Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea mays L.) under Arsenic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Varieties and Cite of Experiment
2.2. Soil Analysis
2.3. Experimental Lay Out
2.4. Seed Priming with Thiamine and IAA
2.5. Arsenic Stress Applications
2.6. Statistical Analysis
2.7. Data Collection
3. Growth Characteristics and Chlorophyll Attributes
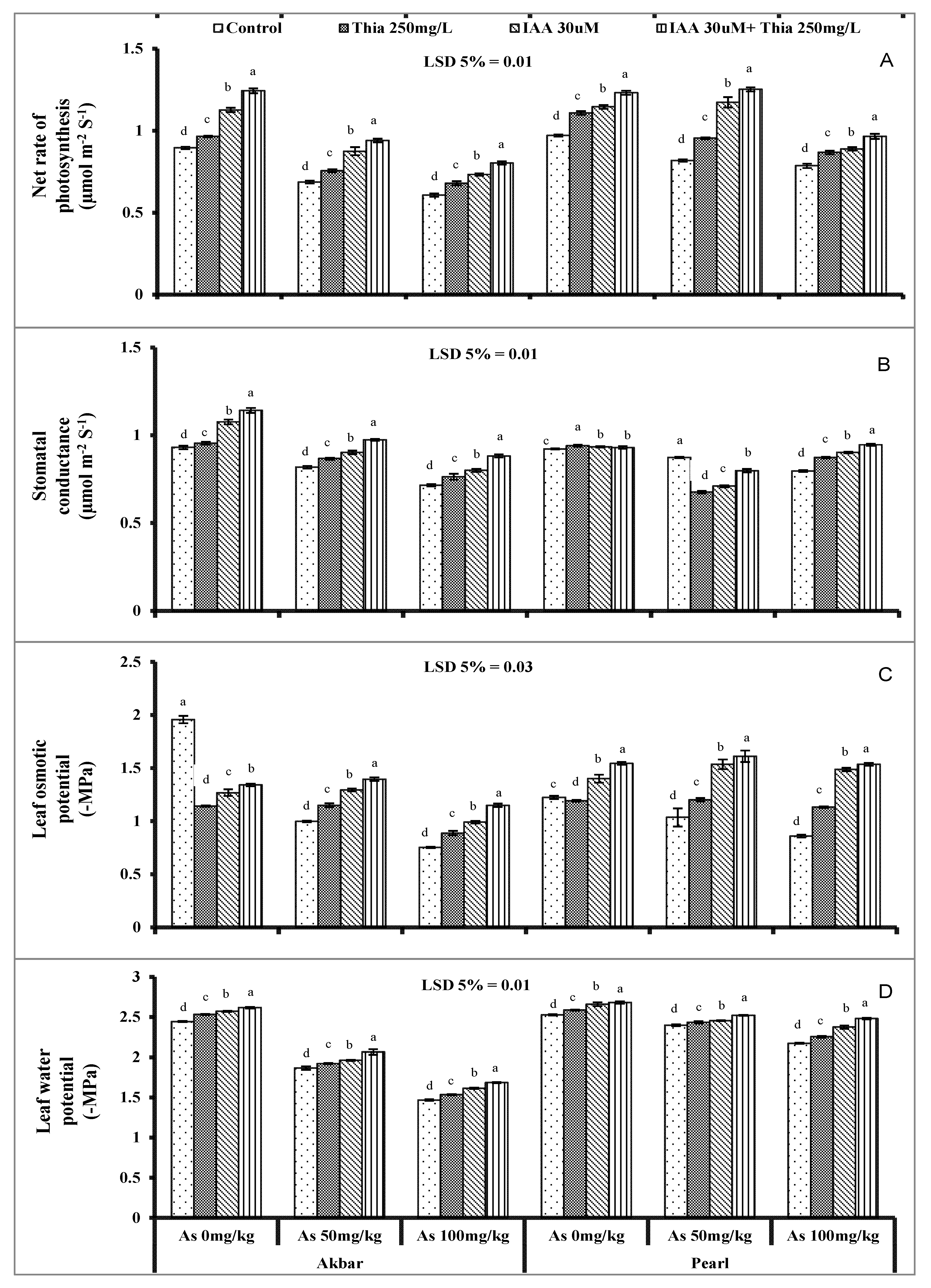
3.1. Leaf Gas Exchange and Plant Water Relation Attributes
3.2. Malondialdehyde (MDA)
3.3. Hydrogen Peroxide (H2O2)
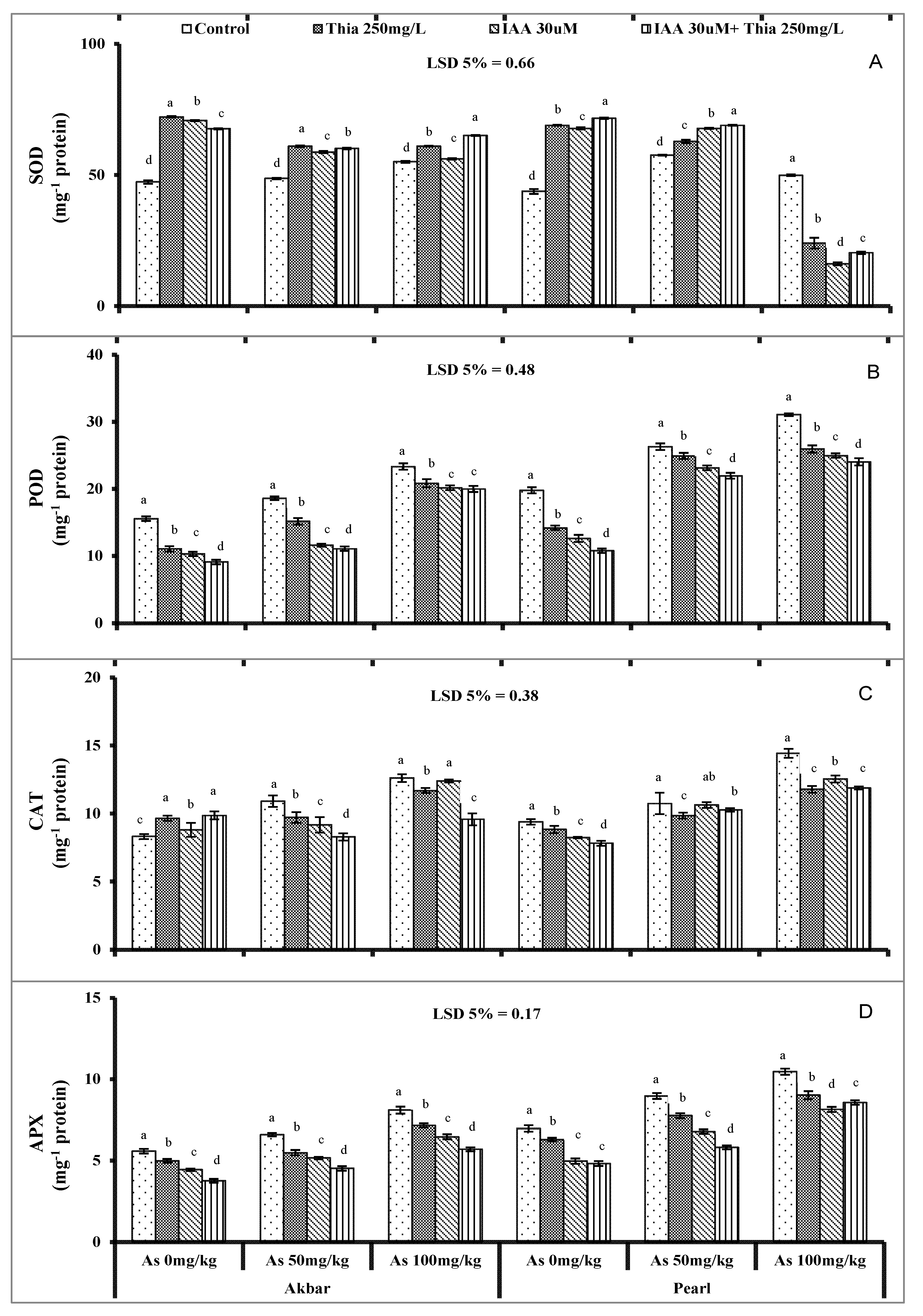
3.4. Enzyme Assays
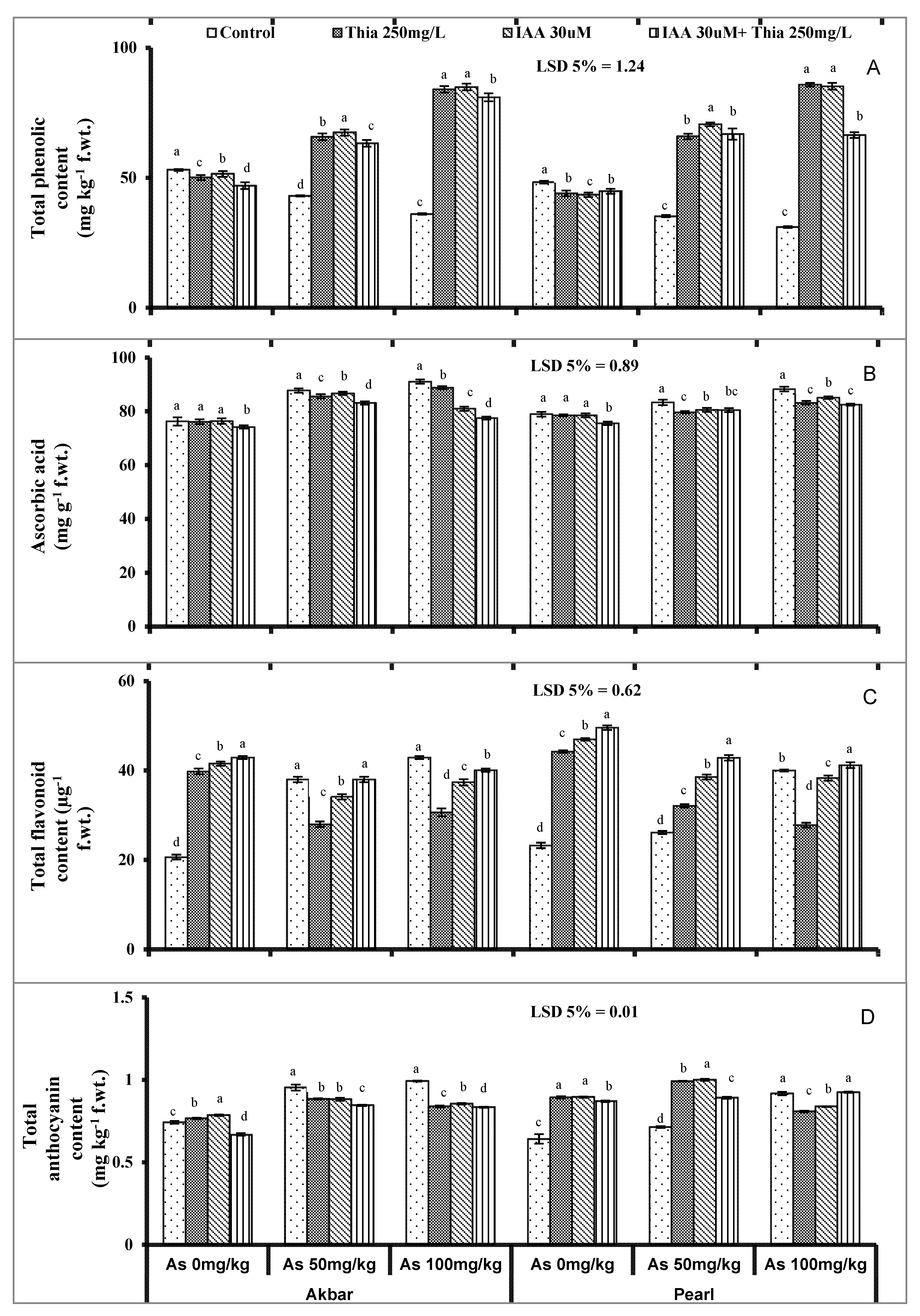
3.5. Total Phenolic and Flavonoid Contents
3.6. Ascorbic Acid (AsA) and Total Soluble Proteins
3.7. Total Soluble Sugars (TSS), Reducing Sugars (RS) and Non-Reducing Sugars (NRS)
3.8. Total Free Amino Acids (TFAA) and Proline
3.9. Anthocyanins
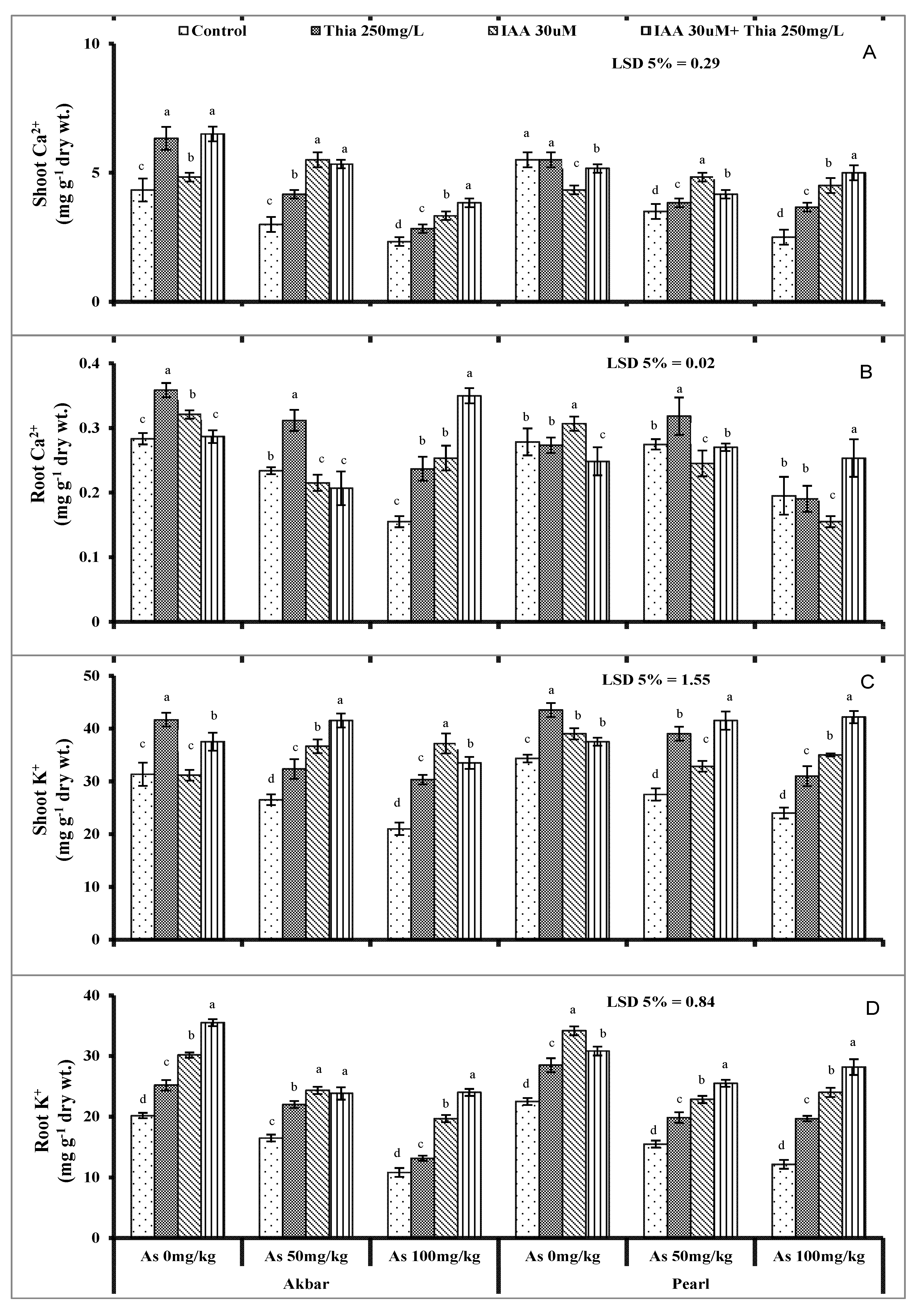
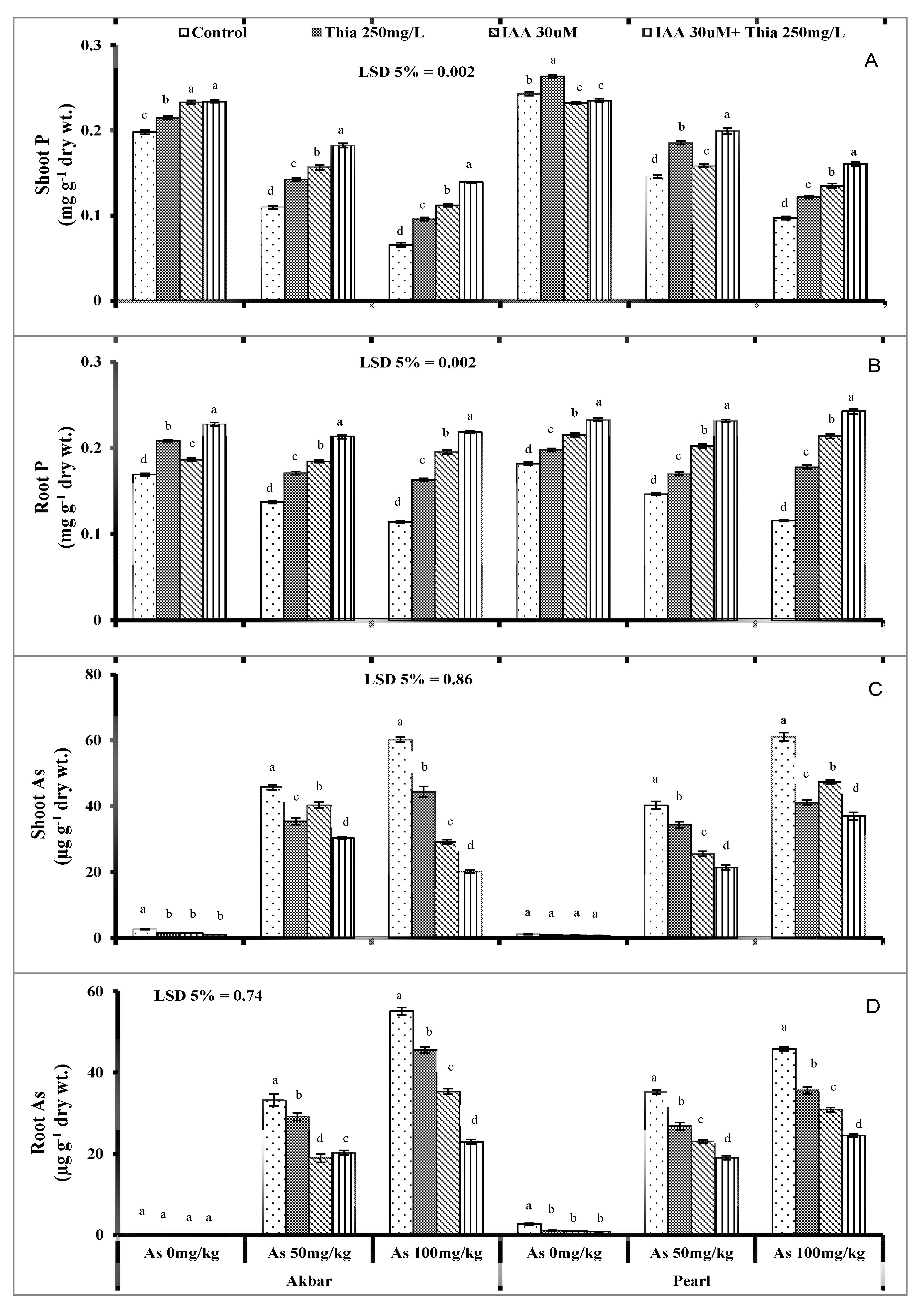
4. Nutritive Ion Analysis and As Determination
Determination of Arsenic, K+, Ca2+, P
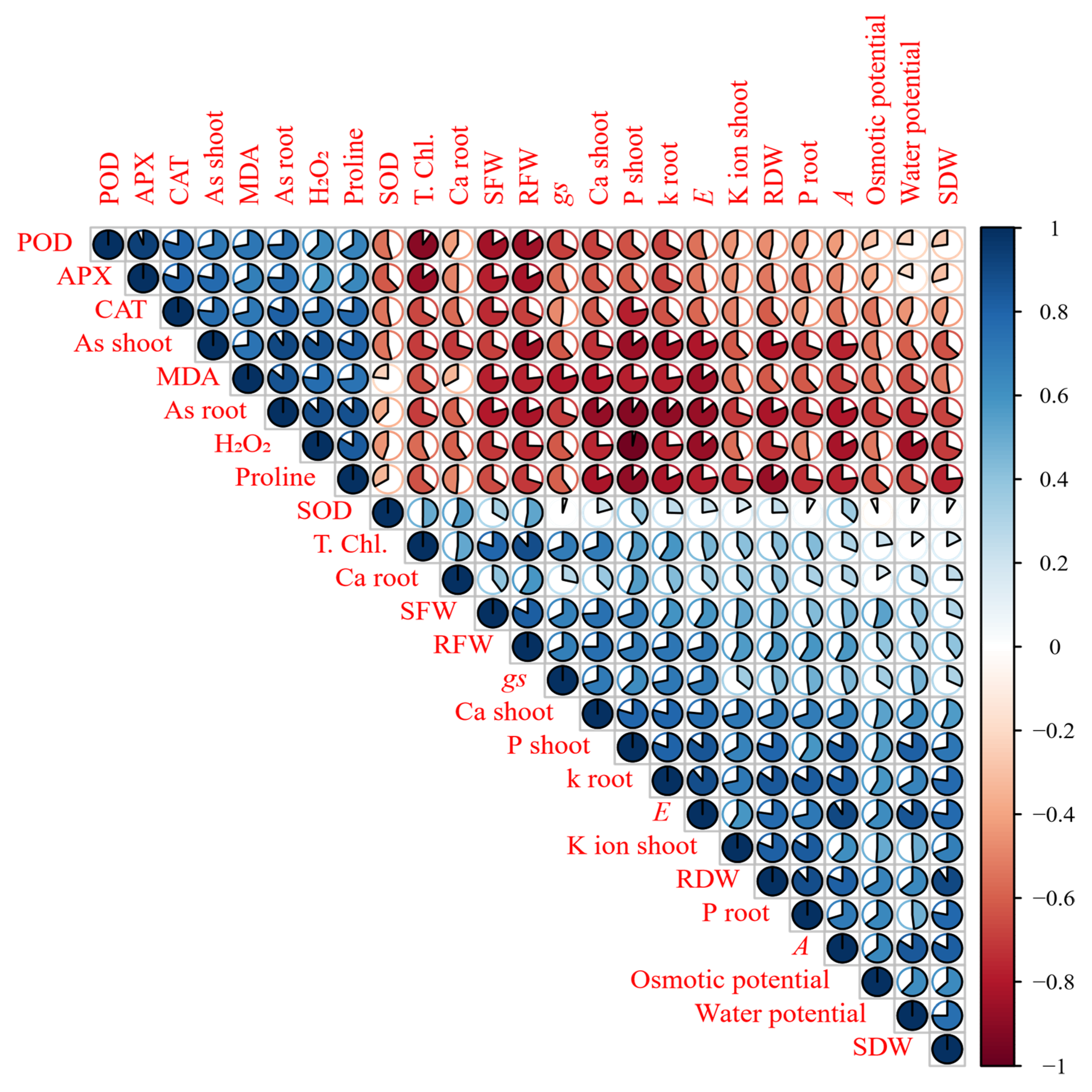
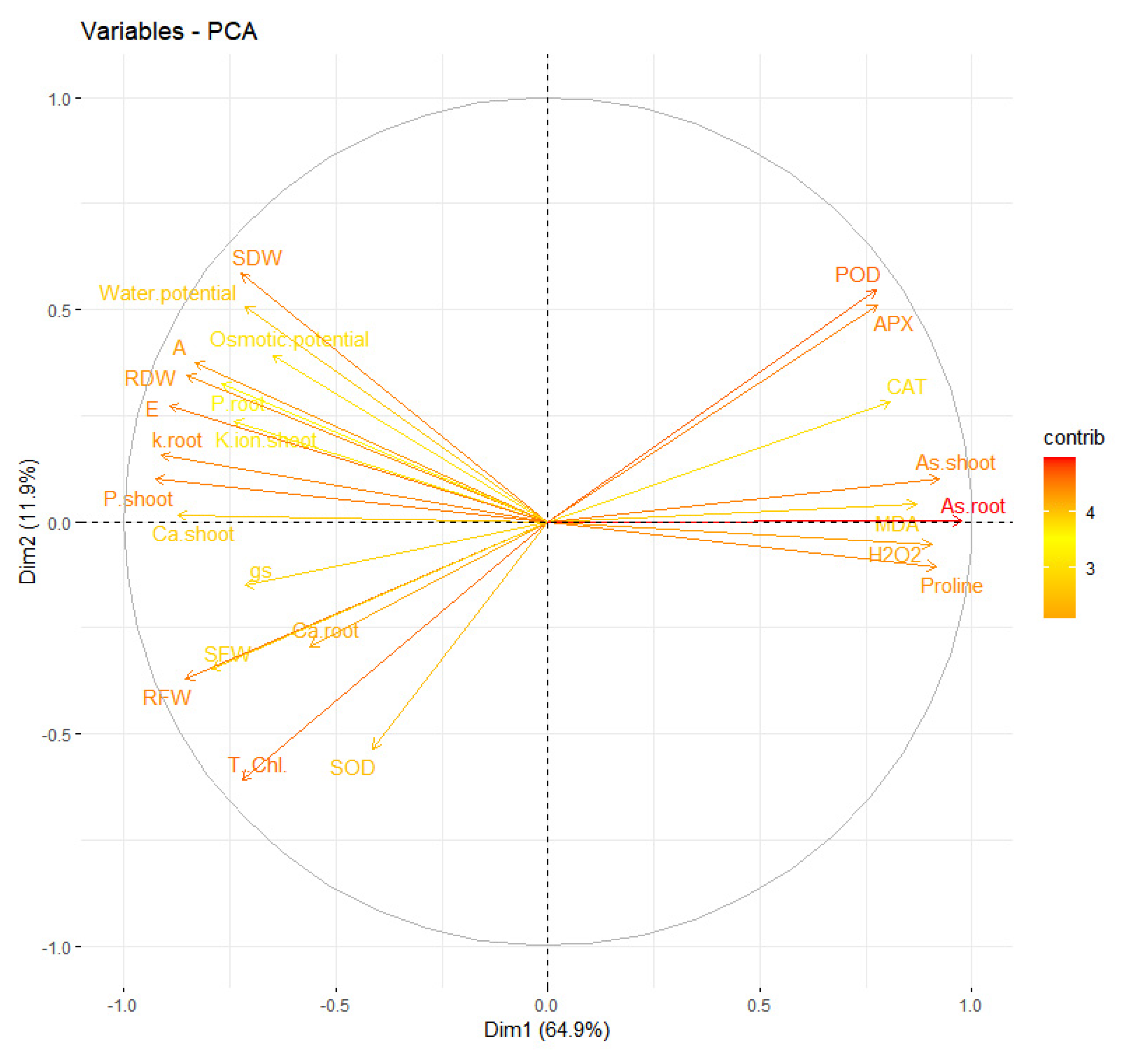
5. Results
5.1. Growth Attributes and Chlorophyll Contents
5.2. Leaf Gas Exchange Attributes
5.3. Plant Water Relation
5.4. Activities of Antioxidant Enzymes
5.5. Non-Enzymatic Antioxidants
5.6. Oxidative Stress Markers
5.7. TSP, TFAA
5.8. Proline TSS, RS, NRS
5.9. Ion Uptake
5.10. Uptake and Accumulation of As
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otero, N.; Sillmann, J.; Schnell, J.L.; Rust, H.W.; Butler, T. Synoptic and meteorological drivers of extreme ozone concentrations over Europe. Environ. Res. Lett. 2016, 11, 024005. [Google Scholar] [CrossRef]
- Kayode, O.T.; Aizebeokhai, A.P.; Odukoya, A.M. Arsenic in agricultural soils and implications for sustainable agriculture. IOP Conf. Ser. Earth Environ. Sci. 2021, 655, 012081. [Google Scholar] [CrossRef]
- González, N.; Esplugas, R.; Marquès, M.; Domingo, J.L. Concentrations of arsenic and vanadium in environmental and biological samples collected in the neighborhood of petrochemical industries: A review of the scientific literature. Sci. Total Environ. 2021, 771, 145149. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Mukherjee, A.B.; Bhattacharya, P. Arsenic flows in the environment of the European Union: A synoptic review. Trace Met. Other Contam. Environ. 2007, 9, 527–547. [Google Scholar]
- Formenton, G.; Gregio, M.; Gallo, G.; Liguori, F.; Peruzzo, M.; Innocente, E.; Masiol, M. M10-bound arsenic emissions from the artistic glass industry in Murano (Venice, Italy) before and after the enforcement of REACH authorisation. J. Hazard Mater. 2021, 406, 124294. [Google Scholar] [CrossRef] [PubMed]
- Itaya, Y.; Kuninishi, K.; Hashimoto, Y. Arsenic, selenium, and chromium speciation in fly ash. J. Mater. Cycles Waste Manag. 2022, 24, 250–258. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Cai, X.; Wang, P.; Li, Z.; Li, Y.; Yin, N.; Du, H.; Cui, Y. Mobilization and transformation of arsenic from ternary complex OM-Fe (III)-As (V) in the presence of As (V)-reducing bacteria. J. Hazard Mater. 2020, 381, 120975. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, R.; Sahoo, P.K.; Mittal, S. Geochemical relationship and translocation mechanism of arsenic in rice plants: A case study from health prone south west Punjab, India. Groundw. Sustain. Dev. 2020, 10, 100333. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sharma, P.; Mitra, S.; Mallick, I.; Ghosh, A. Arsenic uptake and bioaccumulation in plants: A review on remediation and socio-economic perspective in Southeast Asia. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100430. [Google Scholar] [CrossRef]
- Das, T.K.; Sakthivel, T.S.; Jeyaranjan, A.; Seal, S.; Bezbaruah, A.N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere 2020, 253, 126702. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Bhat, J.A.; Zhou, W.; Senan, A.M.; Alam, P.; Ahmad, P. Attenuation mechanisms of arsenic induced toxicity and its accumulation in plants by engineered nanoparticles: A review. Environ. Pollut. 2022, 302, 119038. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Suchismita, D.; da Silva, E.B.; Gao, P.; Vardanyan, L.; Liu, Y.; Ma, L.Q. Interactive effects of chromate and arsenate on their uptake and speciation in Pteris ensiformis. Plant Soil 2018, 422, 515–526. [Google Scholar] [CrossRef]
- Mridha, D.; Paul, I.; De, A.; Ray, I.; Das, A.; Joardar, M.; Roychowdhury, T. Rice seed (IR64) priming with potassium humate for improvement of seed germination, seedling growth and antioxidant defense system under arsenic stress. Ecotoxicol. Environ. Saf. 2021, 219, 112313. [Google Scholar] [CrossRef]
- Nouri, M.; Haddioui, A. Improving seed germination and seedling growth of Lepidium sativum with different priming methods under arsenic stress. Acta Ecol. Sin. 2021, 41, 64–71. [Google Scholar] [CrossRef]
