Efficient Degradation of Carbendazim by Ferrate(VI) Oxidation under Near-Neutral Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Oxidation Experiments
2.3. Analytical Methods
2.4. Theoretical Calculations
3. Results and Discussion
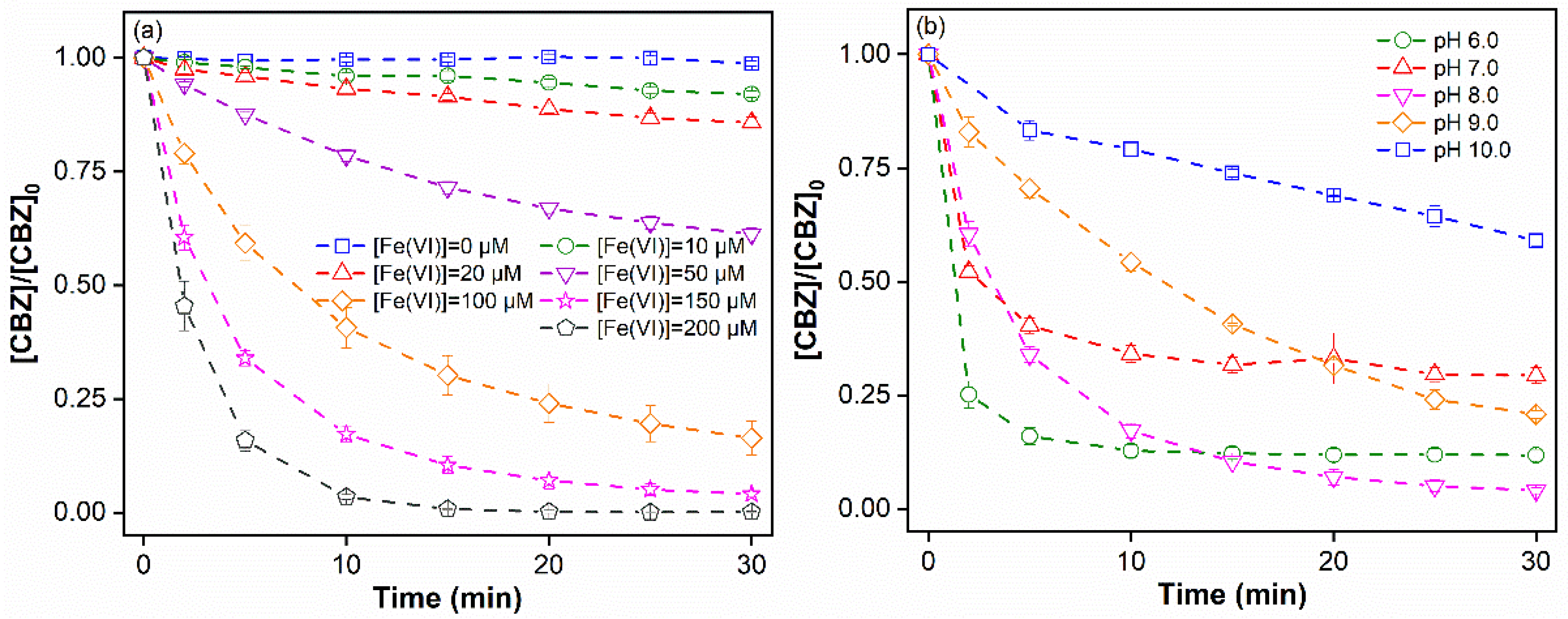
3.1. CBZ Degradation in Fe(VI) Oxidation
3.2. Effects of Inorganic Ions on CBZ Degradation in Fe(VI) Oxidation
3.3. Effects of HA and Water Matrices on CBZ Degradation in Fe(VI) Oxidation
3.4. Pathways and Mechanism of CBZ Degradation in Fe(VI) Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merel, S.; Benzing, S.; Gleiser, C.; Di Napoli-Davis, G.; Zwiener, C. Occurrence and overlooked sources of the biocide carbendazim in wastewater and surface water. Environ. Pollut. 2018, 239, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhou, F.; Li, J.; Zhu, F.; Ma, H. Carbendazim resistance in field isolates of Sclerotinia sclerotiorum in China and its management. Crop Prot. 2016, 81, 115–121. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Nuzzo, S.; Cosma, P. Commercial bentonite clay as low-cost and recyclable “natural” adsorbent for the carbendazim removal/recover from water: Overview on the adsorption process and preliminary photodegradation considerations. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 602, 125060. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Boudina, A.; Emmelin, C.; Baaliouamer, A.; Grenier-Loustalot, M.F.; Chovelon, J.M. Photochemical behaviour of carbendazim in aqueous solution. Chemosphere 2003, 50, 649–655. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Determination of fungicide carbendazim in water and soil samples using dispersive liquid-liquid microextraction and microvolume UV–vis spectrophotometry. Talanta 2015, 134, 24–29. [Google Scholar] [CrossRef]
- Chen, Z.; Ying, G.; Liu, Y.; Zhang, Q.; Zhao, J.; Liu, S.; Chen, J.; Peng, F.; Lai, H.; Pan, C. Triclosan as a surrogate for household biocides: An investigation into biocides in aquatic environments of a highly urbanized region. Water Res. 2014, 58, 269–279. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J.; Liu, Y.; Chen, Z.; Yang, Y.; Zhang, Q.; Ying, G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef]
- Wang, A.; Mahai, G.; Wan, Y.; Jiang, Y.; Meng, Q.; Xia, W.; He, Z.; Xu, S. Neonicotinoids and carbendazim in indoor dust from three cities in China: Spatial and temporal variations. Sci. Total Environ. 2019, 695, 133790. [Google Scholar] [CrossRef]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Martínez-Piernas, A.B.; Sánchez-Pérez, J.A.; Agüera, A. Determination of pesticide levels in wastewater from an agro-food industry: Target, suspect and transformation product analysis. Chemosphere 2019, 232, 152–163. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Pérez-Villanueva, M.E.; Chin-Pampillo, J.S.; Aguilar-Mora, P.; Arias-Mora, V.; Masís-Mora, M. Pesticide occurrence and water quality assessment from an agriculturally influenced Latin-American tropical region. Chemosphere 2021, 262, 127851. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Granger, C.; Dong, H.; Mao, Y.; Duan, S.; Li, J.; Qiang, Z. Occurrences of 29 pesticides in the Huangpu River, China: Highest ecological risk identified in Shanghai metropolitan area. Chemosphere 2020, 251, 126411. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Tran, T.M.; Nguyen, V.T.; Wang, A.; Wang, J.; Kannan, K. Neonicotinoids, fipronil, chlorpyrifos, carbendazim, chlorotriazines, chlorophenoxy herbicides, bentazon, and selected pesticide transformation products in surface water and drinking water from northern Vietnam. Sci. Total Environ. 2021, 750, 141507. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Chen, H.; Fu, Q. Residue and degradation of carbendazim in rice and soil. J. Agro Environ. Sci. 2011, 31, 357–361. [Google Scholar]
- Daam, M.A.; Garcia, M.V.; Scheffczyk, A.; Rombke, J. Acute and chronic toxicity of the fungicide carbendazim to the earthworm Eisenia fetida under tropical versus temperate laboratory conditions. Chemosphere 2020, 255, 126871. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, W.; Li, L.; Jia, L.; Zhao, J.; Zhao, Z.; Peng, S.; Yuan, X.; Chen, Y. Individual and synergistic toxic effects of carbendazim and chlorpyrifos on zebrafish embryonic development. Chemosphere 2021, 280, 130769. [Google Scholar] [CrossRef]
- Baigorria, E.; Fraceto, L.F. Novel nanostructured materials based on polymer/organic-clay composite networks for the removal of carbendazim from waters. J. Clean. Prod. 2022, 331, 129867. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, H. Chemical kinetic modeling of organic pollutant degradation in Fenton and solar photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 123, 175–184. [Google Scholar]
- Machado, R.M.; da Silva, S.W.; Bernardes, A.M.; Ferreira, J.Z. Degradation of carbendazim in aqueous solution by different settings of photochemical and electrochemical oxidation processes. J. Environ. Manag. 2022, 310, 114805. [Google Scholar] [CrossRef]
- Xie, X.; Hu, Y.; Cheng, H. Rapid degradation of p-arsanilic acid with simultaneous arsenic removal from aqueous solution using Fenton process. Water Res. 2016, 89, 59–67. [Google Scholar]
- da Costa, E.P.; Bottrel, S.E.C.; Starling, M.C.V.M.; Leão, M.M.D.; Amorim, C.C. Degradation of carbendazim in water via photo-Fenton in raceway pond reactor: Assessment of acute toxicity and transformation products. Environ. Sci. Pollut. Res. 2019, 26, 4324–4336. [Google Scholar] [CrossRef] [PubMed]
- Mazellier, P.; Leroy, E.; De Laat, J.; Legube, B. Degradation of carbendazim by UV/H2O2 investigated by kinetic modelling. Environ. Chem. Lett. 2003, 1, 68–72. [Google Scholar] [CrossRef]
- Kaur, T.; Sraw, A.; Wanchoo, R.K.; Toor, A.P. Solar assisted degradation of carbendazim in water using clay beads immobilized with TiO2 & Fe doped TiO2. Sol. Energy 2018, 162, 45–56. [Google Scholar]
