Agronomic Evaluation of Recycled Polyurethane Foam-Based Growing Media for Green Roofs
Abstract
1. Introduction
2. Materials and Methods
2.1. PU Foam Preparation
2.2. Mix Preparation
2.3. Experimental Setup
2.4. Analyses
2.4.1. Leachates
2.4.2. Plant Analysis
2.4.3. Growing Medium Analysis
2.5. Statistical Analyses
3. Results
3.1. Agronomic Properties of the Growing Media
| GM1-EU | GM1-HY | GM1-ST | GM1-LP | GM2-EU | GM2-HY | GM2-ST | GM2-LP | GM3-EU | GM3-HY | GM3-ST | GM3-LP | Recommendation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| pH water | 7.6 | 0 | 7.7 | 0 | 7.6 | 0 | 7.7 | 0.1 | 7.8 | 0.1 | 7.8 | 0 | 7.8 | 0 | 7.7 | 0 | 7.9 | 0.1 | 7.8 | 0 | 7.8 | 0.1 | 7.7 | 0 | 6.5–7.5 [53] |
| Electrical conductivity (mS·cm−1) | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.2 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | <0.5 [36] |
| OM (%) | 9.7 | 0.5 | 7.5 | 0.5 | 10.3 | 1.1 | 9.5 | 1 | 7.7 | 0.4 | 8.7 | 0.9 | 9 | 0.2 | 9.6 | 0.9 | 7.6 | 0.4 | 7 | 0.4 | 7.1 | 0.5 | 6.3 | 0.1 | 4–10 [53] |
| NO3−-N (mg·kg−1) | 9.2 | 1.9 | 23.6 | 10.2 | 29.9 | 12.1 | 23.6 | 16.2 | 5.2 | 2.3 | 21.5 | 2.9 | 23.5 | 8.4 | 17.5 | 5.3 | 6.6 | 2.6 | 11.1 | 7.2 | 12.7 | 5.3 | 9.1 | 1.9 | - |
| NH4+-N (mg·kg−1) | 6.5 | 1.3 | 7.7 | 1.1 | 4.3 | 0.2 | 6.2 | 0.6 | 8.3 | 1.1 | 7.1 | 0.5 | 5.3 | 1 | 7 | 0.4 | 2.3 | 1.3 | 4.4 | 0.9 | 3.6 | 1.4 | 3.8 | 0.9 | - |
| CaO (mg·kg−1) | 9182.3 | 888.4 | 10,356 | 875.8 | 9161 | 492.2 | 9299.7 | 166.2 | 9394.3 | 617.1 | 9039.3 | 125.9 | 8298 | 370.2 | 10,534 | 3276.7 | 6478.7 | 586.1 | 6061 | 361.2 | 5479.3 | 429.7 | 4789.7 | 157 | 8379 [36] |
| K2O (mg·kg−1) | 444.3 | 50.9 | 346 | 40 | 326.3 | 7.2 | 315 | 56 | 448.3 | 40.3 | 339.7 | 40.8 | 287.3 | 22.2 | 264.7 | 25.5 | 314 | 30.3 | 230.3 | 25.3 | 229 | 13.5 | 230.3 | 8.3 | >0.18 [54] |
| MgO (mg·kg−1) | 702.3 | 61.9 | 734.7 | 53.5 | 743.3 | 28.6 | 585.3 | 37.7 | 637 | 28.9 | 563 | 35.7 | 526.3 | 9.9 | 540.7 | 35.1 | 457.3 | 50.7 | 393 | 8 | 381 | 23.8 | 363.7 | 14.5 | 119 [36] |
| Na2O (mg·kg−1) | 105.3 | 15 | 102 | 7.6 | 96.3 | 4.6 | 91 | 3.7 | 102 | 5.1 | 94.3 | 7.8 | 89 | 8.2 | 86.7 | 2.5 | 69.3 | 4.6 | 66.7 | 2.5 | 59 | 3.7 | 57.7 | 1.1 | <355 [36] |
| CEC (meq 100 g−1) | 27.2 | 4.9 | 30.4 | 0.8 | 30.8 | 0.9 | 26.5 | 1.4 | 25.3 | 2.8 | 26.5 | 1.9 | 23.3 | 3.2 | 23.5 | 0.9 | 18.5 | 0.9 | 16.4 | 0.6 | 16.5 | 2.9 | 15.3 | 1 | >40 [53] |
| P2O5 (mg·kg−1) | 486.3 | 24.9 | 476.7 | 31.2 | 517 | 61 | 478.3 | 16 | 411 | 50.4 | 415.3 | 11.5 | 430.3 | 12.7 | 406 | 11 | 275 | 8.3 | 254 | 3.1 | 254.3 | 8.2 | 245.7 | 1.8 | >120 [53] |
| B (mg·kg−1) | 0.7 | 0 | 0.7 | 0 | 0.8 | 0.1 | 0.6 | 0 | 0.8 | 0.1 | 0.7 | 0 | 0.7 | 0.1 | 0.7 | 0.1 | 0.5 | 0.1 | 0.5 | 0 | 0.4 | 0 | 0.5 | 0 | 0.4 [36] |
| Fe (mg·kg−1) | 116 | 5.1 | 124.9 | 9.2 | 120.7 | 4.2 | 118.3 | 7.8 | 125.4 | 5.3 | 112.5 | 20.6 | 142.7 | 4.5 | 111.3 | 41.1 | 136.8 | 18.2 | 146 | 16.4 | 157.3 | 12.9 | 184.8 | 18.3 | 9 −300 [55] |