- Kumar, A.; Basu, S.; Kumar, G. Evaluating the effect of seed-priming for improving arsenic tolerance in rice. J. Plant Biochem. Biotech. 2022, 31, 197–201. [Google Scholar] [CrossRef]
- Arikan, B.; Ozfidan-Konakci, C.; Yildiztugay, E.; Zengin, G.; Alp, F.N.; Elbasan, F. Exogenous hesperidin and chlorogenic acid alleviate oxidative damage induced by arsenic toxicity in Zea mays through regulating the water status, antioxidant capacity, redox balance and fatty acid composition. Environ. Pollut. 2022, 292, 118389. [Google Scholar] [CrossRef]
- Shah, A.A.; Riaz, L.; Siddiqui, M.H.; Nazar, R.; Ahmed, S.; Yasin, N.A.; Chaudhry, O. Spermine-mediated polyamine metabolism enhances arsenic-stress tolerance in Phaseolus vulgaris by expression of zinc-finger proteins related genes and modulation of mineral nutrient homeostasis and antioxidative system. Environ. Pollut. 2022, 300, 118941. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Niazi, N.K.; Murtaza, B.; Ahmad, N.; Farooq, A.; Abbas, G. Health risks of arsenic buildup in soil and food crops after wastewater irrigation. Sci. Total Environ. 2021, 772, 145266. [Google Scholar]
- Suhel, M.; Husain, T.; Prasad, S.M.; Singh, V.P. GABA requires nitric oxide for alleviating arsenate stress in tomato and brinjal seedlings. J. Plant Growth Regul. 2022, 1–14. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Alsulimani, A.; Fagoonee, S.; Slama, P.; Kesari, K.K.; Haque, S. Phytochemicals from Indian ethnomedicines: Promising prospects for the management of oxidative stress and cancer. Antioxidants 2021, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Huang, F.; Yu, D.; Ahmad, A.; Ahmad, P. Defense interplay of the zinc-oxide nanoparticles and melatonin in alleviating the arsenic stress in soybean (Glycine max L.). Chemosphere 2022, 288, 132471. [Google Scholar] [CrossRef]
- Shamshir, F.; Abbas, G.; Amjad, M.; Rizwan, M.; Akram, M.; Ahmad, S.; Farooq, A.B.U. Physiological and biochemical characterization of Kalongi (Nigella sativa) against arsenic stress: Implications for human health risk assessment. Environ. Pollut. 2022, 298, 118829. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, A.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Singh, R.P.; Tripathi, R.D. Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol. Environ. Saf. 2012, 79, 189–198. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Chatterjee, S.; Chakrabarty, D.; Trivedi, P.K. Arsenomics: Omics of arsenic metabolism in plants. Front. Physiol. 2012, 3, 275. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Mishra, G.; Singh, R.; Parihar, P.; Kumar, J.; Srivastava, P.K.; Prasad, S.M. Managing arsenic (V) toxicity by phosphate supplementation in rice seedlings: Modulations in AsA-GSH cycle and other antioxidant enzymes. Environ. Sci. Pollut. Res. 2022, 29, 14418–14429. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Bairwa, F.C.; Balyan, J.K.; Laddha, K.C. Balanced Nutrition for Enhancing the Productivity and Sustainability of Maize (Zea mays L.) under Rainfed Condition in Rajasthan. Indian J. Dryland Agric. Res. Dev. 2013, 28, 36–39. [Google Scholar]
- Aftab, A.; Ali, M.; Sahito, M.F.; Mohanty, U.S.; Jha, N.K.; Akhondzadeh, H.; Iglauer, S. Environmental friendliness and high performance of multifunctional tween 80/ZnO-nanoparticles-added water-based drilling fluid: An experimental approach. ACS Sustain. Chem. Eng. 2020, 8, 11224–11243. [Google Scholar] [CrossRef]
- Irfan, M.; Mudassir, M.; Khan, M.J.; Dawar, K.M.; Muhammad, D.; Mian, I.A.; Dewil, R. Heavy metals immobilization and improvement in maize (Zea mays L.) growth amended with biochar and compost. Sci. Rep. 2021, 11, 18416. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Khan, M.A.; Yasmin, H.; Shah, Z.A.; Rinklebe, J.; Alyemeni, M.N.; Ahmad, P. Co application of biofertilizer and zinc oxide nanoparticles upregulate protective mechanism culminating improved arsenic resistance in maize. Chemosphere 2022, 294, 133796. [Google Scholar] [CrossRef]
- Khalid, M.; Kayani, S.I.; Tang, K. The ameliorative effects of exogenous inoculation of Piriformospora indica on molecular, biochemical and physiological parameters of Artemisia annua L. under arsenic stress condition. Ecotoxicol. Environ. Saf. 2020, 206, 111202. [Google Scholar]
- Pavlikova, D.; Pavlik, M.; Staszkova, L.; Motyka, V.; Szakova, J.; Tlustos, P.; Balik, J. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotoxic. Environ. Saf. 2008, 70, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Abidin, A.A.Z.; Wong, S.Y.; Rahman, N.S.A.; Idris, Z.H.C.; Balia Yusof, Z.N. Osmotic, oxidative and salinity stresses upregulate the expressions of thiamine (Vitamin B1) biosynthesis genes (THIC and THI1/THI4) in oil palm (Elaies guineensis). J. Oil Palm. Res. 2016, 28, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Thiamin stimulates growth and secondary metabolites in turnip (Brassica rapa L.) leaf and root under drought stress. Physiol. Plant 2021, 172, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Tuna, A.L.; Polat, T.; Aydemir, S. Exogenous application of thiamin promotes growth and antioxidative defense system at initial phases of development in salt-stressed plants of two maize cultivars differing in salinity tolerance. Acta Physiol. Plant 2015, 37, 1741. [Google Scholar] [CrossRef]
