Differential Physiological Traits, Ion Homeostasis and Cane Yield of Sub-Tropical Sugarcane Varieties in Response to Long-Term Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
3. Results
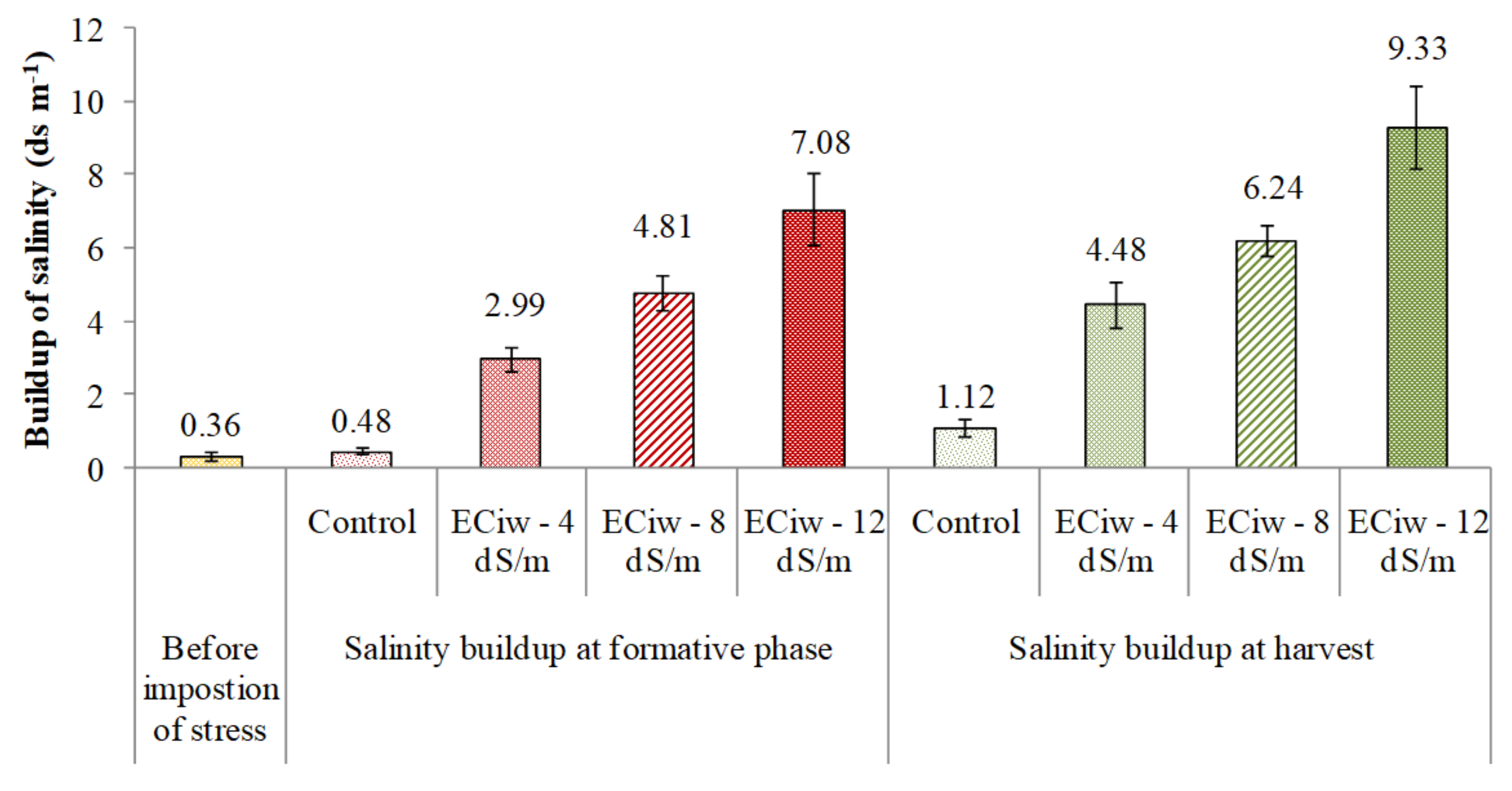
3.1. Build-Up of Salinity in Soil
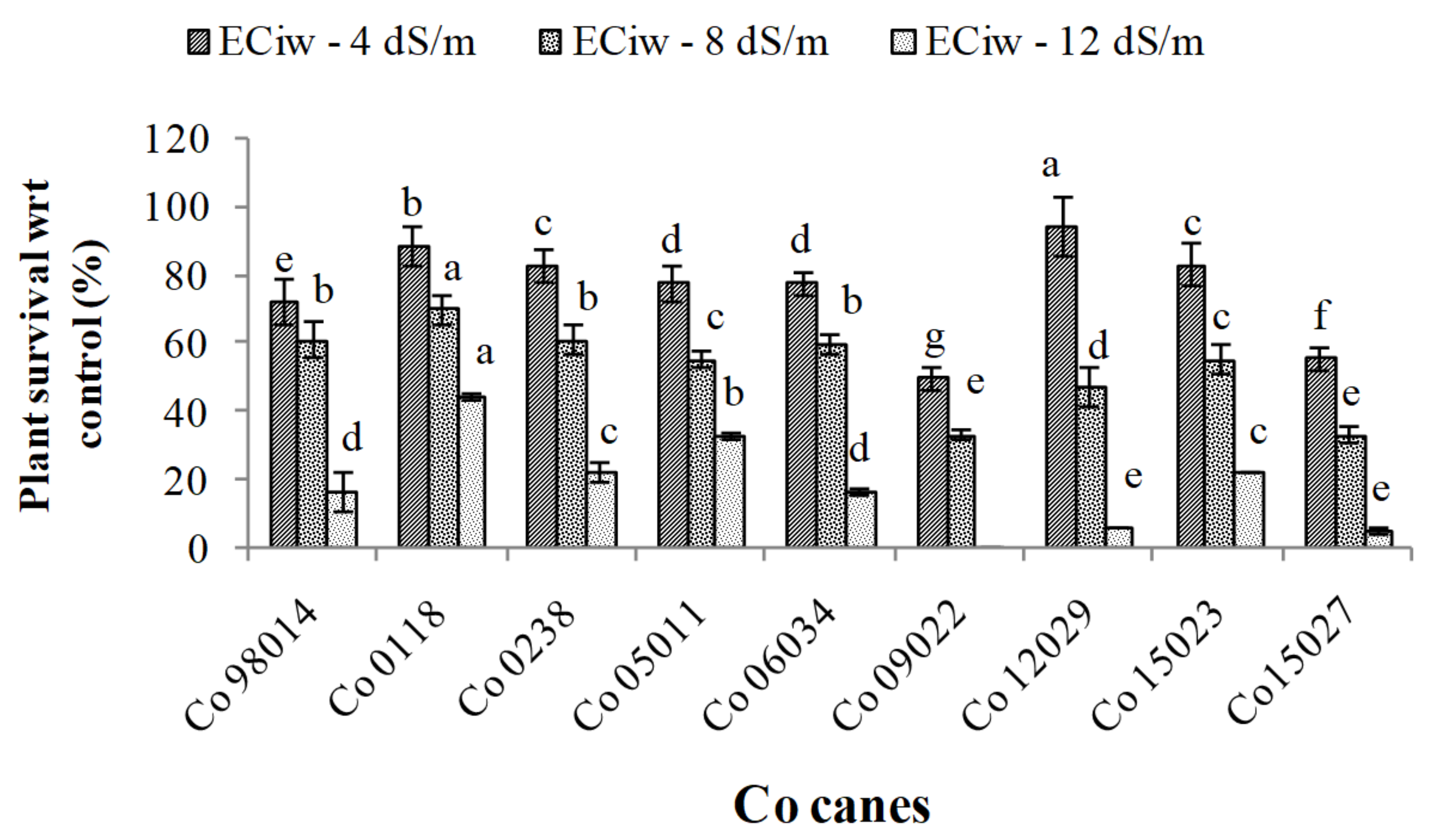
3.2. Plant Survival under Different Levels of Salinity
3.3. Effect of Salinity on Cane Length and RWC
