Effects of Straw Returning on Soil Chemical Properties and Microbial Community Diversity under the Rice-Crayfish Integrated System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Soil Sampling and Storage
2.3. Soil Chemical Properties Analysis
2.4. Soil-Reducing Substances Properties Analysis
2.5. Soil Enzyme Activity Analysis
2.6. Soil Functional Diversity of the Microbial Community Analysis
2.7. Statistical Analysis
3. Results
3.1. Soil Chemical Properties Analysis
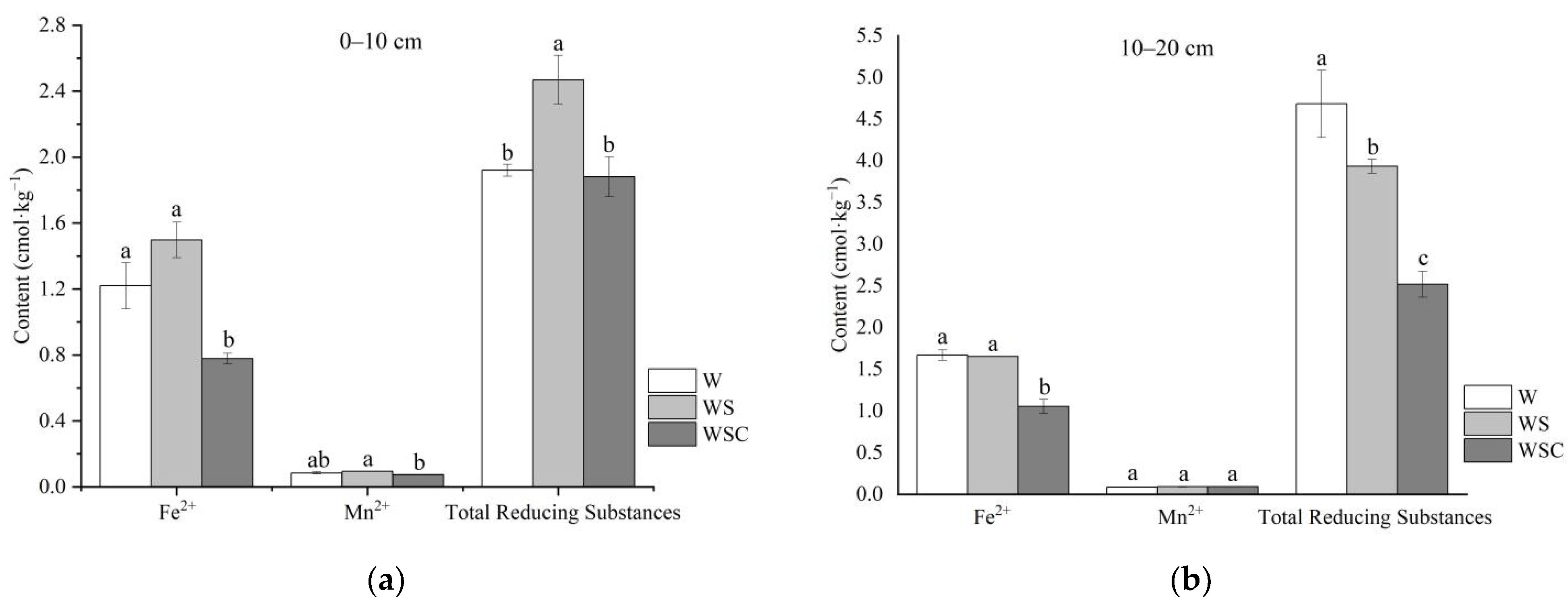
3.2. Soil-Reducing Substances Properties Analysis
3.3. Soil Enzyme Activity Analysis
3.4. Soil Functional Diversity of the Microbial Community Analysis
3.5. Analysis of Soil Microbe Carbon Source Utilization
3.6. Interaction of Chemical and Reducing Substances Properties with Soil Enzyme Activity and Microbial Community Diversity Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien, Y.H.; Avault, J.W., Jr. Production of crayfish in rice fields. Prog. Fish-Cult. 1980, 42, 67–71. [Google Scholar] [CrossRef]
- Brunson, M.W.; Griffin, J.L. Comparison of rice-crayfish and grain sorghum-crayfish double cropping systems. Aquaculture 1988, 72, 265–272. [Google Scholar] [CrossRef]
- Yuan, P.L.; Wang, J.P.; Li, C.F.; Cao, C.G. Long-term rice-crayfish farming aggravates soil gleying and induced changes of soil iron morphology. Soil Use Manag. 2020, 38, 757–770. [Google Scholar] [CrossRef]
- Si, G.H.; Peng, C.L.; Xu, X.Y.; Xu, D.B.; Yuan, J.F.; Li, J.H. Effect of integrated rice-crayfish farming system on soil physicochemical properties in waterlogged paddy soils. Chin. J. Eco-Agric. 2017, 25, 61–68. [Google Scholar]
- Si, G.H.; Peng, C.L.; Yuan, J.F.; Xu, X.Y.; Zhao, S.J.; Xu, D.B.; Wu, J.S. Changes in soil microbial community composition and organic carbon fractions in an integrated rice-crayfish farming system in subtropical China. Sci. Rep. 2017, 7, 2856. [Google Scholar] [CrossRef]
- Yuan, P.L.; Wang, J.P.; Li, C.F.; Xiao, Q.Q.; Liu, Q.J.; Sun, Z.C.; Wang, J.H.; Cao, C.G. Soil quality indicators of integrated rice-crayfish farming in the Jianghan Plain, China using a minimum data set. Soil Tillage Res. 2020, 204, 104732. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, P.N.; Mei, F.J.; Ling, Y.; Qiao, Y.B.; Liu, C.S.; Leghari, S.J.; Guan, X.K.; Wang, T.C. Does continuous straw returning keep China farmland soil organic carbon continued increase? A meta-analysis. J. Environ. Manag. 2021, 288, 112391. [Google Scholar] [CrossRef]
- Li, H.; Cao, Y.; Wang, X.M.; Ge, X.; Li, B.Q.; Jin, C.Q. Evaluation on the Production of Food Crop Straw in China from 2006 to 2014. Bioenergy Res. 2017, 10, 945–957. [Google Scholar] [CrossRef]
- Xue, Y.H. Mechanization technology of corn straw returning. Beijing Agric. 2010, 25, 39. [Google Scholar]