- Xu, L.; Dong, H.; Xu, K.; Li, J.; Qiang, Z. Accelerated degradation of pesticide by permanganate oxidation: A comparison of organic and inorganic activations. Chem. Eng. J. 2019, 369, 1119–1128. [Google Scholar] [CrossRef]
- Sharma, V.K.; Zboril, R.; Varma, R.S. Ferrates: Greener oxidants with multimodal action in water treatment technologies. Acc. Chem. Res. 2015, 48, 182–191. [Google Scholar] [CrossRef]
- Sharma, V.K.; Chen, L.; Zboril, R. Review on high valent FeVI (ferrate): A sustainable green oxidant in organic chemistry and transformation of pharmaceuticals. ACS Sustain. Chem. Eng. 2016, 4, 18–34. [Google Scholar] [CrossRef]
- Dai, M.; Luo, Z.; Luo, Y.; Zheng, Q.; Zhang, B. Degradation of 2,6-dichlorophenol by ferrate(VI) oxidation: Kinetics, performance, and mechanism. Sep. Purif. Technol. 2021, 278, 119475. [Google Scholar] [CrossRef]
- Dong, F.; Li, J.; Lin, Q.; Wang, D.; Li, C.; Shen, Y.; Zeng, T.; Song, S. Oxidation of chloroquine drug by ferrate: Kinetics, reaction mechanism and antibacterial activity. Chem. Eng. J. 2020, 428, 131408. [Google Scholar] [CrossRef]
- Liu, H.; Pan, X.; Chen, J.; Qi, Y.; Qu, R.; Wang, Z. Kinetics and mechanism of the oxidative degradation of parathion by ferrate(VI). Chem. Eng. J. 2019, 365, 142–152. [Google Scholar] [CrossRef]
- Sharma, V.K. Ferrate(VI) and ferrate(V) oxidation of organic compounds: Kinetics and mechanism. Coord. Chem. Rev. 2013, 257, 495–510. [Google Scholar] [CrossRef]
- Wang, K.; Shu, J.; Sharma, V.K.; Liu, C.; Xu, P.; Nesnas, N.; Wang, H. Unveiling the mechanism of imidacloprid removal by ferrate(VI): Kinetics, role of oxidation and adsorption, reaction pathway and toxicity assessment. Sci. Total Environ. 2022, 805, 150383. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cheng, H. A simple treatment method for phenylarsenic compounds: Oxidation by ferrate(VI) and simultaneous removal of the arsenate released with in situ formed Fe(III) oxide-hydroxide. Environ. Int. 2019, 127, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, X.; Zeng, X.; Feng, M.; Qu, R.; Wang, Z.; Nesnas, N.; Sharma, V.K. Ferrate(VI) oxidation of polychlorinated diphenyl sulfides: Kinetics, degradation, and oxidized products. Water Res. 2018, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Baum, J.C.; Nesnas, N.; Lee, Y.; Huang, C.H.; Sharma, V.K. Oxidation of sulfonamide antibiotics of six-membered heterocyclic moiety by ferrate(VI): Kinetics and mechanistic insight into SO2 extrusion. Environ. Sci. Technol. 2019, 53, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, Y.; Ma, J.; Pang, S.; Huang, Z.; Gu, J.; Gao, Y.; Xue, M.; Yuan, Y.; Jiang, J. Transformation of substituted anilines by ferrate(VI): Kinetics, pathways, and effect of dissolved organic matter. Chem. Eng. J. 2018, 332, 245–252. [Google Scholar] [CrossRef]