3.4. Effect of Salinity on Gas Exchange Attributes
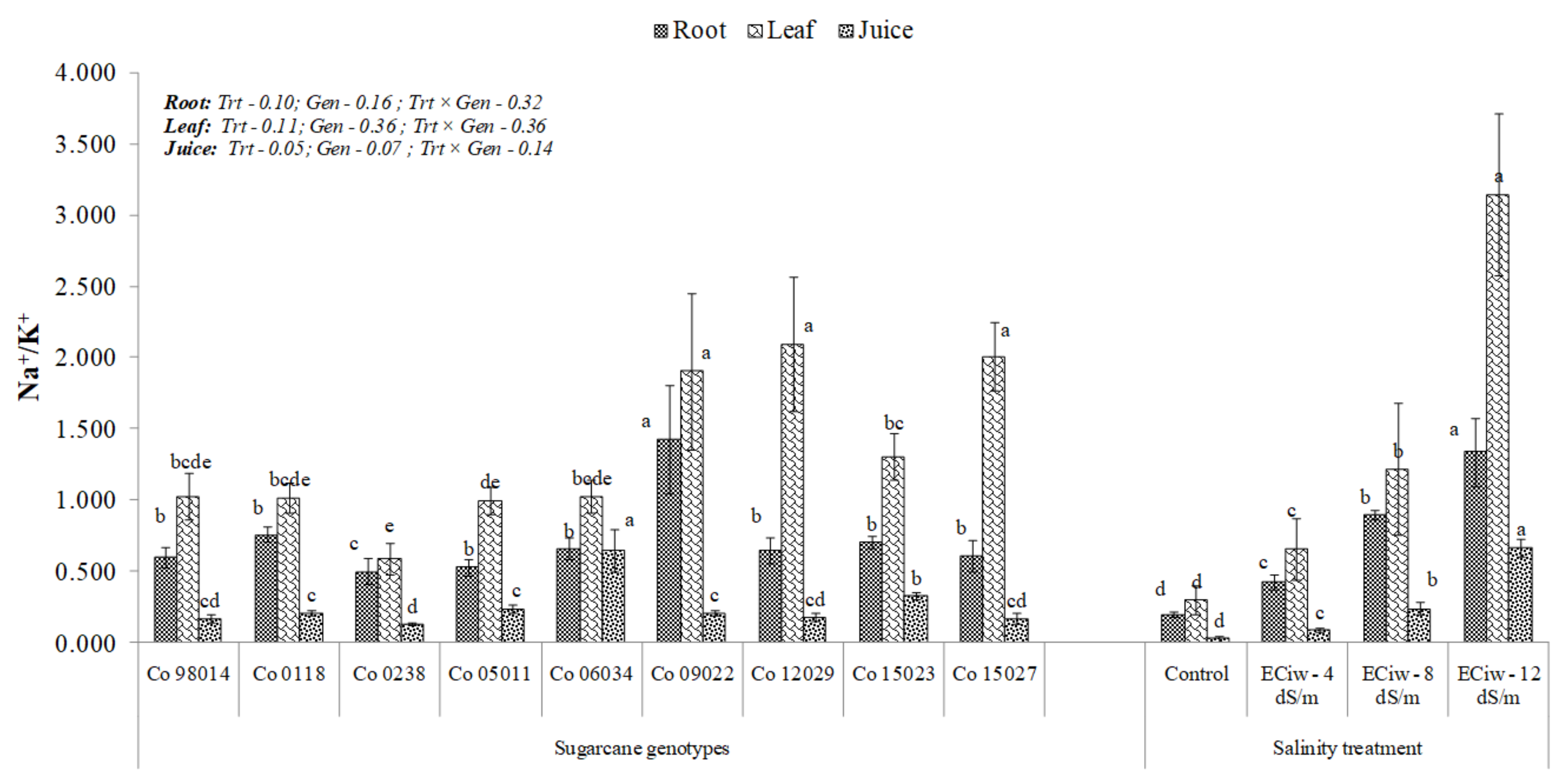
3.5. Effect of Salinity on Ion Dynamics
3.6. Effect of Salinity on SCW
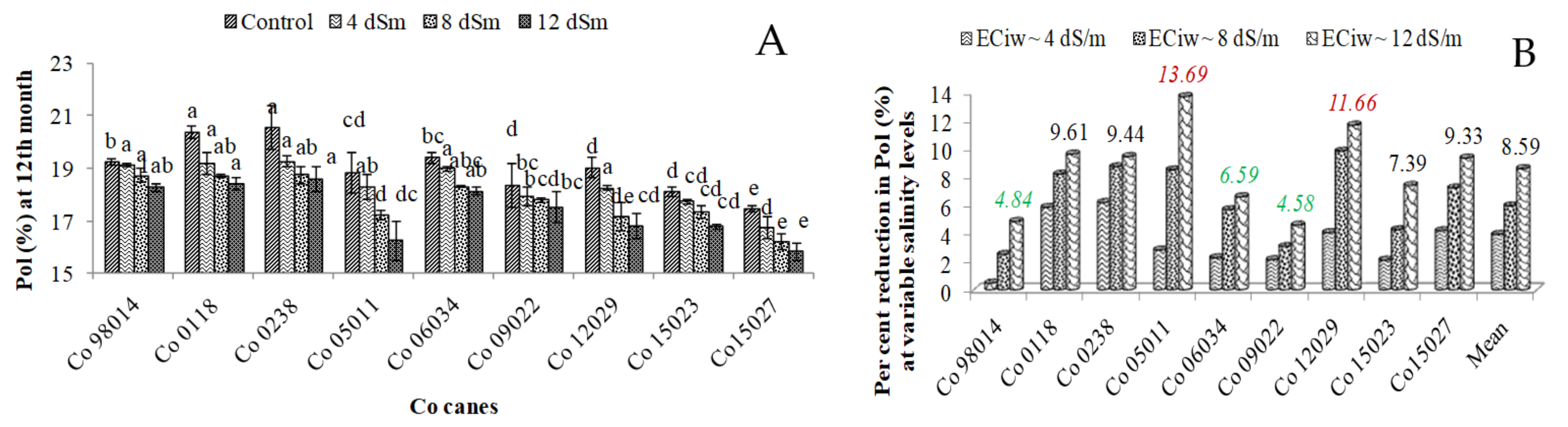
3.7. Effect of Salinity on Sucrose Content
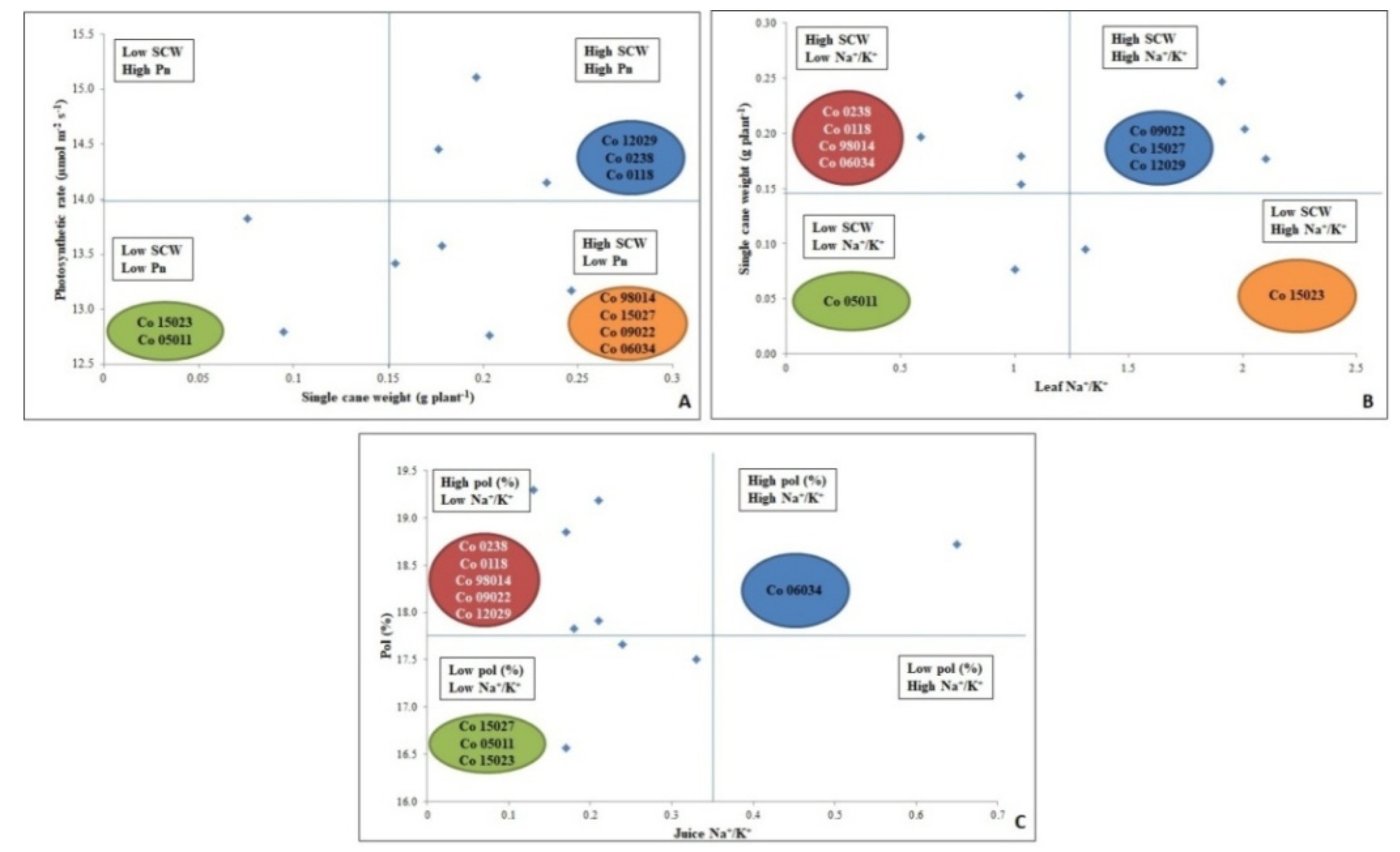
3.8. Biplot Analysis Showing Differential Response of Sugarcane Varieties
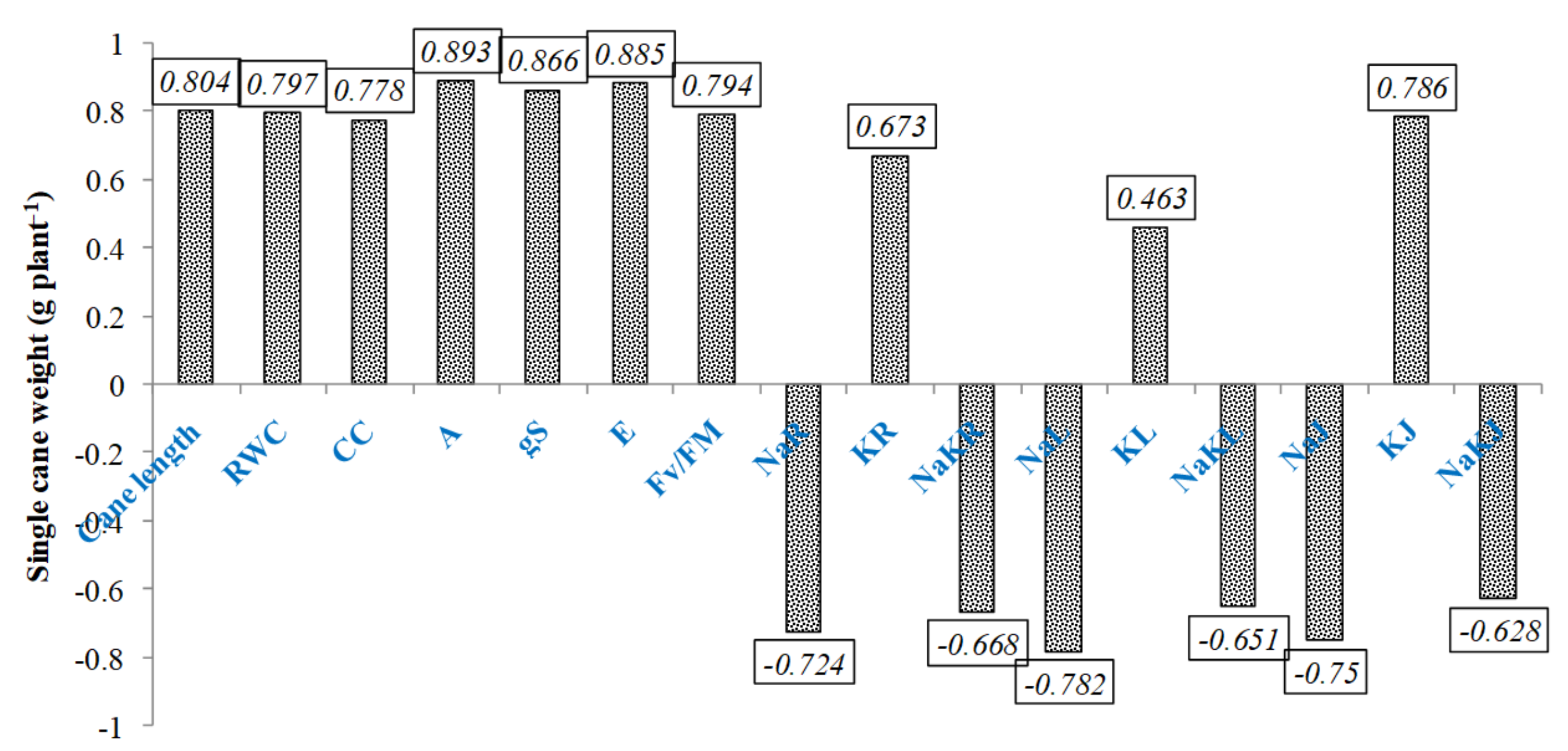
3.9. Traits Modeling for Cane Yield under Salinity Stress
4. Discussion
4.1. Effect of Salinity on Survival (%) and Cane Length
4.2. Effect of Salinity on Physiological Traits
4.3. Effect of Salinity on Ion Dynamics
4.4. Effect of Salinity on Cane Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECse | Electrical conductivity of saturated extract |
| ECiw | Electrical conductivity of irrigation water |
| Pn | photosynthetic rate |
| gS | stomatal conductance |
| E | transpiration rate |
| NMC | Number of millable canes |
| CCS | Commercial cane sugar |
| SCW | Single cane weight |
| FW | Fresh weight |
| DW | Dry weight |
References
- FAO. Area, Production and Productivity of Livestock, Food and Agriculture Organization of United Nations. Food and Agriculture Organization of the United Nations, Database on Crops, 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 May 2022).