- Yang, H.S.; Xu, M.M.; Li, Y.F.; Xu, C.; Zhai, S.L.; Liu, J. The impacts of ditch-buried straw layers on the interface soil physicochemical and microbial properties in a rice-wheat rotation system. Soil Tillage Res. 2020, 202, 104656. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Zhu, Z.K.; Wei, X.M.; Liu, S.L.; Peng, P.Q.; Gunina, A.; Shen, J.L.; Kuzyakov, Y.; Ge, T.D.; Wu, J.S.; et al. Straw and biochar strongly affect functional diversity of microbial metabolism in paddy soils. J. Integr. Agric. 2019, 18, 1474–1485. [Google Scholar] [CrossRef]
- Li, Y.M.; Duan, Y.; Wang, G.L.; Wang, A.Q.; Shao, G.Z.; Meng, X.H.; Hu, H.Y.; Zhang, D.M. Straw alters the soil organic carbon composition and microbial community under different tillage practices in a meadow soil in Northeast China. Soil Tillage Res. 2021, 208, 104879. [Google Scholar] [CrossRef]
- Zhai, S.L.; Xu, C.F.; Wu, Y.C.; Liu, J.; Meng, Y.L.; Yang, H.S. Long-term ditch-buried straw return alters soil carbon sequestration, nitrogen availability and grain production in a rice-wheat rotation system. Crop Pasture Sci. 2021, 72, 245–254. [Google Scholar] [CrossRef]
- Kubar, K.A.; Huang, L.; Lu, J.W.; Li, X.K.; Xue, B.; Yin, Z.Y. Integrative effects of no-tillage and straw returning on soil organic carbón and water stable aggregation under rice-rape rotation. Chil. J. Agric. Res. 2018, 78, 205–215. [Google Scholar] [CrossRef]
- Yan, S.S.; Song, J.M.; Fan, J.S.; Yan, C.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Glob. Ecol. Conserv. 2020, 22, e00962. [Google Scholar] [CrossRef]
- Bu, R.Y.; Ren, T.; Lei, M.J.; Liu, B.; Li, X.K.; Cong, R.H.; Zhang, Y.Y.; Lu, J.W. Tillage and straw-returning practices effect on soil dissolved organic matter, aggregate fraction and bacteria community under rice-rice-rapeseed rotation system. Agric. Ecosyst. Environ. 2020, 287, 106681. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.L.; Wei, T.; Yang, Z.; Jia, Z.K.; Yang, B.P.; Han, Q.F.; Ren, X.L. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Yang, H.S.; Li, Y.F.; Zhai, S.L.; Fang, C.; Liu, J.; Zhang, Q. Long term ditch-buried straw return affects soil fungal community structure and carbon-degrading enzymatic activities in a rice-wheat rotation system. Appl. Soil Ecol. 2020, 155, 103660. [Google Scholar] [CrossRef]
- Wu, L.P.; Ma, H.; Zhao, Q.L.; Zhang, S.R.; Wei, W.L.; Ding, X.D. Changes in soil bacterial community and enzyme activity under five years straw returning in paddy soil. Eur. J. Soil Biol. 2020, 100, 103215. [Google Scholar] [CrossRef]
- Zhang, L.G.; Chen, X.; Xu, Y.J.; Jin, M.C.; Ye, X.X.; Gao, H.J.; Chu, W.Y.; Mao, J.D.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 113118. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Shah, T.; Zhang, L.; Liu, H.Y.; Peng, S.B.; Nie, L.X. Effect of straw returning on soil organic carbon in rice-wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, H.; Ji, X.H.; Li, C.J.; Li, S.N. Effects of reduced inorganic fertilization and rice straw recovery on soil enzyme activities and bacterial community in double-rice paddy soils. Eur. J. Soil Biol. 2019, 94, 103116. [Google Scholar] [CrossRef]