- Ghaffar, A.; Akram, N.A.; Ashraf, M.; Ashraf, Y.; Sadiq, M. Thiamin- induced variations in oxidative defense processes in white clover (Trifolium repens L.) under water deficit stress. Turk. J. Bot. 2019, 43, 58–66. [Google Scholar] [CrossRef]
- Nosaka, K. Recent progress in understanding thiamin biosynthesis and its genetic regulation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006, 72, 30–40. [Google Scholar] [CrossRef]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Sunarić, S.; Pavlović, D.; Stanković, M.; Živković, J.; Arsić, I. Riboflavin and thiamine content in extracts of wild-grown plants for medicinal and cosmetic use. Chem. Pap. 2020, 74, 1729–1738. [Google Scholar] [CrossRef]
- Strydhorst, S.; Hall, L.; Perrott, L. Plant growth regulators: What agronomists need to know. Crops Soils 2018, 51, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Mazid, M. Chickpea responses to application of plant growth regulators, organics and nutrients. Adv. Plants Agric. Res. 2018, 8, 259–273. [Google Scholar] [CrossRef]
- Peat, T.S.; Böttcher, C.; Newman, J.; Lucent, D.; Cowieson, N.; Davies, C. Crystal structure of an indole-3-acetic acid amido synthetase from grapevine involved in auxin homeostasis. Plant Cell 2012, 24, 4525–4538. [Google Scholar] [CrossRef] [Green Version]
- Ji, P.; Jiang, Y.; Tang, X.; Nguyen, T.H.; Tong, Y.A.; Gao, P.; Han, W. Enhancing of phytoremediation efficiency using indole-3-acetic acid (IAA). Soil Sediment Contam. Int. J. 2015, 24, 909–916. [Google Scholar] [CrossRef]
- Hadi, F.; Bano, A.; Fuller, M.P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 2010, 80, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Amoanimaa-Dede, H.; Su, C.; Yeboah, A.; Zhou, H.; Zheng, D.; Zhu, H. Growth regulators promote soybean productivity: A review. PeerJ 2022, 10, e12556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dan, Z.; Gao, F.; Chen, P.; Fan, F.; Li, S. Rice growth-regulating factor 7 modulates plant architecture through regulating GA and indole-3-acetic acid metabolism. Plant Physiol. 2020, 184, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.; Siddiqui, M.H.; Kushwaha, B.K.; Singh, V.P.; Ali, H.M. Mitigation of arsenate toxicity by indole-3-acetic acid in brinjal roots: Plausible association with endogenous hydrogen peroxide. J. Hazard Mater. 2021, 405, 124336. [Google Scholar] [CrossRef]
- Atif, M.; Perveen, S. Comparison of Alteration in Growth, Physiological and Biochemical Attributes of Ten Maize (Zea mays L.) Varieties under Arsenic Stress: Susceptibility and Tolerance. Pol. J. Environ. Stud. 2021, 30, 4913–4923. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis-Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 1969. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max L.). Physiol. Plant 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic peroxidases and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Maehly, A.C. [143]Plant peroxidase. Methods Enzymol. 1955, 2, 801–813. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Henson, C.; Stone, J. Variation in a-amylase and a-amylase inhibitor activities in barley malts. J. Cereal Sci. 1988, 8, 39–46. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mirecki, R.M.; Teramura, A.H. Effects of ultraviolet-B irradiance on soybean: V. The dependence of plant sensitivity on the photosynthetic photon flux density during and after leaf expansion. Plant Physiol. 1984, 74, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.D.; Jack, E.M.; Harrison, C.J.; Claugher, D. Scanning electron microscopy of human metaphase chromosomes. Scanning Electron Microsc. 1986, 1986, 32. [Google Scholar]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Khare, R.; Dhar, Y.V.; Sandhu, G.; Singh, S.; Kumar, S.; Khan, A.; Trivedi, P.K. Genome-wide expression and variation in nucleotide sequences lead to differential response of Arabidopsis thaliana ecotypes towards arsenic stress under sulfur limiting condition. Environ. Exp. Bot. 2022, 195, 104764. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Pavlík, M. Free amino acid regulation in fronds and roots of two Pteris cretica L. ferns under arsenic stress. Plant Soil Environ. 2020, 66, 483–492. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: A review. J. Environ. Manag. 2020, 254, 109814. [Google Scholar] [CrossRef]
