Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Physicochemical Analysis
2.4. Heavy Metal Analysis
2.5. Enzyme Activity Assay
2.6. Statistical Analysis
3. Results and Discussion
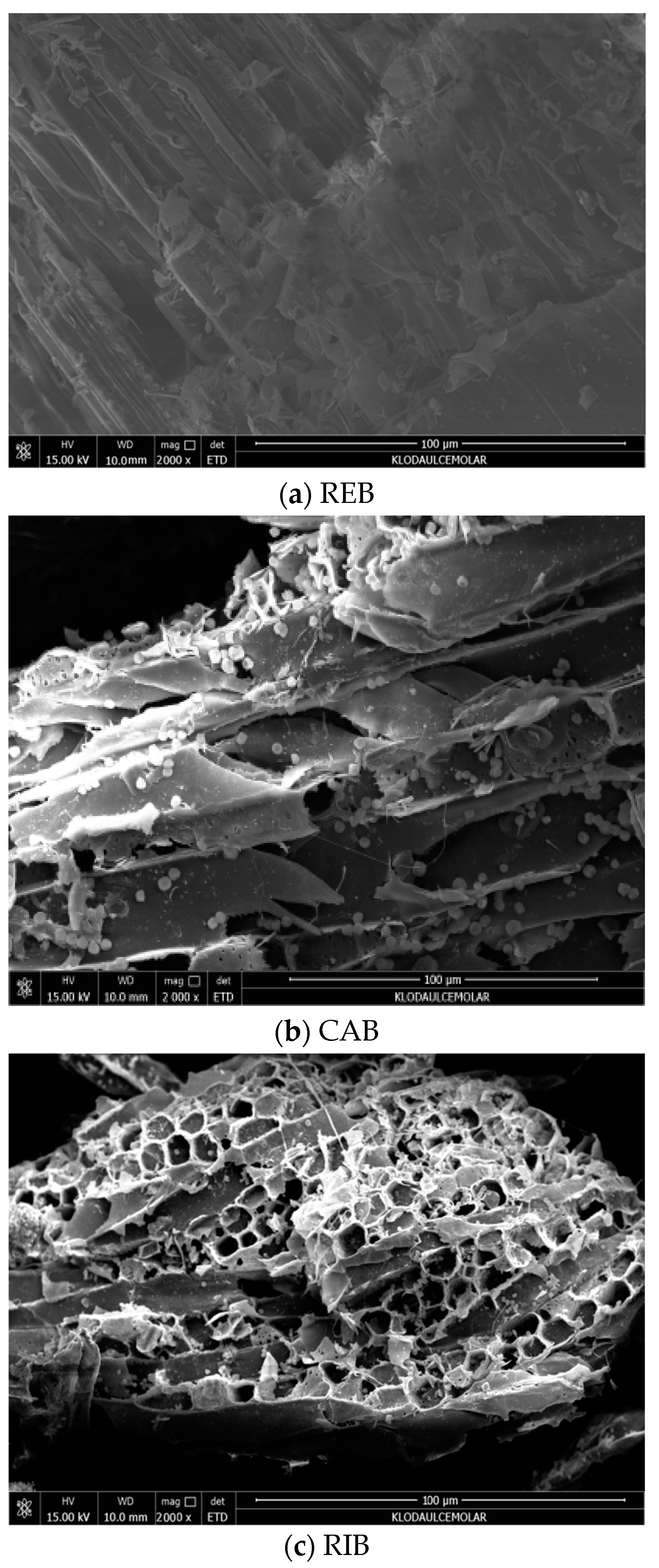
3.1. Comparison of Biochars from Different Feedstocks
3.2. Effects of Biochar Amendments on Soil Physicochemical Properties
3.3. Changes in Soil Enzyme Activities upon Biochar Addition
3.4. Bioavailability of Heavy Metals in Biochar-Amended Soil
3.5. Bioconcentration of Heavy Metals in Plants after Soil Stabilization with Biochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, A.; Wang, Y.; Tan, S.N.; Yusof, M.L.M.; Ghosh, S.; Chen, Z. Phytoremediation, A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.B.; Chen, J.B.; Fu, X.W.; Hu, H.Y.; Li, P.; Qiu, G.L.; Yan, H.Y.; Yin, X.F.; Zhang, H.; Zhu, W. Progress on environmental geochemistry of mercury. Bull. Mineral. Petrol. Geochem. 2013, 32, 503–530. [Google Scholar]
- Marziali, L.; Rosignoli, F.; Drago, A.; Pascariello, S.; Valsecchi, L.; Rossaro, B.; Guzzella, L. Toxicity risk assessment of Hg, DDT and As legacy pollution in sediments: A triad approach under low concentration conditions. Sci. Total Environ. 2017, 593–594, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.W.; Mommsen, T.P. Environmental Toxicology; Cambridge University Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Esdaile, L.J.; Chalker, J.M. The mercury problem in artisanal and small-scale gold mining. Chem. Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Zhao, H.; Rong, Q.; Qin, X.; Nong, X.; Lu, D.; Zhang, C. Study on stabilization of arsenic in soil using FMBO incorporated with peat soil. Environ. Sci. Technol. 2021, 44, 53–59. [Google Scholar]
- Osterwalder, S.; Huang, J.; Shetaya, W.; Agnan, Y.; Frossard, A.; Frey, B.; Alewell, C.; Kretzschmar, R.; Biester, H.; Obrist, D. Mercury emission from industrially contaminated soils in relation to chemical, microbial, and meteorological factors. Environ. Pollut. 2019, 250, 944–952. [Google Scholar] [CrossRef]
- Chai, L.; Tang, J.; Liao, Y.; Yang, Z.; Liang, L.; Li, Q.; Wang, H.; Yang, W. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans and its application in arsenic immobilization in the contaminated soil. J. Soil. Sediments 2016, 16, 2430–2438. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Sheng, L. Preparation of straw biochar and application of constructed wetland in China, A review. J. Clean. Prod. 2020, 273, 123131. [Google Scholar] [CrossRef]
- Bai, B.; Zhao, H.; Zhang, S.; Zhang, X.; Yang, G. Forecasting of agricultural straw burning in the Northeastern China based on neural network. China Environ. Sci. 2020, 40, 5205–5212. [Google Scholar]