- Sun, Z.C.; Guo, Y.; Li, C.F.; Cao, C.G.; Yuan, P.L.; Zou, F.L.; Wang, J.H.; Jia, P.G.; Wang, J.P. Effects of straw returning and feeding on greenhouse gas emissions from integrated rice-crayfish farming in Jianghan Plain, China. Environ. Sci. Pollut. Res. 2019, 26, 11710–11718. [Google Scholar] [CrossRef] [PubMed]
- Si, G.H.; Peng, C.L.; Yuan, J.F.; Xia, X.G.; Cheng, J.P.; Xu, X.Y.; Jia, P.A.; Xie, Y.Y.; Zhou, J.X. Effect of rice straw returning to field on ammonia volatilization in paddy fields under the integrated rice-crayfish system. J. Ecol. Rural Environ. 2021, 37, 360–368. [Google Scholar] [CrossRef]
- Bao, S.D. Methods for Soil Agricultural and Chemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 30–106. [Google Scholar]
- Lu, R.K. Soil and Agricultural Chemistry Analysis, 1st ed.; China Agriculture Science and Technique Press: Beijing, China, 1999; pp. 74–84. [Google Scholar]
- Guan, S.Y. Soil Enzyme and Study Method, 1st ed.; Agricultural Press: Beijing, China, 1986; pp. 274–313. [Google Scholar]
- Schutter, M.; Dick, R. Shift in substrate utilization potential and structure of soil microbial communities in response to carbon substrates. Soil Biol. Biochem. 2001, 33, 1481–1491. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.L.; Cao, Y.F.; Xu, W.X. Decomposition characteristics of different plant straws and soil microbial functional diversity. Acta Pedol. Sin. 2014, 51, 743–752. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Meng, Q.J.; Xu, Y.L.; Li, C.J.; Han, X.Z.; Pei, X.C. Effects of different vegetation on microbial functional diversity in black soil. Chin. J. Ecol. 2008, 27, 1134–1140. [Google Scholar] [CrossRef]
- Xu, Q.F.; Jiang, P.S.; Wang, Q.Z.; Lu, Y.T. Effects of green manure on soil microbial properties of phyllostachys pubescens stands under intensive management. J. Beijing For. Univ. 2009, 31, 43–48. [Google Scholar] [CrossRef]
- Dang, K.; Chen, W.; Zhang, H.Q.; Wang, X.H.; Ai, J.X.; Wang, Y.K.; Zheng, H.T.; Wang, X.H.; Geng, Q.Y.; Jin, F.; et al. Soil research mechanism of combining straw returning with plastic film mulching to increase rice yield. J. Northeast Agric. Sci. 2021, 46, 11–16. [Google Scholar]
- Cai, C.; Li, G.; Peng, L.; Li, J.F.; Wu, Q.X. Effects of rice-crawfish rotation on soil physicochemical properties in Jianghan plain. Acta Pedol. Sin. 2019, 56, 217–226. [Google Scholar] [CrossRef]
- Huang, W.; Wu, J.F.; Pan, X.H.; Tan, X.M.; Zeng, Y.J.; Shi, Q.H.; Liu, T.J.; Zeng, Y.H. Effects of long-term straw return on soil organic carbon fractions and enzyme activities in a double-cropped rice paddy in south China. J. Integr. Agric. 2021, 20, 236–247. [Google Scholar] [CrossRef]
- Haque, K.M.S.; Eberbach, P.L.; Weston, L.A.; Dyall-Smith, M.; Howitt, J.A. Variable impact of rice (Oryza sativa) on soil metal reduction and availability of pore water Fe2+ and Mn2+ throughout the growth period. Chem. Ecol. 2016, 32, 182–200. [Google Scholar] [CrossRef]
- Noellemeyer, E.; Frank, F.; Alvarez, C.; Morazzo, G.; Quiroga, A. Carbon contents and aggregation related to soil physical and biological properties under a land-use sequence in the semiarid region of central Argentina. Soil Tillage Res. 2008, 99, 179–190. [Google Scholar] [CrossRef]