- Tanveer, Y.; Yasmin, H.; Nosheen, A.; Ali, S.; Ahmad, A. Ameliorative effects of plant growth promoting bacteria, zinc oxide nanoparticles and oxalic acid on Luffa acutangula grown on arsenic enriched soil. Environ. Pollut. 2022, 300, 118889. [Google Scholar] [CrossRef]
- Suohui, T.; Yanping, C.; Shuhui, Z.; Zhihua, L.; Honggang, J.; Jun, L.; Tao, L. Thiamine induces resistance in tobacco against black shank. Australas. Plant Pathol. 2022, 51, 231–243. [Google Scholar] [CrossRef]
- Goyer, A. Thiamine in plants: Aspects of its metabolism and functions. Phytochemistry 2010, 71, 1615–1624. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhao, S.; Fu, Y.; Zhu, L. Homeobox protein 24 mediates the conversion of indole-3-butyric acid to indole-3-acetic acid to promote root hair elongation. New Phytol. 2021, 232, 2057–2070. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ashraf, U.; Khan, I.; Wang, L. Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut. 2017, 228, 13. [Google Scholar] [CrossRef]
- Li, C.X.; Feng, S.L.; Yun, S.; Jiang, L.N.; Lu, X.Y.; Hou, X.L. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 2007, 19, 725–732. [Google Scholar] [CrossRef]
- Šimonová, E.; Henselová, M.; Masarovičová, E.; Kohanová, J. Comparison of tolerance of Brassica juncea and Vigna radiata to cadmium. Biol. Plant 2007, 51, 488–492. [Google Scholar] [CrossRef]
- Vezza, M.E.; Llanes, A.; Travaglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant 2016, 38, 257. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Differential responses of growth, photosynthesis, oxidative stress, metals accumulation and NRAMP genes in contrasting Ricinus communis genotypes under arsenic stress. Environ. Sci. Pollut. Res. 2019, 26, 31166–31177. [Google Scholar] [CrossRef]
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 11. [Google Scholar] [CrossRef]
- Sanjari, S.; Keramat, B.; Nadernejad, N.; Mozafari, H. Ameliorative effects of 24-epibrassinolide and thiamine on excess cadmium-induced oxidative stress in Canola (Brassica napus L.) plants. J. Plant Interact. 2019, 14, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Mridha, D.; Gorain, P.C.; Joardar, M.; Das, A.; Majumder, S.; De, A.; Roychowdhury, T. Rice grain arsenic and nutritional content during post harvesting to cooking: A review on arsenic bioavailability and bioaccessibility in humans. Food Res. Int. 2022, 2022, 111042. [Google Scholar] [CrossRef]
- Jung, H.I.; Kong, M.S.; Lee, B.R.; Kim, T.H.; Chae, M.J.; Lee, E.J.; Kim, Y.H. Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate–glutathione homeostasis in rice seedlings. Front. Plant Sci. 2019, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Faize, M.; Burgos, L.; Faize, L.; Piqueras, A.; Nicolas, E.; BarbaEspin, G.; ClementeMoreno, M.; Alcobendas, R.; Artlip, T.; Hernandez, J. Involvement of cytosolic ascorbate peroxidase and Cu/Znsuperoxide dismutasefor improved tolerance against drought stress. J. Exp. Bot. 2011, 62, 2599–2613. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzymefamily with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros-Barcelo, A.; Pomar, F.; Ferrer, M.A.; Martinez, P.; Ballesta, M.A.; Pedreno, M.A. In situ characterization of a NO-sensitive peroxidase in the lignifying xylem of Zinnia elegans. Physiol. Plant 2002, 114, 33–40. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, A.; Xing, D. Modulation of cellular redox status by thiamine-activated NADPH oxidase confers Arabidopsis resistance to Sclerotinia sclerotiorum. J. Exp. Bot. 2013, 64, 3261–3272. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bashri, G.; Prasad, S.M. Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and antioxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiol. Plant 2015, 37, 49. [Google Scholar] [CrossRef]
- Boubakri, H.; Poutaraud, A.; Wahab, M.A.; Clayeux, C.; Baltenweck-Guyot, R.; Steyer, D.; Soustre-Gacougnolle, I. Thiamine modulates metabolism of the phenylpropanoid pathway leading to enhanced resistance to Plasmopara viticola in grapevine. BMC Plant Biol. 2013, 13, 31. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.; Abdelaal, K.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; El Nahhas, N. Exogenous ascorbic acid induced chilling tolerance in tomato plants through modulating metabolism, osmolytes, antioxidants, and transcriptional regulation of catalase and heat shock proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Madany, M.M.; Zinta, G.; Abuelsoud, W.; Hozzein, W.N.; Selim, S.; Asard, H.; Abd Elgawad, H. Hormonal seed-priming improves tomato resistance against broomrape infection. J. Plant Physiol. 2020, 250, 153184. [Google Scholar] [CrossRef]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium (VI) toxicity in legume plants: Modulation effects of rhizobial symbiosis. BioMed Res. Int. 2018, 2018, 8031213. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.A.; Kim, D.S.; Park, H.S.; Jang, K.Y.; Kang, M.J.; Lee, D.G.; Chung, M.J. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Sarıoglu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The combined supplementation of melatonin and salicylic acid effectively detoxifies arsenic toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants. Environ. Pollut. 2022, 297, 118727. [Google Scholar] [CrossRef] [PubMed]