- Pooja; Nandwal, A.S.; Chand, M.; Kumari, A.; Rani, B.; Goel, V.; Singh, S. Genotypic differences in growth behavior and quality parameters of sugarcane (Saccharum officinarum) varieties under moisture stress conditions. Ind. J. Agr. Sci. 2019, 89, 65–72. [Google Scholar]
- Vision S.B.I. 2030; Vision Document; Sugarcane Breeding Institute: Coimbatore, India, 2011.
- Kumar, P.; Sharma, P.K. Soil Salinity and Food Security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Vision C.S.S.R.I. 2050; Vision Document; ICAR—Central Soil Salinity Research Institute: Karnal, India, 2017.
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance current assessment. J. Irrig. Drain. Div. Amer. Soc. Civil Eng. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Rao, V.P.; Sengar, R.S.; Singh, S.; Sharma, V. Molecular and metabolic perspectives of sugarcane under salinity stress pressure. Prog. Agr. 2015, 15, 77–84. [Google Scholar]
- Plaut, Z.; Meinzer, F.C.; Federman, E. Leaf development, transpiration and ion uptake and distribution in Sugarcane cultivarsgrown under salinity. Plant Soil 2000, 218, 59–69. [Google Scholar] [CrossRef]
- Santana, M.J.; Carvalho, J.A.; Souza, K.J.; Sousa, A.M.G.; Vasconcelos, C.L.; Andrade, L.A.B. Lab effects of irrigationwater salinity on sprouting and initial development of sugarcane (saccharum sp.) and in soils with different textural levels. Rev. Cienc. Agríc. 2007, 31, 1470–1476. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; ArtMed: Guelph, ON, Canada, 2017. [Google Scholar]
- Zuffo, A.M.; Steiner, F.; Aguilera, J.G.; Teodoro, P.E.; Teodoro, L.P.R.; Busch, A. Multi-trait stability index: A tool for simultaneous selection of soya bean in drought and saline stress. J. Agron. Crop Sci. 2020, 206, 815–822. [Google Scholar] [CrossRef]
- Simões, W.L.; Calgaro, M.; Coelho, D.S.; Santos, D.B.D.; Souza, M.A.D. Growth of sugar cane varieties under salinity. Rev. Ceres 2016, 63, 265–271. [Google Scholar] [CrossRef]
- Brindha, C.; Vasantha, S.; Arunkumar, R. The response of sugarcane subjected to salinity stress at differentgrowth phases. J. Plant Stress Physiol. 2019, 5, 28–33. [Google Scholar] [CrossRef][Green Version]
- Simões, W.L.; Coelho, D.S.; Mesquita, A.C.; Calgaro, M.; da Silva, J.S. Physiological and biochemical responses of sugarcane varieties to salt stress. Rev. Caatinga 2019, 32, 1069–1076. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relation of cotton plants. The field measurement of water deficit in leaves. New Phytol. 1950, 49, 81–87. Available online: https://www.jstor.org/stable/2428690 (accessed on 14 June 2022). [CrossRef]
- IRRI. International Rice Research Institute (IRRI): Philippines. 2013. Available online: http://bbi.irri.org/products (accessed on 21 May 2017).