- De la Paz Jimenez, M.; de la Horra, A.M.; Pruzzo, L.; Palma, R.M. Soil quality: A new index based on microbiological and biochemical parameters. Biol. Fertil. Soils 2002, 35, 302–306. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.Y.; Ren, G.X.; Khan, A.; Feng, Y.Z.; Yang, G.H. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Indirect effects of nitrogen amendments on organic substrate quality increase enzymatic activity driving decomposition in a Mesic Grassland. Ecosystems 2011, 14, 234–247. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Zhou, D.P.; Zhu, C.B.; Fan, J.Q.; Jiang, Z.F.; Wu, S.H. Microbial community structure and function in greenhouse soils of asparagus. Soils 2014, 46, 1076–1082. [Google Scholar] [CrossRef]
- Rogers, B.F.; Tate, R.L.T. Temporal analysis of the soil microbial community along a toposequence in Pineland soils. Soil Biol. Biochem. 2001, 33, 1389–1401. [Google Scholar] [CrossRef]
- Yang, H.S.; Fang, C.; Meng, Y.; Dai, Y.J.; Liu, J. Long term ditch-buried straw return increases functionality of soil microbial communities. Catena 2021, 202, 105316. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Mo, R.L.; Deng, W.; Zhu, Z.X.; Liu, D.B.; Hu, X.M. Effects of long-term straw retention on soil microorganisms under a rice-wheat cropping system. Arch. Microbiol. 2020, 202, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Grove, J.A.; Gehder, M.; Anderson, W.A.; Legge, R.L. Data transformations in the analysis of community-level substrate utilization data from microplates. J. Microbiol. Methods. 2007, 69, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wei, H.; Lü, C.; Ke, Q.; Li, Y.; Jing, P. Allelopathic effect of rice straw on rape (Brassica napus L.) seedlings. J. Agric. Resour. Environ. 2016, 33, 253–261. [Google Scholar] [CrossRef]
- Chou, C.H.; Lin, H.J. Autointoxication mechanism of Oryza sativa I. Phytotoxic effects of decomposing rice residues in soil. J. Chem. Ecol. 1976, 2, 353–367. [Google Scholar] [CrossRef]
- Wang, Z.T.; Lu, L.; Chen, Q.; Wen, X.x.; Liao, Y.C. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef]



| Soil Depth | pH | Total Organic Carbon | Available N | Available P | Available K | Total N | Total P | Total K | |
|---|---|---|---|---|---|---|---|---|---|
| (cm) | (g·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (g·kg−1) | (g·kg−1) | (g·kg−1) | ||
| 0–10 | W | 6.81 ± 0.06a | 24.14 ± 0.19b | 114.88 ± 4.42b | 11.16 ± 0.83a | 197.05 ± 4.88a | 2.50 ± 0.01a | 0.67 ± 0.02a | 23.01 ± 0.16a |
| WS | 6.84 ± 0.04a | 25.34 ± 0.64b | 127.00 ± 3.45ab | 12.79 ± 0.16a | 205.52 ± 5.08a | 2.56 ± 0.01a | 0.68 ± 0.00a | 23.12 ± 0.21a | |
| WSC | 6.42 ± 0.03b | 26.90 ± 0.35a | 136.04 ± 4.24a | 12.01 ± 0.75a | 194.09 ± 12.25a | 2.55 ± 0.07a | 0.67 ± 0.02a | 23.00 ± 0.50a | |
| 10–20 | W | 6.98 ± 0.01a | 19.88 ± 0.51b | 105.12 ± 4.95ab | 10.61 ± 0.24a | 171.23 ± 5.08a | 2.18 ± 0.03b | 0.61 ± 0.01a | 23.18 ± 0.30a |