- Zahra, S.S.; Khan, M.I.; Imran, M.; Aman, Q.; Ali, R. The relationship between job stress and turnover intentions in the pesticide sector of Pakistan: An employee behavior perspective. Manag. Issues Healthc. Syst. 2018, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Dolui, D.; Hasanuzzaman, M.; Saha, I.; Ghosh, A.; Adak, M.K. Amelioration of sodium and arsenic toxicity in Salvinia natans L. with 2,4-D priming through physiological responses. Environ. Sci. Pollut. Res. 2022, 29, 9232–9247. [Google Scholar] [CrossRef]
- Khalid, A.; Aftab, F. Effect of exogenous application of IAA and GA 3 on growth, protein content, and antioxidant enzymes of Solanum tuberosum L. grown in vitro under salt stress. Vitr. Cell. Dev. Biol. Plant 2020, 56, 377–389. [Google Scholar] [CrossRef]
- Samanta, J.; Mondal, A.; Saha, S.; Chakraborty, S.; Sengupta, A. Oleic acid protects from arsenic-induced cardiac hypertrophy via AMPK/FoxO/NFATc3 pathway. Cardiovasc. Toxicol. 2020, 20, 261–280. [Google Scholar] [CrossRef]
- Samanta, S.; Roychoudhury, A. Arsenic stress and mineral nutrition in plants. In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Cambridge, MA, USA, 2022; pp. 361–375. [Google Scholar]
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin antagonistic control of the shoot/root growth ratio and its relevance for adaptation to drought and nutrient deficiency stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Rapala-Kozik, M.; Wolak, N.; Kujda, M.; Banas, A.K. The upregulation of thiamine (vitamin B1) biosynthesis in Arabidopsis thaliana seedlings under salt and osmotic stress conditions is mediated by abscisic acid at the early stages of this stress response. BMC Plant Biol. 2012, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Huerta, E.A.; Gómez-Bernal, J.M.; Barbosa-Martínez, C.; Armienta-Hernández, M.A.; Martínez-Villegas, N.V. Morphological characteristics and accumulation of arsenic in Argyrochosma formosa (Liebm.) Windham developed in a highly contaminated site with arsenic in Matehuala, SLP, México. Environ. Sci. Pollut. Res. 2022, 29, 2685–2698. [Google Scholar] [CrossRef] [PubMed]
- Gulz, P.A.; Gupta, S.K.; Schulin, R. Arsenic accumulation of common plants from contaminated soils. Plant Soil 2005, 272, 337–347. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Zou, S.W.; Nanayakkara, K.N.; Matsuura, T.; Chen, J.P. Adsorptive removal of arsenic from aqueous solution by a PVDF/zirconia blend flat sheet membrane. J. Membr. Sci. 2011, 374, 1–11. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, T.; Liang, Y.C. Transport via xylem of trichloroethylene in wheat, corn, and tomato seedlings. J. Hazard Mater. 2010, 182, 472–476. [Google Scholar] [CrossRef] [PubMed]
| SFW (g) | SDW (g) | |||||
|---|---|---|---|---|---|---|
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 20.47 ± 0.47 a | 15.63 ± 0.39 d | 13.44 ± 0.56 b | 1.37 ± 0.02 c | 1.09 ± 0.01 d | 0.97 ± 0.01 d |
| Thia. 250 mg/L | 20.24 ± 0.58 a | 16.51 ± 0.63 c | 13.74 ± 0.27 b | 1.50 ± 0.02 a | 1.19 ± 0.01 c | 1.16 ± 0.01 c |
| IAA 30 µM | 17.40 ± 0.49 c | 17.33 ± 0.58 b | 13.37 ± 0.40 b | 1.41 ± 0.01 b | 1.24 ± 0.01 b | 1.30 ± 0.02 b |
| IAA 30 µM + Thia 250 mg/L | 19.48 ± 0.53 b | 19.48 ± 0.52 a | 14.64 ± 0.47 a | 1.49 ± 0.01 a | 1.29 ± 0.01 a | 1.39 ± 0.02 a |
| Pearl | ||||||
| Control | 16.44 ± 0.57 c | 12.60 ± 0.38 d | 12.60 ± 0.42 d | 1.61 ± 0.02 d | 1.18 ± 0.03 d | 1.15 ± 0.03 d |
| Thia. 250 mg/L | 17.54 ± 0.37 b | 14.42 ± 0.46 b | 14.37 ± 0.54 b | 1.77 ± 0.03 c | 1.40 ± 0.03 c | 1.42 ± 0.03 c |
| IAA 30 µM | 16.03 ± 0.24 c | 13.53 ± 0.61 c | 13.26 ± 0.50 c | 1.94 ± 0.01 b | 1.63 ± 0.02 b | 1.63 ± 0.02 b |
| IAA 30 µM + Thia 250 mg/L | 18.33 ± 0.41 a | 16.77 ± 0.55 a | 15.70 ± 0.62 a | 2.03 ± 0.03 a | 1.76 ± 0.02 a | 1.71 ± 0.01 a |
| LSD 5% | 0.57 | 0.02 | ||||
| RFW (g) | RDW (g) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 2.55 ± 0.11 d | 2.11 ± 0.03 c | 1.89 ± 0.03 d | 0.28 ± 0.006 d | 0.17 ± 0.006 d | 0.11 ± 0.009 d |
| Thia. 250 mg/L | 2.85 ± 0.09 b | 2.41 ± 0.05 b | 2.35 ± 0.04 b | 0.31 ± 0.006 b | 0.26 ± 0.006 b | 0.17 ± 0.006 c |
| IAA 30 µM | 2.64 ± 0.05 c | 2.35 ± 0.04 b | 2.23 ± 0.03 c | 0.29 ± 0.006 c | 0.25 ± 0.006 c | 0.27 ± 0.017 b |
| IAA 30 µM + Thia 250 mg/L | 3.00 ± 0.07 a | 2.54 ± 0.05 a | 2.43 ± 0.03 a | 0.34 ± 0.006 a | 0.30 ± 0.009 a | 0.35 ± 0.012 a |
| Pearl | ||||||
| Control | 2.20 ± 0.06 d | 1.98 ± 0.06 c | 2.00 ± 0.11 c | 0.33 ± 0.011 d | 0.19 ± 0.009 d | 0.15 ± 0.006 d |
| Thia. 250 mg/L | 2.52 ± 0.05 b | 2.30 ± 0.05 a | 2.06 ± 0.03 bc | 0.42 ± 0.009 b | 0.25 ± 0.006 c | 0.22 ± 0.009 c |