| Mn (mg·kg−1) | 19 | 2.7 | 19.3 | 2.2 | 17.5 | 1.5 | 19 | 1.2 | 25.8 | 2.5 | 21.3 | 1.6 | 21 | 2.2 | 20.9 | 2.8 | 34.9 | 2.1 | 29.9 | 2.7 | 30.5 | 0.9 | 33.3 | 3.9 | 9 [36] |
| Mo (mg·kg−1) | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | 0.1 | 0 | - |
| Co (mg·kg−1) | 0.5 | 0.1 | 0.4 | 0.1 | 0.3 | 0.1 | 0.5 | 0.1 | 0.7 | 0.1 | 0.4 | 0.2 | 0.3 | 0.1 | 0.5 | 0.2 | 0.5 | 0.1 | 0.4 | 0.1 | 0.4 | 0 | 0.4 | 0 | - |
| Bulk density (g·cm−3) | 0.53 | 0.04 | 0.58 | 0.02 | 0.57 | 0.06 | 0.5 | 0.01 | 0.5 | 0.06 | 0.56 | 0.04 | 0.55 | 0.03 | 0.49 | 0.03 | 0.81 | 0.04 | 0.82 | 0.07 | 0.88 | 0 | 0.82 | 0.06 | <1.2 [53] |
| Available water (mm·cm−1) | 1.06 | 0.28 | 1.12 | 0.32 | 1.22 | 0.24 | 0.68 | 0.03 | 1.16 | 0.2 | 1.13 | 0.15 | 0.99 | 0.22 | 0.92 | 0.07 | 0.85 | 0.17 | 0.88 | 0.16 | 0.94 | 0.11 | 1.07 | 0.25 | >1.5 [53] |
| Macroporosity (% vol) | 17.03 | 3.92 | 14.22 | 11.25 | 6.27 | 1.17 | 19.73 | 7.27 | 9.89 | 1.92 | 15.25 | 6.08 | 16.78 | 9.42 | 13.84 | 7 | 31.36 | 4.26 | 31.71 | 1.85 | 31.85 | 1.54 | 28.45 | 4.55 | >20 [53] |
| GM loading at water retention capacity (kg·m−2) | 100.1 | 11.4 | 108.5 | 9 | 108.6 | 13.6 | 88.5 | 2.2 | 97 | 12.1 | 105.6 | 8.6 | 101.5 | 8.1 | 90.8 | 6.3 | 137.7 | 8.9 | 140.4 | 14.2 | 150.3 | 2.4 | 143.4 | 13.2 | <180 French regulation [26] <150 [13] |
3.2. Plant Production
3.3. Leachate Quality
3.4. Correlation between Plant Biomass and the Agronomic Properties of the GM
4. Discussion
4.1. Agro-Environmental Quality of the Growing Media
4.2. Ability of the Plants to Grow in Polyurethane Foam-Based GM
4.3. Implications for the Foam Recycling and Green Roof Industries
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Revision of World Urbanization Prospects Multimedia Library—United Nations Department of Economic and Social Affairs; Department of Economic and Social Affairs: New York, NY, USA, 2018. [Google Scholar]
- Kelly, C. Acute Food Insecurity Inmega-Cities: Issues and Assistance Options; Benfield Hazard Research Centre: London, UK, 2003. [Google Scholar]
- Levin, M.J.; Kim, K.-H.J.; Morel, J.L.; Burghardt, W.; Charzynski, P.; Shaw, R.K. Soils within Cities; Catena soil sciences: Stuttgart, Germany, 2007. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of The Regions Next Steps for a Sustainable European future—European Action for Sustainability; COM/2016/0739; European Commission: Luxembourg, 2016. [Google Scholar]
- Berardi, U.; Ghaffarian, H.A. State-of-the-art analysis of the environmental benefits of green roofs. Appl. Energy 2014, 115, 411–428. [Google Scholar] [CrossRef]