| WS | 6.91 ± 0.03a | 21.21 ± 0.19a | 89.43 ± 2.07b | 10.29 ± 0.64a | 178.00 ± 2.96a | 2.21 ± 0.04b | 0.62 ± 0.01a | 23.14 ± 0.32a | |
| WSC | 6.73 ± 0.05b | 21.28 ± 0.27a | 108.93 ± 5.44a | 11.05 ± 0.85a | 185.62 ± 1.53a | 2.62 ± 0.15a | 0.64 ± 0.01a | 23.03 ± 0.17a |
| Soil Depth (cm) | AWCD of 96 h | Shannon Index | Simpson Index | McIntosh Index | |
|---|---|---|---|---|---|
| 0–10 | W | 0.87 ± 0.06b | 2.89 ± 0.03b | 0.939 ± 0.002b | 6.68 ± 0.10c |
| WS | 1.27 ± 0.02a | 3.11 ± 0.05a | 0.951 ± 0.003a | 8.71 ± 0.08b | |
| WSC | 1.42 ± 0.03a | 3.21 ± 0.02a | 0.957 ± 0.001a | 9.08 ± 0.04a | |
| 10–20 | W | 0.51 ± 0.09b | 2.50 ± 0.20b | 0.897 ± 0.006c | 4.81 ± 0.48b |
| WS | 0.69 ± 0.10b | 2.73 ± 0.17ab | 0.922 ± 0.004b | 5.40 ± 0.65b | |
| WSC | 1.23 ± 0.13a | 3.16 ± 0.04a | 0.954 ± 0.002a | 8.10 ± 0.69a |
| AWCD of 96 h | Acid Phosphatase | Urease | Sucrase | McIntosh Index | Simpson Index | Shannon Index | |
|---|---|---|---|---|---|---|---|
| pH | −0.719 ** | −0.812 ** | 0.317 | −0.644 ** | −0.697 ** | −0.702 ** | −0.654 ** |
| TOC | 0.503 * | 0.507 * | −0.31 | 0.256 | 0.498 * | 0.483 * | 0.514 * |
| AP | 0.537 * | 0.466 | 0.013 | 0.429 | 0.565 * | 0.442 | 0.421 |
| AK | 0.712 ** | 0.654 ** | 0.006 | 0.435 | 0.752 ** | 0.778 ** | 0.685 ** |
| AN | 0.721 ** | 0.641 ** | −0.321 | 0.454 | 0.727 ** | 0.588 * | 0.583 * |
| TK | −0.259 | −0.07 | 0.014 | −0.081 | −0.258 | −0.221 | −0.346 |
| TN | 0.748 ** | 0.644 ** | −0.416 | 0.637 ** | 0.762 ** | 0.789 ** | 0.668 ** |
| TP | 0.616 ** | 0.572 * | −0.151 | 0.404 | 0.629 ** | 0.651 ** | 0.501 * |
| Fe2+ | −0.766 ** | −0.723 ** | 0.373 | −0.556 * | −0.767 ** | −0.781 ** | −0.688 ** |
| Mn2+ | −0.132 | −0.212 | 0.093 | −0.011 | −0.148 | −0.028 | −0.034 |
| Total Reducing Substances | −0.772 ** | −0.780 ** | 0.147 | −0.540 * | −0.770 ** | −0.890 ** | −0.773 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Peng, C.; Si, G.; Sha, A.; Yuan, J.; Zhao, S.; Xu, D.; Liu, W. Effects of Straw Returning on Soil Chemical Properties and Microbial Community Diversity under the Rice-Crayfish Integrated System. Sustainability 2022, 14, 13539. https://doi.org/10.3390/su142013539
Zhu X, Peng C, Si G, Sha A, Yuan J, Zhao S, Xu D, Liu W. Effects of Straw Returning on Soil Chemical Properties and Microbial Community Diversity under the Rice-Crayfish Integrated System. Sustainability. 2022; 14(20):13539. https://doi.org/10.3390/su142013539
Chicago/Turabian StyleZhu, Xiuxiu, Chenglin Peng, Guohan Si, Aihua Sha, Jiafu Yuan, Shujun Zhao, Dabing Xu, and Wei Liu. 2022. "Effects of Straw Returning on Soil Chemical Properties and Microbial Community Diversity under the Rice-Crayfish Integrated System" Sustainability 14, no. 20: 13539. https://doi.org/10.3390/su142013539
APA StyleZhu, X., Peng, C., Si, G., Sha, A., Yuan, J., Zhao, S., Xu, D., & Liu, W. (2022). Effects of Straw Returning on Soil Chemical Properties and Microbial Community Diversity under the Rice-Crayfish Integrated System. Sustainability, 14(20), 13539. https://doi.org/10.3390/su142013539