- Shukla, P.; Giri, B.S.; Mishra, R.K.; Pandey, A.; Chaturvedi, P. Lignocellulosic biomass-based engineered biochar composites, A facile strategy for abatement of emerging pollutants and utilization in industrial applications. Renew. Sustain. Energy Rev. 2021, 152, 111643. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; AL-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Lian, F.; Xing, B. Black carbon (biochar) in water/soil environments: Molecular structure, sorption, stability, and potential risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Chen, Z.; Tong, F.; Shen, H.; Gao, Y.; Liu, L.; Zhang, Z.; Lu, X. Effects of different biomass and pyrolysis technique on biochar characterization and immobilization of heavy metal in contaminated soil. J. Ecol. Rural Environ. 2021, 37, 86–95. [Google Scholar]
- Hu, H.; Xi, B.; Tan, W. Effects of sulfur-rich biochar amendment on microbial methylation of Hg in rhizosphere paddy soil and methyl Hg accumulation in rice. Environ. Pollut. 2021, 286, 117290. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Wu, Q.; Wang, F.; Shen, B. Hg sorption study of halides modified biochars derived from cotton straw. Chem. Eng. J. 2016, 302, 305–313. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, Y.; Sun, X.; Wang, H.; Teng, X. Corn stover biochar increased edible safety of spinach by reducing the migration of Hg from soil to spinach. Sci. Total Environ. 2020, 758, 143883. [Google Scholar] [CrossRef]
- Kamran, M.A.; Bibi, S.; Chen, B. Preventative effect of crop straw-derived biochar on plant growth in an As polluted acidic ultisol. Sci. Total Environ. 2022, 812, 151469. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Removal of As by wheat straw biochar from soil. Bull. Environ. Contam. Toxicol. 2022, 108, 415–422. [Google Scholar] [CrossRef]
- Soares, M.B.; Santos, F.H.; Alleoni, L.R.F. Iron-modified biochar from sugarcane straw to remove As and lead from contaminated water. Water Air Soil Pollut. 2021, 232, 391. [Google Scholar] [CrossRef]
- Garau, G.; Porceddu, A.; Sanna, M.; Silvetti, M.; Castaldi, P. Municipal solid wastes as a resource for environmental recovery, impact of water treatment residuals and compost on the microbial and biochemical features of As and trace metal-polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, L.; Zeng, G.; Xu, P.; Huang, C.; Deng, L.; Wang, R.; Wan, J. The effects of rice straw biochar on indigenous microbial community and enzymes activity in heavy metal-contaminated sediment. Chemosphere 2017, 174, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Shi, X.; Liu, Z. Synthesis and characterization of reed-based biochar and its adsorption properties for Cu2+ and bisphenol A (BPA). Environ. Chem. 2020, 39, 2196–2205. [Google Scholar]
- Haddad, S.A.; Lemanowicz, J. Benefits of corn-cob biochar to the microbial and enzymatic activity of soybean plants grown in soils contaminated with heavy metals. Energies 2021, 14, 5763. [Google Scholar] [CrossRef]
- Bundy, L.G.; Bremner, J.M. A simple titrimetric method for determination of inorganic carbon in soils. Soil Sci. Soc. Am. J. 1972, 36, 273–275. [Google Scholar] [CrossRef]
- Sumner, M.; Miller, W. Cation Exchange Capacity and Exchange Coefficients. Methods of Soil Analysis Part 3-Chemical Methods; (methodsofsoilan3); U.S. Environmental Protection Agency: Madison, WI, USA, 1996; pp. 1201–1229.