- Sutton, R.K. Introduction to green roof ecosystems. In Green Roof Ecosystems; Sutton, R.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Grunwald, L.; Heusinger, J.; Weber, S. A GIS-based mapping methodology of urban green roof ecosystem services applied to a Central European city. Urban For. Urban Green. 2017, 22, 54–63. [Google Scholar] [CrossRef]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Köhler, M.; Liu, K.K.Y.; Rowe, B. Green roofs as urban ecosystems: Ecological structures, functions, and services. BioScience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Lassalle, F. Panorama technique, historique et géographique. In Quelle Place Pour Les Toitures Végétalisées Dans La Ville De Demain; HAL Open Science: Paris, France, 2012. [Google Scholar]
- FBB. Bundesweite Strategie Gebäudegrün; Fachvereinigung Bauwerksbegrünung e.V. (FBB): Saarbrücken, Germany, 2015; p. 6. [Google Scholar]
- Pfoser, N.; Henrich, J.; Jenner, N.; Schreiner, J.; Kanashiro, C.; Heusinger, J.; Weber, S.; Hegger, M.; Dettmar, J. Gebäude Begrünung Energie—Potenziale und Wechselwirkungen. Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau e.V. (FLL); Bundesinstitut für Bau-, Stadt- und Raumforschung(BBSR), FLL-Schriftenreihe, FV2014/01: Bonn, Germany, 2014; p. 305. [Google Scholar]
- Wilkinson, S.; Feitosa, R.C. Retrofitting housing with lightweight green roof technology in Sydney, Australia, and Rio de Janeiro, Brazil. Sustainability 2015, 7, 1081–1098. [Google Scholar] [CrossRef]
- Cascone, S. Green Roof Design: State of the Art on Technology and Materials. Sustainability 2019, 11, 3020. [Google Scholar] [CrossRef]
- European Parliament. Directive 2000/53/EC of the European Parliament and of the Council on 18 September 2000 on End-of-Life Vehicles; European Parliament: Strasbourg, France, 2000. [Google Scholar]
- Scheirs, J. Polymer Recycling; Chapter 10; John Wiley & Sons: Chichester, UK, 1998. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2011: An Analysis of European Plastics Production, Demand and Recovery for 2010; Ave E. van Nieuwenhuyse 4/3; Association of Plastics Manufacturers: Bruxelles, Belgium, 2011. [Google Scholar]
- Boujard, C.; Foray, N.; Caudron, J.-C. Panorama du Marché du Polyuréthane et état de l’art de ses Techniques de Recyclage; ADEME: Angers, France, 2014; 106p. [Google Scholar]
- Howard, G.T. Biodegredation of polyurethane: A review. Int. Biodeterior. Biodegrad. 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Weigand, E. Properties and applications of recycled polyurethanes. In Recycling and Recovery of Plastics; Section, 7.10; Branderup, J., Bittner, M., Menges, G., Micheali, W., Eds.; Hanser Publishers: Münich, Germany, 1996. [Google Scholar]
- Cregut, M.; Bedas, M.; Durand, M.J.; Thouand, G. New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol. Adv. 2003, 31, 1634–1647. [Google Scholar] [CrossRef]
- Bikard, J. Fabrication Des Mousses en Polyuréthane. 2009. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/plasturgie-procedes-specifiques-aux-composites-42474210/fabrication-des-mousses-en-polyurethane-am3714/ (accessed on 12 November 2018).