- Kumar, R.; Meena, M.R.; Kulshreshtha, N.; Kumar, A.; Ram, B. Genotypic response of recently evolved sugarcane “Co” clones under different levels of saline irrigation water. J. Sugarcane Res. 2017, 7, 159–168. [Google Scholar]
- Pooja; Nandwal, A.S.; Chand, M.; Singh, K.; Mishra, A.K.; Kumar, A.; Kumari, A.; Rani, B. Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Exp. Biol. 2019, 57, 721–732. [Google Scholar]
- Pooja; Kulshreshtha, N.; Kumar, R.; Raja, A.K.; Pandey, S.K.; Goel, V.; Ram, B. Identification of drought-tolerant genotypes based on physiological traits, yield attributes and drought tolerance indices. Sugar Tech 2021, 23, 741–767. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef]
- Vasantha, S.; Venkataramana, S.; Rao, P.N.G.; Gomathi, R. Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Tech 2010, 12, 5–8. [Google Scholar] [CrossRef]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, N.; Kaur, G.; Kumar, A.; Mann, A. Variability of durum wheat in terms of physio-biochemical traits against salinity stress. Cereal Res. Commun. 2021, 49, 45–54. [Google Scholar] [CrossRef]
- Pooja; Nandwal, A.S.; Chand, M.; Pal, A.; Kumari, A.; Rani, B.; Goel, V.; Kulshreshtha, N. Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (saccharum officinarum) varieties differing in maturity. Ind. J. Agr. Sci. 2020, 90, 507–512. [Google Scholar]
- Garg, N.; Singla, R. Growth, photosynthesis, nodule nitrogen and carbon fixationin the chickpea cultivars under salt stress. Braz. J. Plant Physiol. 2004, 16, 137–146. [Google Scholar] [CrossRef]
- Steduto, P.; Albrizio, R.; Giorio, P.; Sorrentino, G. Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000, 44, 243–255. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Zeng, Y.; Li, D.M.; Guo, D.J.; Rajput, V.D.; Chen, G.L.; Barakhov, A.; Minkina, T.M.; Li, Y.R. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.P.; Verma, C.L.; Malviya, M.K.; Guo, D.J.; Rajput, V.D.; Sharma, A.; Wei, K.J.; Chen, G.L.; Solomon, S.; et al. Predication of photosynthetic leaf gas exchange of sugarcane (saccharum spp) leaves in response to leaf positions to foliar spray of potassium salt of active phosphorus under limited water irrigation. ACS Omega 2021, 6, 2396–2409. [Google Scholar] [CrossRef] [PubMed]
- Stepien, P.; Klobus, G. Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biologia Plant. 2006, 50, 610–616. [Google Scholar] [CrossRef]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Hassan, M.; Sun, N.; et al. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Pimentel, C. Photoinhibition in a C4 plant, Zea mays L.: A minireview. Theor. Expt. Plant Physiol. 2014, 26, 157–165. [Google Scholar] [CrossRef]
- Silva, M.d.A.; Pincelli, R.P.; Barbosa, M.d.A. Water stress effects on chlorophyll fluorescence and chlorophyll content in sugarcanecultivars with contrasting tolerance. Biosci. J. 2018, 34, 75–87. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Yeo, A.R. Ion accumulation in the cell walls of rice plantsgrowing under saline conditions: Evidence for the Oertli hypothesis. Plant Cell Environ. 1991, 14, 319–325. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinaur Associates Inc.: Sunderlan, MA, USA, 2006. [Google Scholar]
- Kumar, A.; Mishra, A.K.; Singh, K.; Lata, C.; Kumar, A.; Krishnamurthy, S.L.; Kumar, P. Diurnal changes and effect of elevated CO2 on gas exchange under individual and interactive salt and water stress in wheat (Triticum aestivum). Ind. J. Agr. Sci. 2019, 89, 763. [Google Scholar]
- Kumar, A.; Mann, A.; Kumar, A.; Kumar, N.; Meena, B.L. Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Internat. J. Phytoremed. 2021, 23, 1041–1051. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef]
- Lata, C.; Soni, S.; Kumar, N.; Kumar, A.; Pooja; Mann, A.; Rani, S. Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agr. Sci. 2019, 89, 1050–1052. [Google Scholar]