- Elliott, C.; Snyder, G.H. Autoclave-induced digestion for the colorimetric determination of siliconin rice straw. J. Agric. Food Chem. 1991, 39, 1118–1119. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.; Wang, H.; Shu, W. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- O’Dell, R.; Silk, W.; Green, P.; Claassen, V. Compost amendment of Cu-Zn minespoil reduces toxic bioavailable heavy metal concentrations and promotes establishment and biomass production of Bromus carinatus (Hook and Arn.). Environ. Pollut. 2007, 148, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Casida, L.E. Microbial metabolic activity in soil as measured by dehydrogenase determinations. Appl. Environ. Microbiol. 1977, 34, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Trasar-Cepeda, C.; Camiña, F.; Leirós, M.C.; Gil-Sotres, F. An improved method to measure catalase activity in soils. Soil Biol. Biochem. 1999, 31, 483–485. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzyme and Its Research Methods; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Yang, C.; Lu, S. Straw and straw biochar differently affect phosphorus availability; enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Suárez, G.; Paz-Ferreiro, J.; Askeland, M.P.J.; Méndez, A.; Gascó, G. Remediation of mining soils by combining Brassica napus growth and amendment with chars from manure waste. Chemosphere 2020, 261, 127798. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Li, L.; Zheng, C.; Fu, Y.; Wu, D.; Yang, X.; Shen, H. Silicate-mediated alleviation of Pb toxicity in banana grown in Pb-contaminated soil. Biol. Trace Element Res. 2012, 145, 101. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Gong, J.; Xue, X.; Yang, H. Preparation of zinc chloride and sulfur modified cornstalk biochar and its stabilization effect on mercury contaminated soil. Chin. J. Environ. Eng. 2021, 15, 1403–1408. [Google Scholar]
- Li, Q.; Liang, W.; Liu, F.; Wang, G.; Wan, J.; Zhang, W.; Peng, C.; Yang, J. Simultaneous immobilization of arsenic; lead and cadmium by magnesium-aluminum modified biochar in mining soil. J. Environ. Manag. 2022, 310, 114792. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zheng, J.; Zhang, X. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, S.; Hao, X.; Li, G. Biochar effects on metal bioaccumulation and arsenic speciation in alfalfa (Medicago sativa L.) grown in contaminated soil. Int. J. Environ. Sci. Technol. 2016, 13, 2467–2474. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, H.; Liang, J.; Guo, S.; Huang, L.; Xu, P.; Liu, Y.; Yuan, Y.; He, X.; He, Y. Efficiency of biochar and compost (or composting) combined amendments for reducing Cd, Cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. Rsc Adv. 2015, 5, 34541–34548. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.; Lee, Y.H.; Tsang, D.C.W.; Rinklebe, J.; Kwon, E.; Ok, Y.S. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Y.; Qi, Y.; Li, J. Biochar-enhanced composts reduce the potential leaching of nutrients and heavy metals and suppress plant-parasitic nematodes in excessively fertilized cucumber soils. Environ. Sci. Pollut. Res. 2018, 25, 7589–7599. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils, mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and green waste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Machmuller, M.B.; Kramer, M.G.; Cyle, T.K.; Hill, N.; Hancock, D.; Thompson, A. Emerging land use practices rapidly increase soil organic matter. Nat. Commun. 2015, 6, 6995. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Maqbool, Z.; Khan, K.S.; Hussain, Q.; Ijaz, S.S.; Iqbal, M.; Aziz, I.; Hussain, A.; Abbas, M.S.; Rafa, H.U. Integrated use of biochar and compost to improve soil microbial activity; nutrient availability, and plant growth in arid soil. Arab. J. Geosci. 2019, 12, 232. [Google Scholar] [CrossRef]