- Huber, J.J.; Zheng, Y.; Dixon, M.A. Hydroponic Cucumber Production Using Urethane Foam as a Growth Substrate. Acta Hortic. 2005, 697, 139–145. [Google Scholar] [CrossRef]
- Wright, H.C.; Cameron, D.D.; Ryan, A.J. Experimental design as a framework for optimising polyurethane foam as a soilless growing media. Acta Hortic. 2021, 1317, 125–132. [Google Scholar] [CrossRef]
- Artous, M.; Guénon, R.; Lemmel, O.; Buord, H.; Vidal-Beaudet, L.; Cannavo, P. Construction of fertile growing media from recycled polyurethane foam. Environ. Ingénierie Dev. 2022, 86, 22–29. [Google Scholar] [CrossRef]
- AFNOR. NFU44-095; Amendements Organiques—Composts Contenant des Matières D’intérêt Agronomique, Issues du Traitement des Eaux. AFNOR: Paris, France, 2002; 22p.
- ADIVET; PDE; EMB. Règles Professionnelles Pour la Conception et la Réalisation des Terrasses et Toitures Végétalisées; ADIVET: Paris, France, 2018; 84p. [Google Scholar]
- AFNOR. NF EN 13037; Amendements du Sol et Supports de Culture—Détermination du pH—Amendements Organiques et Supports de Culture. AFNOR: Paris, France, 2012.
- AFNOR. NF EN 13038; Amendements du Sol et Supports de Culture—Détermination de la Conductivité électrique—Amendements Organiques et Supports de Culture. AFNOR: Paris, France, 2012.
- AFNOR. NF EN 13039; Amendements du Sol et Supports de Culture—Détermination de la Matière Organique et des Cendres. AFNOR: Paris, France, 2011.
- AFNOR. NF EN 13040; Amendements Organiques et Supports de Culture—Préparation des échantillons Pour les Essais Physiques et Chimiques, Détermination de la Teneur en Matière Sèche, du Taux D’humidité et de la Masse Volumique Compactée en Laboratoire. AFNOR: Paris, France, 2007.
- AFNOR. NF EN 13654-2; Amendements du Sol et Supports de Culture—Détermination de L’azote—Partie 2: Méthode de Dumas. AFNOR: Paris, France, 2002.
- AFNOR. NF EN ISO 13395; Qualité de L’eau—Détermination de L’azote Nitreux et de L’azote Nitrique et de la Somme des Deux Par Analyse en Flux (CFA et FIA) et Détection Spectrométrique. AFNOR: Paris, France, 2006; 24p.
- AFNOR. NF 31-122; Qualité des Sols—Extraction et Dosage du Bore Soluble à L’eau Bouillante. AFNOR: Paris, France, 1999; 13p.
- AFNOR. NF EN ISO 11885; Qualité de L’eau—Dosage D’éléments Choisis Par Spectroscopie D’émission Optique Avec Plasma Induit Par Haute Fréquence (ICP-OES). AFNOR: Paris, France, 2009; 38p.
- AFNOR. ISO 14870:2001; Qualité du Sol—Extraction des éléments en Traces Par Une Solution Tamponnée de DTPA. AFNOR: Paris, France, 2001; 4p.
- AFNOR. NF U44-175; Supports de Culture—Détermination de la Capacité de Rétention Pour L’eau, L’air et de la Masse Volumique Apparente Sèche—Application de la Mesure du Volume. AFNOR: Paris, France, 1992.
- AFNOR. NF EN 13650; Amendements du Sol et Supports de Culture—Extraction D’éléments Solubles Dans L’eau Régale. AFNOR: Paris, France, 2002.
- AFNOR. NF X31-147; Qualité des Sols—Sols, Sédiments—Mise en Solution Totale Par Attaque Acide. AFNOR: Paris, France, 1996; 12p.
- AFNOR. ISO 14507:2003; Qualite du Sol. Pretraitement des échantillons Pour La Determination des Contaminants Organiques. AFNOR: Paris, France, 2003; 24p.