- Feng, M.; Wang, X.; Chen, J.; Qu, R.; Sui, Y.; Cizmas, L.; Wang, Z.; Sharma, V.K. Degradation of fluoroquinolone antibiotics by ferrate(VI): Effects of water constituents and oxidized products. Water Res. 2016, 103, 48–57. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.; Zhao, J.; Liu, S.; Zhou, L.; Chen, F. Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate(VI) treatment of secondary wastewater effluents. Water Res. 2012, 46, 2194–2204. [Google Scholar] [CrossRef]
- Jiang, J.; Durai, H.B.P.; Winzenbacher, R.; Petri, M.; Seitz, W. Drinking water treatment by in situ generated ferrate(VI). Desalination Water Treat. 2015, 55, 731–739. [Google Scholar] [CrossRef]
- Zheng, Q.; Wu, N.; Qu, R.; Albasher, G.; Cao, W.; Li, B.; Alsultan, N.; Wang, Z. Kinetics and reaction pathways for the transformation of 4-tert-butylphenol by ferrate(VI). J. Hazard. Mater. 2021, 401, 123405. [Google Scholar] [CrossRef]
- Li, C.; Li, X.Z.; Graham, N. A study of the preparation and reactivity of potassium ferrate. Chemosphere 2005, 61, 537–543. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Thomas, M.J. Studies of hypervalent iron in aqueous solutions. 1. Radiation-induced reduction of iron(VI) to iron(V) by CO2-. J. Am. Chem. Soc. 1987, 109, 7761–7764. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, J.; von Gunten, U. Spectrophotometric determination of ferrate (Fe(VI)) in water by ABTS. Water Res. 2005, 39, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.D.; Bielski, B.H.J. Kinetics of ferrate(V) decay in aqeuous solution. Inorg. Chem. 1989, 28, 3947–3951. [Google Scholar] [CrossRef]
- Rush, J.D.; Bielski, B.H.J. Decay of ferrate(V) in neutral and acidic solutions. A premix pulse radiolysis study. Inorg. Chem. 1994, 33, 5499–5502. [Google Scholar] [CrossRef]
- Rush, J.D.; Zhao, Z.; Bielski, B.H.J. Reaction of ferrate(VI)/ferrate(V) with hydrogen peroxide and superoxide anion- a stopped-flow and premix pulse radiolysis study. Free. Radic. Res. 1996, 24, 187–198. [Google Scholar] [CrossRef]
- Lee, Y.; Kissner, R.; von Gunten, U. Reaction of ferrate(VI) with ABTS and self-decay of ferrate(VI): Kinetics and mechanisms. Environ. Sci. Technol. 2014, 48, 5154–5162. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Shao, B.; Zhu, J.; Hu, Z.; Guan, X. Three kinetic patterns for the oxidation of emerging organic contaminants by Fe(VI): The critical roles of Fe(V) and Fe(IV). Environ. Sci. Technol. 2021, 55, 11338–11347. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, F.; Meng, J.; Shao, B.; Dong, H.; Chu, W.; Cao, T.; Wei, G.; Wang, H.; Guan, X. Overlooked role of Fe(IV) and Fe(V) in organic contaminant oxidation by Fe(VI). Environ. Sci. Technol. 2020, 54, 9702–9710. [Google Scholar] [CrossRef]