- Watanabe, K.; Takaragawa, H.; Ueno, M.; Kawamitsu, Y. Changes in agronomic and physiological traits of sugarcane grown with saline irrigation water. Agronomy 2020, 10, 722. [Google Scholar] [CrossRef]
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318. [Google Scholar] [CrossRef]
- Ashraf, M.; Rahmatullah; Kanwar, S.; Tahi, M.A.; Sarwar, A.; Ali, L. Differential salt tolerance of sugarcane genotypes. Pak. J. Agri. Sci. 2007, 44, 85–89. [Google Scholar]
- Musavi, F.; Lak, S.; Shokuhfar, A.; Modhaj, A. Evaluation of sugarcane (Saccharum officinarum L.) different to the uptake and transport of ions in salt stress conditions. Biol. Forum—Int. J. 2015, 7, 1719–1724. [Google Scholar]
- Granja, M.M.C.; Medeiros, M.J.L.; Silva, M.M.A.; Camara, T.R.; Willadino, L.; Ulisses, C. Response to in vitro salt stress in sugarcane is conditioned by concentration and condition of exposure to NaCl. Acta Biol Colomb. 2018, 23, 30–38. [Google Scholar] [CrossRef]
- Vasantha, S.; Gomathi, R.; Rakkiyappan, P. Sodium content juice and jaggery quality of Sugarcane under salinity. Electron. J. Biol. Sci. 2009, 1, 33–38. [Google Scholar]
- Zhao, D.; Zhu, K.; Momotaz, A.; Gao, X. Sugarcane plant growth and physiological responses to soil salinity during tillering and stalk elongation. Agriculture 2020, 10, 608. [Google Scholar] [CrossRef]
| Soil Parameter | Values |
|---|---|
| pH2 | 7.03 ± 0.47 |
| ECe (dS m−1) | 0.45 ± 0.02 |
| Texture | Sandy loam |
| Organic carbon (%) | 0.697 ± 0.02 |
| Available Nitrogen (N) kg ha−1 | 133.8 ±14.04 |
| Available Phosphorus (P) kg ha−1 | 33.77 ± 4.28 |
| Available Potassium (K) kg ha−1 | 358.4 ± 10.59 |
| Source of Variation | DF | Cane Length | RWC | Chlorophyll Content | Pn | gS | E | Fv/Fm |
|---|---|---|---|---|---|---|---|---|
| Mean Sum of Squares | ||||||||
| Rep | 2 | 447.7 | 1.947 | 0.315 | 1.799 | 0.0001 | 0.289 | 0.007 |
| Treatments (T) | 3 | 63,258.58 ** | 58.79 ** | 594.88 ** | 2412.54 ** | 0.064 ** | 136.87 ** | 0.537 ** |
| Genotypes (G) | 8 | 3912.33 ** | 1894.96 ** | 31.14 ** | 7.28 ** | 0.0009 ** | 0.533 ** | 0.008 ** |
| T × G | 24 | 452.93 ** | 15.84 ** | 17.91 ** | 8.06 ** | 0.0003 ** | 0.342 ** | 0.003 |
| Salinity treatments | ||||||||
| Control | 184.57 A | 84.94 A | 32.49 A | 23.14 A | 0.127 A | 5.52 A | 0.75 A | |
| ECiw ~ 4 dS m−1 | 134.93 B | 80.77 B | 29.74 B | 19.24 B | 0.108 B | 4.74 B | 0.71 B | |
| ECiw ~ 8 dS m−1 | 94.78 C | 72.26 C | 25.43 C | 10.43 C | 0.056 C | 2.46 C | 0.62 C | |
| ECiw ~12 dS m−1 | 75.04 D | 66.31 D | 21.85 D | 1.97 D | 0.02 D | 0.56 D | 0.43 D | |
| CV (%) | 7.565 | 2.57 | 3.189 | 10.643 | 10.573 | 17.779 | 8.982 | |
| LSD @ 5% (T) | 6.16 | 1.69 | 0.58 | 0.97 | 0.01 | 0.39 | 0.04 | |
| Genotypes | ||||||||
| Co 98014 | 153.58 A | 77.45 AB | 27.93 BC | 13.58 BCDE | 0.081 BC | 3.23 CD | 0.67 A | |
| Co 0118 | 131.67 C | 72.8 E | 28.14 BC | 14.15 BC | 0.082 BC | 3.22 CD | 0.63 ABC | |
| Co 0238 | 131.21 C | 76.77 B | 29.67 A | 15.11 A | 0.094 A | 3.72 A | 0.61 BCD | |
| Co 05011 | 97.83 F | 74.79 CD | 28.11 BC | 13.82 BCD | 0.078 CD | 3.6 AB | 0.64 AB | |
| Co 06034 | 141.0 B | 77.61 AB | 28.43 B | 13.41 CDE | 0.073 DE | 3.25 CD | 0.58 D | |
| Co 09022 | 115.25 D | 73.11 DE | 26.66 D | 13.17 DE | 0.069 EF | 3.39 BC | 0.6 CD | |
| Co 12029 | 111.92 DE | 78.89 A | 24.41 F | 14.45 AB | 0.073 DE | 3.13 CD | 0.63 BC | |
| Co 15023 | 106.42 EF | 78.16 AB | 27.57 C | 12.8 E | 0.085 B | 3.27 CD | 0.64 AB | |
| Co 15027 | 112.08 DE | 75.06 C | 25.48 E | 12.76 E | 0.066 F | 3.09 D | 0.64 AB | |
| CV (%) | 8.692 | 3.13 | 2.679 | 8.203 | 9.044 | 10.787 | 7.921 | |
| HSD @ 5% (G) | 8.67 | 1.30 | 0.60 | 0.92 | 0.01 | 0.29 | 0.04 | |
| HSD @ 5% (T × G) | 17.34 | 3.78 | 1.27 | 1.98 | 0.01 | 0.67 | NS | |
| Source of Variation | DF | Na+ Content (%DW) | K+ Content (%DW) | ||||
|---|---|---|---|---|---|---|---|
| Root | Leaf | Juice | Root | Leaf | Juice | ||
| Mean Sum of Squares | |||||||
| Rep | 2 | 0.0008 | 0.084 | 0.0067 | 0.0586 | 0.1364 | 0.0104 |