| IAA 30 µM | 2.42 ± 0.05 c | 2.24 ± 0.06 ab | 2.09 ± 0.06 b | 0.38 ± 0.006 c | 0.31 ± 0.014 b | 0.29 ± 0.011 b |
| IAA 30 µM + Thia 250 mg/L | 2.76 ± 0.04 a | 2.23 ± 0.06 b | 2.28 ± 0.03 a | 0.45 ± 0.006 a | 0.41 ± 0.014 a | 0.37 ± 0.009 a |
| LSD 5% | 0.07 | 0.01 | ||||
| SL (cm) | RL (cm) | |||||
|---|---|---|---|---|---|---|
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 90.03 ± 1.05 b | 74.77 ± 1.10 c | 59.93 ± 0.89 d | 19.47 ± 0.41 d | 15.73 ± 0.46 c | 13.47 ± 0.48 d |
| Thia. 250 mg/L | 92.17 ± 1.50 a | 85.53 ± 0.66 b | 82.93 ± 0.95 b | 21.30 ± 0.55 b | 18.43 ± 0.57 a | 15.83 ± 0.23 b |
| IAA 30 µM | 90.87 ± 0.58 b | 86.03 ± 0.90 b | 81.67 ± 0.55 c | 20.47 ± 0.67 c | 17.47 ± 0.41 b | 14.80 ± 0.55 c |
| IAA 30 µM + Thia 250 mg/L | 92.87 ± 0.82 a | 89.17 ± 0.46 a | 84.70 ± 0.84 a | 23.37 ± 0.54 a | 18.73 ± 0.56 a | 16.80 ± 0.55 a |
| Pearl | ||||||
| Control | 94.03 ± 0.77 c | 83.50 ± 2.84 c | 84.33 ± 0.63 c | 20.80 ± 0.68 c | 18.37 ± 0.66 c | 14.90 ± 0.26 d |
| Thia. 250 mg/L | 95.63 ± 0.66 b | 88.47 ± 0.56 b | 87.57 ± 0.50 b | 23.53 ± 0.66 b | 21.40 ± 0.66 a | 19.27 ± 0.43 b |
| IAA 30 µM | 96.63 ± 0.69 ab | 89.33 ± 0.56 b | 87.33 ± 0.82 b | 20.50 ± 0.65 c | 19.50 ± 0.70 b | 17.63 ± 0.63 c |
| IAA 30 µM + Thia 250 mg/L | 97.10 ± 0.96 a | 92.70 ± 0.94 a | 91.47 ± 0.56 a | 24.47 ± 0.52 a | 20.80 ± 0.58 a | 20.80 ± 0.76 a |
| LSD 5% | 1.15 | 0.66 | ||||
| Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 1.07 ± 0.03 d | 0.67 ± 0.01 d | 0.57 ± 0.01 d | 0.32 ± 0.02 a | 0.30 ± 0.01 a | 0.30 ± 0.01 a |
| Thia. 250 mg/L | 1.28 ± 0.02 b | 0.93 ± 0.02 b | 0.67 ± 0.01 b | 0.30 ± 0.01 c | 0.26 ± 0.01 c | 0.23 ± 0.01 b |
| IAA 30 µM | 1.17 ± 0.00 c | 0.82 ± 0.01 c | 0.61 ± 0.02 c | 0.32 ± 0.00 a | 0.19 ± 0.01 d | 0.22 ± 0.01 c |
| IAA 30 µM + Thia 250 mg/L | 1.36 ± 0.01 a | 0.99 ± 0.01 a | 0.79 ± 0.01 a | 0.31 ± 0.01 b | 0.28 ± 0.01 b | 0.23 ± 0.01 b |
| Pearl | ||||||
| Control | 1.05 ± 0.01 d | 0.57 ± 0.00 c | 0.45 ± 0.00 d | 0.34 ± 0.01 b | 0.31 ± 0.00 c | 0.26 ± 0.01 d |
| Thia. 250 mg/L | 1.15 ± 0.01 b | 0.64 ± 0.00 b | 0.57 ± 0.00 b | 0.36 ± 0.00 a | 0.35 ± 0.00 b | 0.33 ± 0.01 a |
| IAA 30 µM | 1.08 ± 0.01 c | 0.64 ± 0.00 b | 0.51 ± 0.01 c | 0.36 ± 0.01 a | 0.30 ± 0.01 d | 0.31 ± 0.01 b |
| IAA 30 µM+ Thia 250 mg/L | 1.23 ± 0.04 a | 0.79 ± 0.01 a | 0.71 ± 0.01 a | 0.34 ± 0.01 b | 0.36 ± 0.01 a | 0.29 ± 0.01 c |
| LSD 5% | 0.02 | 0.01 | ||||
| Chl a/b | Chl a + b (mg g−1 FW) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 3.38 ± 0.29 c | 2.24 ± 0.14 c | 1.94 ± 0.12 c | 1.39 ± 0.01 d | 0.96 ± 0.00 d | 0.87 ± 0.00 c |
| Thia. 250 mg/L | 4.34 ± 0.24 a | 3.56 ± 0.24 b | 2.96 ± 0.17 b | 1.58 ± 0.01 b | 1.20 ± 0.01 b | 0.89 ± 0.00 b |
| IAA 30 µM | 3.68 ± 0.08 b | 3.91 ± 0.48 a | 2.79 ± 0.23 b | 1.49 ± 0.00 c | 1.04 ± 0.04 c | 0.83 ± 0.00 d |
| IAA 30 µM + Thia 250 mg/L | 4.43 ± 0.19 a | 3.50 ± 0.11 b | 3.49 ± 0.20 a | 1.67 ± 0.00 a | 1.28 ± 0.00 a | 1.02 ± 0.01 a |
| Pearl | ||||||
| Control | 3.06 ± 0.08 bc | 1.83 ± 0.05 b | 1.72 ± 0.01 b | 1.39 ± 0.00 d | 0.88 ± 0.00 d | 0.71 ± 0.01 d |
| Thia. 250 mg/L | 3.20 ± 0.03 b | 1.83 ± 0.01 b | 1.80 ± 0.05 b | 1.51 ± 0.01 b | 1.00 ± 0.01 b | 0.88 ± 0.00 b |
| IAA 30 µM | 2.96 ± 0.08 c | 2.12 ± 0.04 a | 1.84 ± 0.02 b | 1.44 ± 0.00 c | 0.94 ± 0.01 c | 0.82 ± 0.00 c |
| IAA 30 µM + Thia 250 mg/L | 3.69 ± 0.31 a | 2.20 ± 0.10 a | 2.43 ± 0.11 a | 1.57 ± 0.02 a | 1.15 ± 0.00 a | 1.00 ± 0.00 a |
| LSD 5% | 0.21 | 0.01 | ||||
| Carotenoids (mg g−1 FW) | Trans rate (µmol m−2 s−1) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 0.014 ± 0.000 a | 0.012 ± 0.000 a | 0.009 ± 8.89E a | 0.43 ± 0.01 d | 0.32 ± 0.01 d | 0.25 ± 0.01 d |
| Thia. 250 mg/L | 0.015 ± 0.000 a | 0.013 ± 3.43E a | 0.012 ± 4.29E a | 0.47 ± 0.00 c | 0.35 ± 0.00 c | 0.29 ± 0.01 c |
| IAA 30 µM | 0.014 ± 0.000 a | 0.014 ± 0.001 a | 0.010 ± 6.44E a | 0.55 ± 0.00 b | 0.38 ± 0.01 b | 0.34 ± 0.01 b |
| IAA 30 µM + Thia 250 mg/L | 0.015 ± 0.000 a | 0.013 ± 5.73E a | 0.013 ± 0.000 a | 0.62 ± 0.01 a | 0.42 ± 0.01 a | 0.37 ± 0.01 a |
| Pearl | ||||||
| Control | 0.011 ± 0.000 a | 0.011 ± 5.72E a | 0.010 ± 0.000 a | 0.45 ± 0.01 d | 0.38 ± 0.01 d | 0.33 ± 0.00 d |
| Thia. 250 mg/L | 0.012 ± 0.000 a | 0.011 ± 0.000 a | 0.011 ± 0.000 a | 0.46 ± 0.00 c | 0.42 ± 0.01 c | 0.41 ± 0.01 c |
| IAA 30 µM | 0.011 ± 0.000 a | 0.014 ± 0.000 a | 0.010 ± 0.000 a | 0.51 ± 0.00 b | 0.43 ± 0.00 b | 0.45 ± 0.00 b |
| IAA 30 µM + Thia 250 mg/L | 0.013 ± 0.000 a | 0.014 ± 0.000 a | 0.013 ± 4.6E a | 0.59 ± 0.01 a | 0.47 ± 0.00 a | 0.46 ± 0.01 a |
| LSD 5% | 3.42 | 0.01 | ||||
| H2O2 (µmolg−1 FW) | MDA (nmolg−1 FW) | |||||
|---|---|---|---|---|---|---|
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 11.28 ± 0.07 a | 15.03 ± 0.11 a | 15.93 ± 0.08 a | 7.36 ± 0.82 c | 8.95 ± 0.36 a | 11.67 ± 0.42 a |
| Thia. 250 mg/L | 10.32 ± 0.11 c | 13.61 ± 0.09 c | 14.73 ± 0.39 c | 8.68 ± 0.60 b | 9.13 ± 0.66 a | 10.16 ± 0.61 b |