- Jia, W.; Wang, B.; Wang, C.; Sun, H. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2013, 65, 28–39. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Kucharski, M. Activity of β-glucosidase; arylsulfatase and phosphatases in soil contaminated with copper. J. Elem. 2010, 15, 213–226. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrolysis 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Sun, H.; Li, H.; Ma, L. Lead bioavailability in different fractions of mining- and smelting-contaminated soils based on a sequential extraction and mouse kidney model. Environ. Pollut. 2020, 262, 114253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, K.; Cave, M.; Li, H.B.; Ma, L.Q. Lead bioaccessibility in 12 contaminated soils from China, correlation to lead relative bioavailability and lead in different fractions. J. Hazard. Mater. 2015, 295, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Herrera, J.A.; Ríos-Reyes, C.A.; Vargas-Fiallo, L.Y. Mercury speciation in mine tailings amended with biochar, Effects on mercury bioavailability; methylation potential and mobility. Sci. Total Environ. 2021, 760, 143959. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tan, X.; Cai, X.; Liu, S. Remediation of As and Cd contaminated sediment by biochars, Accompanied with the change of microbial community. J. Environ. Chem. Eng. 2022, 10, 106912. [Google Scholar] [CrossRef]
- Yu, Z.; Qiu, W.; Wang, F.; Lei, M.; Wang, D.; Song, Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 2017, 168, 341–349. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhang, Z.; Ye, Z.; Wu, Q.; Tang, Y.; Xiao, T. Roles of soluble minerals in Cd sorption onto rice straw biochar. J. Environ. Sci. 2022, 113, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. The role of tailored biochar in increasing plant growth; and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, Q.; Su, Y.; Sun, L.; Wu, T.; Wang, G.; Kelly, R.M. Rice busk biochar treatment to cobalt-polluted fluvo-aquic soil, Speciation and enzyme activities. Ecotoxicology 2019, 28, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Dave, G.; Murimboh, J.D. A review of metal (Pb and Zn) sensitive and pH tolerant bioassay organisms for risk screening of metal contaminated acidic soils. Environ. Pollut. 2013, 179, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducinglead and copper mobility and uptake to ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Shaaban, M.; Hu, H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 2018, 211, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Liu, F.; Liao, R.; Hu, Y. Accumulation of heavy metals in soil-crop systems, a review for wheat and corn. Environ. Sci. Pollut. Res. Int. 2017, 24, 15209–15225. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Y.; Zhang, C.; Yi, J.; An, S.; Wang, D. Effect of sewage sludge compost products application on total mercury and methylmercury in soil and plants. Environ. Sci. 2017, 38, 405–411. [Google Scholar]
- Zama, E.F.; Reid, B.J.; Sun, G.X.; Yuan, H.Y.; Li, X.M.; Zhu, Y.G. Silicon (Si) biochar for the mitigation of arsenic (As) bioaccumulation in spinach (Spinacia oleracean) and improvement in the plant growth. J. Clean. Prod. 2018, 189, 386–395. [Google Scholar] [CrossRef]





| Particle Size Distribution (%) | Texture | pH | Organic Matter (g kg−1) | CaCO3 (%) | Total Hg (mg kg−1) | Total As (mg kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| Clay (0–2 μm) | Silt (2–50 μm) | Sand (50–2000 μm) | ||||||
| 11.29 | 71.16 | 17.55 | Silty loam | 8.96 | 8.73 | 8.95 | 8.74 | 106 |
| Treatment | Biochar Type | Biochar Dose (g pot−1) | Plant |
|---|---|---|---|
| 1 | Reed straw biochar (REB) | 30 (1%) | Spinach |
| 2 | Cassava straw biochar (CAB) | ||
| 3 | Rice straw biochar (RIB) | ||
| 4 | Control | 0 |
| Property | Biochar | ||
|---|---|---|---|
| REB | CAB | RIB | |
| Yield (%) | 29.24 ± 2.57 c | 32.20 ± 1.35 b | 45.71 ± 0.61 a |
| pH | 10.42 ± 0.13 b | 10.11 ± 0.09 c | 10.81 ± 0.24 a |
| Electrical conductivity (μS cm−1) | 689.21 ± 1.36 b | 1028.05 ± 2.73 a | 1005.36 ± 2.59 a |
| S (%) | 0.32 ± 0.02 c | 0.51 ± 0.14 b | 0.69 ± 0.25 a |
| SiO2 (%) | 4.02 ± 0.91 b | 3.58 ± 0.24 b | 11.08 ± 0.37 a |
| Average pore size (μm) | 10.36 ± 1.24 a | 8.25 ± 1.69 b | 3.09 ± 0.83 c |
| Specific surface area (m2 g−1) | 10.61 ± 0.37 c | 18.45 ± 0.27 b | 23.05 ± 1.36 a |
| Zeta potential (mV) | −12.69 ± 0.21 c | −14.18 ± 0.44 b | −15.96 ± 0.18 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, R.; Lu, N.; Zhang, B. Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar. Sustainability 2022, 14, 13637. https://doi.org/10.3390/su142013637
Wei Y, Li R, Lu N, Zhang B. Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar. Sustainability. 2022; 14(20):13637. https://doi.org/10.3390/su142013637
Chicago/Turabian StyleWei, Yang, Risheng Li, Nan Lu, and Baoqiang Zhang. 2022. "Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar" Sustainability 14, no. 20: 13637. https://doi.org/10.3390/su142013637
APA StyleWei, Y., Li, R., Lu, N., & Zhang, B. (2022). Stabilization of Soil Co-Contaminated with Mercury and Arsenic by Different Types of Biochar. Sustainability, 14(20), 13637. https://doi.org/10.3390/su142013637