| Treatments (T) | 3 | 0.1051 ** | 18.077 ** | 1.663 ** | 1.111 ** | 26.742 ** | 13.868 ** |
| Genotypes (G) | 8 | 0.0069 ** | 1.991 ** | 0.1834 ** | 0.1351 | 7.403 ** | 1.341 ** |
| T × G | 24 | 0.0016 ** | 0.323 ** | 0.0333 ** | 0.1081 | 0.428 ** | 0.209 ** |
| Salinity treatments | |||||||
| Control | 0.12 D | 0.97 D | 0.08 D | 0.64 A | 3.45 A | 2.8 A | |
| ECiw ~ 4 dS m−1 | 0.15 C | 1.43 C | 0.18 C | 0.49 B | 2.45 B | 2.08 B | |
| ECiw ~ 8 dS m−1 | 0.19 B | 1.98 B | 0.36 B | 0.27 C | 1.82 C | 1.56 C | |
| ECiw ~ 12 dS m−1 | 0.26 A | 2.88 A | 0.65 A | 0.2 C | 1.1 D | 1.14 D | |
| CV (%) | 7.831 | 4.81 | 13.72 | 61.82 | 8.93 | 7.815 | |
| LSD @ 5% (T) | 0.02 | 0.06 | 0.03 | 0.16 | 0.13 | 0.10 | |
| Genotypes | |||||||
| Co 98014 | 0.15 E | 1.53 DE | 0.17 D | 0.53 | 2.14 C | 2.33 A | |
| Co 0118 | 0.17 CD | 2.13 B | 0.27 C | 0.32 | 2.57 B | 1.95 B | |
| Co 0238 | 0.16 D | 1.66 CD | 0.27 C | 0.50 | 3.98 A | 2.38 A | |
| Co 05011 | 0.16 DE | 2.02 B | 0.25 C | 0.44 | 2.64 B | 1.88 B | |
| Co 06034 | 0.17 D | 1.35 E | 0.61 A | 0.34 | 1.94 CD | 1.73 C | |
| Co 09022 | 0.19 C | 1.59 D | 0.33 B | 0.18 | 1.55 E | 1.93 B | |
| Co 12029 | 0.21 AB | 1.57 D | 0.26 C | 0.41 | 1.51 E | 1.25 D | |
| Co 15023 | 0.19 BC | 1.79 C | 0.35 B | 0.42 | 1.58 DE | 1.72 C | |
| Co 15027 | 0.22 A | 2.68 A | 0.33 B | 0.47 | 1.93 CD | 1.89 B | |
| CV (%) | 9.258 | 10.77 | 11.89 | 71.17 | 13.928 | 7.611 | |
| HSD @ 5% (G) | 0.01 | 0.16 | 0.03 | NS | 0.28 | 0.12 | |
| HSD @ 5% (T × G) | 0.03 | 0.31 | 0.06 | NS | 0.49 | 0.24 | |
| Treatments/Genotypes | Control | ECiw–4 dS m−1 | ECiw–8 dS m−1 | ECiw–12 dS m−1 | Mean |
|---|---|---|---|---|---|
| Co 98014 | 0.399 | 0.316 (20.8%) | 0.179 (55.1%) | 0.155 (61.2%) | 0.262 C |
| Co 0118 | 0.564 | 0.388 (31.2%) | 0.234 (61.8%) | 0.064 (88.7%) | 0.313 A |
| Co 0238 | 0.576 | 0.388 (32.6%) | 0.197 (65.8%) | 0.145 (74.8%) | 0.327 AB |
| Co 05011 | 0.527 | 0.313(40.6%) | 0.076 (58.6%) | 0.063 (88.1%) | 0.245 BC |
| Co 06034 | 0.500 | 0.440 (12.0%) | 0.154 (69.2%) | 0.110 (78.0%) | 0.301 ABC |
| Co 09022 | 0.580 | 0.326 (43.8%) | 0.247(57.4%) | - | 0.288 A |
| Co 12029 | 0.487 | 0.305 (37.4%) | 0.177 (63.7%) | 0.090 (81.5%) | 0.265 BC |
| Co 15023 | 0.567 | 0.213 (62.4%) | 0.095 (83.2%) | 0.060(89.4%) | 0.234 C |
| Co15027 | 0.765 | 0.469 (38.7%) | 0.204 (73.3%) | 0.130 (83.0%) | 0.392 A |
| General Mean | 0.552 A | 0.351 B (36.4%) | 0.174 C (68.5%) | 0.091 D (83.5%) | |
| CD @ 5% | Treatment (T)–0.04; Genotypes (CC)–0.05; T × CC–0.11 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhansu, P.; Kumar, R.; Kumar, A.; Vengavasi, K.; Raja, A.K.; Vasantha, S.; Meena, M.R.; Kulshreshtha, N.; Pandey, S.K. Differential Physiological Traits, Ion Homeostasis and Cane Yield of Sub-Tropical Sugarcane Varieties in Response to Long-Term Salinity Stress. Sustainability 2022, 14, 13246. https://doi.org/10.3390/su142013246
Dhansu P, Kumar R, Kumar A, Vengavasi K, Raja AK, Vasantha S, Meena MR, Kulshreshtha N, Pandey SK. Differential Physiological Traits, Ion Homeostasis and Cane Yield of Sub-Tropical Sugarcane Varieties in Response to Long-Term Salinity Stress. Sustainability. 2022; 14(20):13246. https://doi.org/10.3390/su142013246
Chicago/Turabian StyleDhansu, Pooja, Ravinder Kumar, Ashwani Kumar, Krishnapriya Vengavasi, Arun K. Raja, Srinivasavedantham Vasantha, Mintu Ram Meena, Neeraj Kulshreshtha, and Shashi K. Pandey. 2022. "Differential Physiological Traits, Ion Homeostasis and Cane Yield of Sub-Tropical Sugarcane Varieties in Response to Long-Term Salinity Stress" Sustainability 14, no. 20: 13246. https://doi.org/10.3390/su142013246
APA StyleDhansu, P., Kumar, R., Kumar, A., Vengavasi, K., Raja, A. K., Vasantha, S., Meena, M. R., Kulshreshtha, N., & Pandey, S. K. (2022). Differential Physiological Traits, Ion Homeostasis and Cane Yield of Sub-Tropical Sugarcane Varieties in Response to Long-Term Salinity Stress. Sustainability, 14(20), 13246. https://doi.org/10.3390/su142013246