| IAA 30 µM | 10.64 ± 0.10 b | 13.96 ± 0.07 b | 15.21 ± 0.12 b | 9.87 ± 0.59 a | 7.65 ± 0.30 b | 9.58 ± 0.30 b |
| IAA 30 µM + Thia 250 mg/L | 9.96 ± 0.11 d | 13.06 ± 0.12 d | 14.02 ± 0.07 d | 5.09 ± 0.66 d | 6.10 ± 1.15 c | 8.91 ± 0.27 c |
| Pearl | ||||||
| Control | 10.78 ± 0.10 a | 13.10 ± 0.11 a | 14.76 ± 0.09 a | 7.81 ± 0.32 a | 8.43 ± 0.10 b | 10.12 ± 0.68 a |
| Thia. 250 mg/L | 10.27 ± 0.07 c | 12.51 ± 0.06 c | 14.17 ± 0.10 c | 8.28 ± 0.57 a | 8.70 ± 0.58 b | 8.41 ± 0.54 bc |
| IAA 30 µM | 10.48 ± 0.08 b | 12.85 ± 0.06 b | 14.39 ± 0.09 b | 7.11 ± 0.16 b | 9.47 ± 0.59 a | 8.14 ± 0.20 c |
| IAA 30 µM + Thia 250 mg/L | 10.09 ± 0.11 d | 11.97 ± 0.08 d | 13.39 ± 0.09 d | 5.77 ± 0.73 c | 8.28 ± 0.39 b | 8.88 ± 0.71 b |
| LSD 5% | 0.14 | 0.66 | ||||
| TSP (mg g−1 FW) | TFAA (mg g−1 FW) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 536.46 ± 4.80 c | 488.13 ± 4.76 a | 404.14 ± 5.66 d | 7.07 ± 0.44 a | 11.03 ± 0.44 b | 15.86 ± 0.15 a |
| Thia. 250 mg/L | 588.91 ± 4.76 a | 490.53 ± 6.28 a | 479.56 ± 5.64 b | 4.54 ± 0.06 b | 4.32 ± 0.07 d | 12.74 ± 0.16 b |
| IAA 30 µM | 517.26 ± 4.75 d | 479.90 ± 5.12 b | 522.06 ± 4.80 a | 4.25 ± 0.08 c | 5.49 ± 0.07 c | 11.69 ± 0.15 c |
| IAA 30 µM + Thia 250 mg/L | 560.46 ± 4.71 b | 471.67 ± 5.95 c | 438.08 ± 4.48 c | 4.52 ± 0.10 b | 14.78 ± 0.20 a | 10.85 ± 0.11 d |
| Pearl | ||||||
| Control | 575.54 ± 5.05 b | 511.44 ± 5.39 d | 493.61 ± 7.99 d | 7.64 ± 0.29 c | 14.71 ± 0.11 a | 14.66 ± 0.17 a |
| Thia. 250 mg/L | 610.84 ± 5.66 a | 562.51 ± 4.75 b | 537.15 ± 10.42 c | 13.61 ± 0.12 a | 13.69 ± 0.15 b | 13.55 ± 0.14 b |
| IAA 30 µM | 571.08 ± 4.38 b | 540.23 ± 4.53 c | 570.74 ± 4.28 b | 13.63 ± 0.13 a | 14.59 ± 0.10 a | 14.56 ± 0.07 a |
| IAA 30 µM + Thia mg/L | 615.99 ± 3.89 a | 606.73 ± 8.37 a | 591.65 ± 3.86 a | 12.90 ± 0.22 b | 14.72 ± 0.10 a | 13.12 ± 0.10 c |
| LSD 5% | 6.47 | 0.21 | ||||
| TSS (mg g−1 FW) | Reducing sugars (mg g−1 FW) | |||||
|---|---|---|---|---|---|---|
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 90.13 ± 0.87 d | 142.15 ± 0.94 a | 149.42 ± 0.60 a | 15.60 ± 1.53 d | 11.57 ± 0.66 d | 7.80 ± 0.25 d |
| Thia 250 mg/L | 146.41 ± 2.00 b | 115.47 ± 3.72 c | 115.82 ± 17.73 c | 23.39 ± 0.87 c | 14.34 ± 0.87 c | 10.56 ± 0.43 c |
| IAA 30 µM | 128.80 ± 2.40 c | 100.88 ± 1.96 d | 131.07 ± 4.12 b | 28.93 ± 0.91 b | 19.12 ± 0.66 b | 14.34 ± 0.87 b |
| IAA 30 µM+ Thia 250 mg/L | 169.56 ± 2.63 a | 130.31 ± 3.52 b | 153.46 ± 2.55 a | 31.70 ± 0.87 a | 24.40 ± 0.66 a | 17.61 ± 0.66 a |
| Pearl | ||||||
| Control | 142.86 ± 0.90 d | 166.90 ± 1.39 a | 179.79 ± 0.74 a | 22.14 ± 0.66 d | 14.09 ± 0.66 d | 9.81 ± 0.87 d |
| Thia 250 mg/L | 171.06 ± 3.09 b | 135.34 ± 3.70 c | 110.69 ± 2.63 d | 28.17 ± 1.53 c | 20.38 ± 1.31 c | 20.38 ± 1.74 c |
| IAA 30 µM | 159.74 ± 1.65 c | 122.01 ± 1.33 d | 139.87 ± 3.49 c | 35.72 ± 1.53 b | 32.45 ± 0.87 b | 30.94 ± 0.87 b |
| IAA 30 µM+ Thia 250 mg/L | 177.86 ± 3.49 a | 153.96 ± 2.86 b | 146.91 ± 2.40 b | 43.77 ± 1.57 a | 38.24 ± 1.10 a | 38.99 ± 1.10 a |
| LSD 5% | 5.1 | 1.2 | ||||
| Non reducing sugar (mg g−1 FW) | Proline (µmolg−1 FW) | |||||
| As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | As 0 mg/kg | As 50 mg/kg | As 100 mg/kg | |
| Akbar | ||||||
| Control | 129.05 ± 3.80 b | 77.48 ± 1.00 d | 64.65 ± 2.97 d | 0.98 ± 0.01 a | 1.21 ± 0.01 a | 1.33 ± 0.01 a |
| Thia 250 mg/L | 123.02 ± 2.65 c | 101.13 ± 3.46 b | 89.81 ± 2.00 c | 0.96 ± 0.00 b | 0.98 ± 0.01 b | 1.10 ± 0.01 b |
| IAA 30 µM | 99.87 ± 1.53 d | 81.76 ± 2.63 c | 116.73 ± 4.39 b | 0.96 ± 0.01 b | 0.98 ± 0.00 b | 1.10 ± 0.01 b |
| IAA 30 µM+ Thia 250 mg/L | 137.86 ± 1.76 a | 105.91 ± 2.90 a | 135.85 ± 2.86 a | 0.95 ± 0.00 c | 0.96 ± 0.00 c | 1.07 ± 0.03 c |
| Pearl | ||||||
| Control | 130.56 ± 3.46 b | 100.63 ± 1.76 b | 76.98 ± 3.14 c | 0.90 ± 0.01 a | 1.10 ± 0.00 a | 1.21 ± 0.01 a |
| Thia 250 mg/L | 142.89 ± 4.62 a | 114.96 ± 4.53 a | 90.31 ± 3.90 b | 0.87 ± 0.00 b | 1.11 ± 0.01 a | 1.12 ± 0.01 b |
| IAA 30 µM | 124.02 ± 2.97 c | 89.56 ± 2.01 c | 108.93 ± 3.21 a | 0.90 ± 0.01 a | 0.99 ± 0.00 b | 1.09 ± 0.01 c |
| IAA 30 µM+ Thia 250 mg/L | 134.09 ± 4.30 b | 115.72 ± 2.51 a | 107.92 ± 1.90 a | 0.83 ± 0.00 c | 0.98 ± 0.01 c | 0.98 ± 0.00 d |
| LSD 5% | 3.59 | 0.01 | ||||
| Source of Variation | Varieties (Var) | Stress (S) | Treatment (T) | Var × S | Var × T | S × T | Var × S × T | Error |
|---|---|---|---|---|---|---|---|---|
| Shoot f. wt. | 50.601 *** | 114.254 *** | 19.963 *** | 16.108 *** | 2.353 * | 4.465 *** | 1.059 ns | 0.731 |
| Shoot dry wt. | 1.820 *** | 0.711 *** | 0.496 *** | 0.027 *** | 0.076 *** | 0.018*** | 0.006 *** | 0.001 |
| Root f. wt. | 0.647 *** | 1.346 *** | 0.557 *** | 0.042 * | 0.014 ns | 0.018 ns | 0.022 ns | 0.010 |
| Root dry wt. | 0.060 *** | 0.077 *** | 0.084 *** | 0.005 *** | 0.001 ** | 0.008*** | 0.002 *** | 2.5 × 10−4 |
| Shoot length | 749.490 *** | 777.910 *** | 350.603 *** | 69.033 *** | 71.654 *** | 54.546*** | 36.789 *** | 2.932 |