- Sharma, V.K. Potassium ferrate(VI): An environmentally friendly oxidant. Adv. Environ. Res. 2002, 6, 143–156. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, D.; Gao, Z.; Ji, X.; Zhang, J.; Jin, C. Enhanced ferrate oxidation of organic pollutants in the presence of Cu(II) Ion. J. Hazard. Mater. 2022, 433, 128772. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G. Oxidation of benzophenone-3 during water treatment with ferrate(VI). Water Res. 2013, 47, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Q.; Fu, Y.; Peng, B.; Zhou, G. Kinetics and mechanism of diclofenac removal using ferrate(VI): Roles of Fe3+, Fe2+, and Mn2+. Environ. Sci. Pollut. Res. 2018, 25, 22998–23008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, M.; Luo, C.; Nesnas, N.; Huang, C.H.; Sharma, V.K. Effect of metal ions on oxidation of micropollutants by ferrate(VI): Enhancing role of FeIV species. Chem. Eng. J. 2021, 55, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Manoli, K.; Nakhla, G.; Ray, A.K.; Sharma, V.K. Oxidation of caffeine by acid-activated ferrate(VI): Effect of ions and natural organic matter. AIChE J. 2017, 63, 4998–5006. [Google Scholar] [CrossRef]
- Schreyer, J.M.; Ockerman, L.T. Stability of the ferrate(VI) ion in aqueous solution. Anal. Chem. 1951, 23, 1312–1314. [Google Scholar] [CrossRef]
- Luo, C.; Feng, M.; Sharma, V.K.; Huang, C.-H. Oxidation of pharmaceutials by ferrate(VI) in hydrolyzed urine: Effects of major inorganic constituents. Environ. Sci. Technol. 2019, 53, 5272–5281. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Shu, H.; Dai, Y. Removal of bromide by aluminium chloride coagulant in the presence of humic acid. J. Hazard. Mater. 2007, 147, 457–462. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Wang, Y.; Zhao, Y.; Xu, Y.; Liu, X. DFT insights into the degradation mechanism of carbendazim by hydroxyl radicals in aqueous solution. J. Hazard. Mater. 2022, 431, 128577. [Google Scholar] [CrossRef]
- Kaur, T.; Toor, A.P.; Wanchoo, R.K. Parametric study on degradation of fungicide carbendazim in dilute aqueous solutions using nano TiO2. Desalination Water Treat. 2014, 54, 122–131. [Google Scholar] [CrossRef]
- Xiao, R.; Gao, L.; Wei, Z.; Spinney, R.; Luo, S.; Tang, C.; Yang, W. Mechanistic insight into degradation of endocrine disrupting chemical by hydroxyl radical: An experimental and theoretical approach. Environ. Pollut. 2017, 231, 1446–1452. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, H. Efficient Degradation of Carbendazim by Ferrate(VI) Oxidation under Near-Neutral Conditions. Sustainability 2022, 14, 13678. https://doi.org/10.3390/su142013678
Li Y, Cheng H. Efficient Degradation of Carbendazim by Ferrate(VI) Oxidation under Near-Neutral Conditions. Sustainability. 2022; 14(20):13678. https://doi.org/10.3390/su142013678
Chicago/Turabian StyleLi, Yu, and Hefa Cheng. 2022. "Efficient Degradation of Carbendazim by Ferrate(VI) Oxidation under Near-Neutral Conditions" Sustainability 14, no. 20: 13678. https://doi.org/10.3390/su142013678
APA StyleLi, Y., & Cheng, H. (2022). Efficient Degradation of Carbendazim by Ferrate(VI) Oxidation under Near-Neutral Conditions. Sustainability, 14(20), 13678. https://doi.org/10.3390/su142013678