- AFNOR. NF EN 16167; Sols, Biodéchets Traités et Boues—Dosage des Polychlorobiphényles (PCBs) Par Chromatographie en Phase Gazeuse-Spectrométrie Gazeuse Couplée Avec un Détecteur de Masse (CG-SM) ou un Détecteur Par Capture D’électrons (CG-ECD). AFNOR: Paris, France, 2018.
- AFNOR. XP X33-012; Caractérisation des Boues—Dosage des Hydrocarbures Aromatiques Polycycliques (HAP) et des Polychlorobiphényles (PCB). AFNOR: Paris, France, 2000.
- AFNOR. NF EN 27888; Qualité de L’eau—Détermination de la Conductivité électrique. AFNOR: Paris, France, 1994; 10p.
- AFNOR. NF EN ISO 11732; Qualité de L’eau—Dosage de L’azote Ammoniacal—Méthode Par Analyse en Flux (CFA et FIA) et Détection Spectrométrique. AFNOR: Paris, France, 2005; 28p.
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2017; 631p. [Google Scholar]
- AFNOR. NF EN ISO 16198; Qualité du Sol—Test Végétal Pour L’évaluation de la Biodisponibilité Environnementale des Eléments Traces Pour les Végétaux. AFNOR: Paris, France, 2015.
- CEN. EN 13040; Soil Improvers and Growing Media. Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density. European Committee for Standardization: Bruxelles, Belgium, 1999.
- De Boodt, M.; Verdonck, O.; Cappaert, I. Method for Measuring the Water Release Curve of Organic Substrates. Acta Hortic. 1974, 37, 2054–2063. [Google Scholar] [CrossRef]
- AFNOR. NF EN ISO 22032; Qualité de L’eau—Dosage D’une Sélection D’éthers Diphényliques Polybromés Dans des Sédiments et des Boues D’épuration—Méthode Par Extraction et Chromatographie en Phase Gazeuse/Spectrométrie de Masse. AFNOR: Paris, France, 2009; 37p.
- AFNOR. NFU44-051; Amendements Organiques—Dénominations, Spécifications et Marquage. AFNOR: Paris, France, 2006; 37p.
- INERIS. Hydrocarbures Aromatiques Polycycliques (HAP); Rapport Final, DRC-03-47026-ETSC–Bdo-N03DR177; INERIS: Verneuil-en-Halatte, France, 2003. [Google Scholar]
- European Parliament. Commission Decision 2005/747/EC Amending for the Purposes of Adapting to Technical Progress the Annex to Directive 2002/95/EC of the European Parliament and of the Council on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment; European Parliament: Strasbourg, France, 2005. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Vidal-Beaudet, L.; Cannavo, P.; Schwartz, C.; Séré, G.; Béchet, B.; Legret, M.; Peyneau, P.-E.; Bataillard, P.; Coussy, S.; Damas, O. Using wastes for fertile urban soil construction2014 The French Research Project SITERRE. In Soils within Cities. Global Approaches to Their Sustainable Management—Composition, Properties, and Functions of Soils of the Urban Environment; Levin, M.J., Kim, K.-H.J., Morel, J.L., Burghardt, W., Charzynski, P., Shaw, R.K., Eds.; Edited on behalf of IUSS Working Group SUITMA; Catena-Schweizerbart: Stuttgart, Germany, 2017; 253p. [Google Scholar]
- Kramer, P.; Kozlowski, T. Mineral nutrition and salt absorption. In Physiology of Woody Plants; Academic Press: Cambridge, MA, USA, 1979; 39p. [Google Scholar]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Lellei-Kovács, E.; Kovács-Láng, E.; Botta-Dukát, Z.; Kalapos, T.; Emmett, B.; Beier, C. Thresholds and interactive effects of soil moisture on the temperature response of soil respiration. Eur. J. Soil Biol. 2011, 47, 247–255. [Google Scholar] [CrossRef]
- Alsup, S.E.; Ebbs, S.D.; Battaglia, L.L.; Retzlaff, W.A. Heavy metals in leachate from simulated green roof systems. Ecol. Eng. 2011, 37, 1709–1717. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Joshi, U.M. Can green roof act as a sink for contaminants? A methodological study to evaluate runoff quality from green roofs. Environ. Pollut. 2014, 194, 121–129. [Google Scholar] [CrossRef]