| Root length | 85.151 *** | 154.348 *** | 48.803 *** | 4.875 * | 1.460 ns | 1.997 ns | 0.949 ns | 0.961 |
| Chl. a | 0.304 *** | 2.054 *** | 0.205 *** | 0.018 *** | 0.006 *** | 0.001* | 0.003 *** | 6.6 × 10−4 |
| Chl. b | 0.052 *** | 0.023 *** | 0.001 ** | 0.001 ** | 0.006 *** | 0.003*** | 0.001 ** | 3.0× 10−4 |
| Chlorophyll ratio | 16.677 *** | 9.854 *** | 2.641 *** | 0.567 ** | 0.913 *** | 0.337** | 0.072 ns | 0.098 |
| Total Chlorophyll | 0.109 *** | 2.499 *** | 0.210 *** | 0.011 *** | 0.001 * | 0.003*** | 0.006 *** | 3.9× 10−4 |
| Carotenoids | 1.52 × 10−5 *** | 3.55 × 10−5 *** | 1.81 × 10−5 *** | 1.3 × 10−5 *** | 1.8 × 10−6 *** | 3.63 × 10−6 *** | 7.96 × 10−7 * | 2.6 × 10−7 |
| A | 0.429 *** | 0.520 *** | 0.264 *** | 0.049 *** | 0.001 * | 0.010*** | 0.008 *** | 5.0× 10−4 |
| E | 0.042 *** | 0.144 *** | 0.055 *** | 0.020 *** | 4.38 × 10−4 ns | 0.002*** | 4.61 × 10−4 * | 1.7 × 10−4 |
| gs | 0.034 *** | 0.175 *** | 0.041 *** | 0.081 *** | 0.019 *** | 0.007 *** | 0.009 *** | 2.0 × 10−4 |
| Ψw | 3.454 *** | 2.398 *** | 0.126 *** | 0.696 *** | 2.671 ns | 0.005 *** | 0.002 ** | 5.2 × 10−4 |
| Ψs | 0.256 *** | 0.495 *** | 0.411 *** | 0.235 *** | 0.226 *** | 0.211 *** | 0.058 *** | 0.002 |
| MDA | 0.047 ns | 38.618 *** | 11.702 *** | 6.976 ** | 1.902 ns | 1.965ns | 1.304 ns | 1.009 |
| H2O2 | 10.083 *** | 105.028 *** | 6.173 *** | 2.049 *** | 0.429 *** | 0.145 ** | 0.023 ns | 0.044 |
| AsA | 12.416 * | 427.615 *** | 88.657 *** | 76.755 *** | 15.739 *** | 20.209 *** | 14.825 *** | 1.775 |
| TSP | 82,082.92 *** | 30,318.97 *** | 8005.24 *** | 3120.6 *** | 4467.6 *** | 3953.3 *** | 1262.33 *** | 93.297 |
| TFAA | 367.610 *** | 145.775 *** | 9.897 *** | 52.619 *** | 35.337 *** | 23.227 *** | 13.585 *** | 0.103 |
| TSS | 6817.01 *** | 1336.84 *** | 2451.77 *** | 782.33 *** | 650.06 *** | 3066.6*** | 152.128 * | 57.861 |
| RS | 1673.90 *** | 614.41 *** | 1241.20 *** | 38.382 *** | 131.00 *** | 2.067 ns | 18.416 *** | 3.211 |
| NRS | 674.499 *** | 6740.893 *** | 2269.101 *** | 646.75 *** | 375.2 *** | 1300.0 *** | 202.300 *** | 28.628 |
| TPC | 193.187 *** | 2786.794 *** | 2651.804 *** | 42.611 *** | 21.757 ** | 1054.8*** | 55.380 *** | 3.421 |
| TFC | 36.528 *** | 94.788 *** | 438.796 *** | 53.768 *** | 64.258 *** | 319.749*** | 23.996 *** | 0.848 |
| TAC | 0.014 *** | 0.087 *** | 0.009 *** | 0.014 *** | 0.057 *** | 0.027*** | 0.008 *** | 2.2 × 10−4 |
| Proline | 0.033 *** | 0.251 *** | 0.080 *** | 0.014 *** | 0.013 *** | 0.012*** | 0.004 *** | 3.4 × 10−4 |
| CAT | 3.666 ** | 65.547 *** | 6.367 *** | 4.934 *** | 1.086 * | 2.488*** | 2.462 *** | 0.324 |
| POD | 664.127 *** | 712.353 *** | 137.162 *** | 78.399 *** | 0.835 ns | 3.221** | 4.579 *** | 0.506 |
| SOD | 1352.80 *** | 2872.00 *** | 274.408 *** | 2500.6 *** | 155.86 *** | 522.25 *** | 184.085 *** | 0.983 |
| APX | 52.833 *** | 44.595 *** | 17.466 *** | 2.011 *** | 0.461 *** | 0.214 * | 0.297 ** | 0.068 |
| Shoot Ca2+ | 0.003 ns | 19.815 *** | 6.846 *** | 3.024 *** | 0.873 ** | 2.297 *** | 0.978 *** | 0.187 |
| Root Ca2+ | 0.005 * | 0.030 *** | 0.007 *** | 0.012 *** | 0.003 ** | 0.011 *** | 0.002 * | 8.8 × 10−4 |
| Shoot K+ | 88.888 *** | 165.010 *** | 440.657 *** | 7.774 ns | 5.620 ns | 81.112 *** | 35.561 *** | 5.347 |
| Root K+ | 42.013 *** | 577.166 *** | 483.782 *** | 35.388 *** | 4.986 * | 13.310 *** | 15.013 *** | 1.562 |
| Shoot P | 0.010 *** | 0.082 *** | 0.007 *** | 6.08 × 10−6 ns | 0.001 *** | 0.001 *** | 2.38 × 10−4 *** | 1.3–5 |
| Root P | 0.002 *** | 0.003 *** | 0.022 *** | 4.68 × 10−5 * | 3.6 × 10−4 *** | 0.0014 *** | 1.4 × 10−4 *** | 1.0 × 10−5 |
| Shoot As | 64.723 *** | 4805.875 *** | 1365.578 *** | 414.80 ** | 32.241 *** | 105.58 *** | 105.636 *** | 2.391 |
| Root As | 4.869 ns | 3643.594 *** | 894.909 *** | 125.17 *** | 17.307 *** | 110.45 *** | 20.659 *** | 1.704 |
| Df | 1 | 2 | 3 | 2 | 3 | 6 | 6 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atif, M.; Perveen, S.; Parveen, A.; Mahmood, S.; Saeed, M.; Zafar, S. Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea mays L.) under Arsenic Stress. Sustainability 2022, 14, 13288. https://doi.org/10.3390/su142013288
Atif M, Perveen S, Parveen A, Mahmood S, Saeed M, Zafar S. Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea mays L.) under Arsenic Stress. Sustainability. 2022; 14(20):13288. https://doi.org/10.3390/su142013288
Chicago/Turabian StyleAtif, Muhammad, Shagufta Perveen, Abida Parveen, Saqib Mahmood, Muhammad Saeed, and Sara Zafar. 2022. "Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea mays L.) under Arsenic Stress" Sustainability 14, no. 20: 13288. https://doi.org/10.3390/su142013288
APA StyleAtif, M., Perveen, S., Parveen, A., Mahmood, S., Saeed, M., & Zafar, S. (2022). Thiamine and Indole-3-Acetic Acid Induced Modulations in Physiological and Biochemical Characteristics of Maize (Zea mays L.) under Arsenic Stress. Sustainability, 14(20), 13288. https://doi.org/10.3390/su142013288






