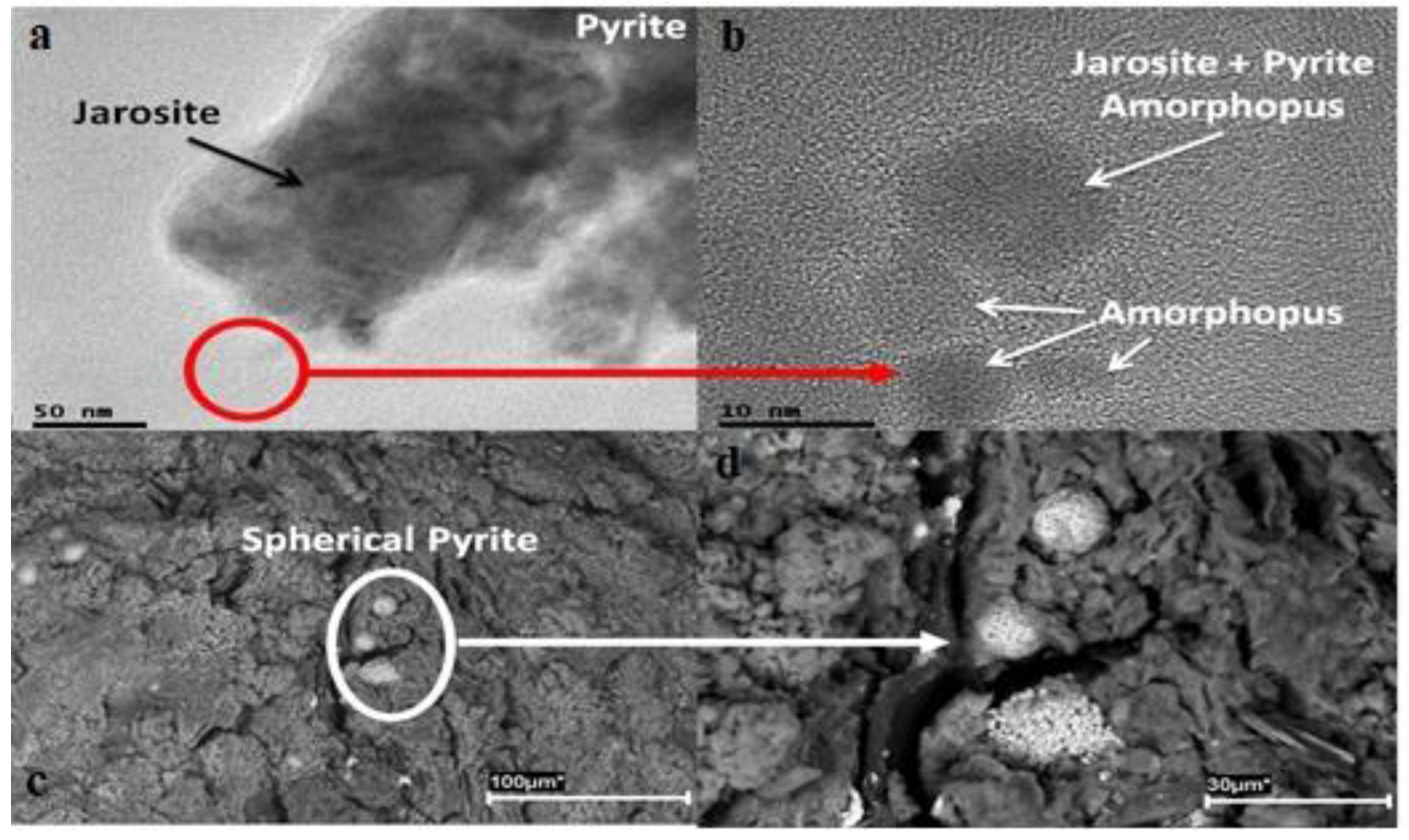

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyemi, S.A.; Mercado-Caruso, N.; Nyakuma, B.B.; Oliveira, M.L.S. Geochemical and Advanced Electron Microscopical Characterisations of Artisanal Gold Mining Rejects in Colombia. Sustainability 2022, 14, 13245. https://doi.org/10.3390/su142013245
Akinyemi SA, Mercado-Caruso N, Nyakuma BB, Oliveira MLS. Geochemical and Advanced Electron Microscopical Characterisations of Artisanal Gold Mining Rejects in Colombia. Sustainability. 2022; 14(20):13245. https://doi.org/10.3390/su142013245
Chicago/Turabian StyleAkinyemi, Segun A., Nohora Mercado-Caruso, Bemgba B. Nyakuma, and Marcos L. S. Oliveira. 2022. "Geochemical and Advanced Electron Microscopical Characterisations of Artisanal Gold Mining Rejects in Colombia" Sustainability 14, no. 20: 13245. https://doi.org/10.3390/su142013245
APA StyleAkinyemi, S. A., Mercado-Caruso, N., Nyakuma, B. B., & Oliveira, M. L. S. (2022). Geochemical and Advanced Electron Microscopical Characterisations of Artisanal Gold Mining Rejects in Colombia. Sustainability, 14(20), 13245. https://doi.org/10.3390/su142013245