- López-Uceda, A.; Galvín, A.P.; Ayuso, J.; Jiménez, J.R.; Vanwalleghem, T.; Peña, A. Risk assessment by percolation leaching tests of extensive green roofs with fine fraction of mixed recycled aggregates from construction and demolition waste. Environ. Sci. Pollut. Res. 2018, 25, 36024–36034. [Google Scholar] [CrossRef]
- Stovin, V.; Vesuviano, G.; Kasmin, H. The hydrological performance of a green roof test bed under UK climatic conditions. J. Hydrol. 2012, 414–415, 148–161. [Google Scholar] [CrossRef]
- Viola, F.; Hellies, M.; Deidda, R. Retention performance of green roofs in representative climates worldwide. J. Hydrol. 2017, 553, 763–772. [Google Scholar] [CrossRef]
- Monterusso, M.A.; Rowe, D.B.; Rugh, C.L.; Russell, D.K. Runoff Water Quantity and Quality from Green Roof Systems; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2004; Volume 639, pp. 369–376. [Google Scholar]
- Farrell, C.; Szota, C.; Williams Nicholas, S.G.; Arndt Stefan, K. High water users can be drought tolerant: Using physiological traits for green roof plant selection. Plant Soil 2013, 372, 177–193. [Google Scholar] [CrossRef]
- Monterusso, M.A.; Rowe, D.B.; Rugh, C.L. Establishment and persistence of Sedum spp. and native taxa for green roof applications. HortScience 2005, 40, 391–396. [Google Scholar] [CrossRef]
- Snodgrass, E.C.; Snodgrass, L.L. Green Roof Plants: A Resource and Planting Guide; Timber Press: Portland, OR, USA, 2006; p. 203. [Google Scholar]
- Farrell, C.; Mitchell, R.E.; Szota, C.; Rayner, J.P.; Williams, N.S.G. Green roofs for hot and dry climates: Interacting effects of plant water use, succulence and substrate. Ecol. Eng. 2012, 49, 270–276. [Google Scholar] [CrossRef]
- Szota, C.; Farrell, C.; Williams, N.S.G.; Arndt, S.K.; Fletcher, T.D. Drought-avoiding plants with low water use can achieve high rainfall retention without jeopardising survival on green roofs. Sci. Total Environ. 2017, 603–604, 340–351. [Google Scholar] [CrossRef]
- Van Mechelen, C.; Dutoit, T.; Kattge, J.; Hermy, M. Plant trait analysis delivers an extensive list of potential green roof species for Mediterranean France. Ecol. Eng. 2014, 67, 48–59. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Colpaert, J.V.; Van Tichelen, K.K. Reclamation of a bare industrial area contaminated by non-ferrous metals: Physico-chemical and biological evaluation of the durability of soil treatment and revegetation. Environ. Pollut. 1996, 94, 131–140. [Google Scholar] [CrossRef]
- Norland, M.R.; Veith, D.L. Revegetation of coarse taconite iron ore tailing using municipal solid waste compost. J. Hazard. Mater. 1995, 41, 123–134. [Google Scholar] [CrossRef]
- Córdova, S.; Neaman, A.; González, I.; Ginocchio, R.; Fine, P. The effect of lime and compost amendments on the potential for the revegetation of metal-polluted, acidic soils. Geoderma 2011, 166, 135–144. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kita, A.; Janoska, P.; Połowniak, M.; Kozik, V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012, 135, 494–501. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B. Media depth influences Sedum green roof establishment. Urban Ecosyst. 2008, 11, 361–372. [Google Scholar] [CrossRef]
- Martens, R.; Domsch, K.H. Microbial degradation of polyurethane foams and isocyanate based polyureas in different media. Water Air Soil Pollut. 1981, 15, 503–509. [Google Scholar] [CrossRef]
- Dubelley, F. Mécanismes de dégradation des enveloppes barrières pour application panneaux isolants sous vide. Ph.D. Thesis, Grenoble Alpes University, Grenoble, France, 2017; p. 227. [Google Scholar]
- Lovett, D.; Eastop, D. The degradation of polyester polyurethane: Preliminary study of 1960s foam-laminated dresses. Stud. Conserv. 2004, 49, 100–104. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. Assessment of the degradation of polyurethane foams after artificial and natural ageing by using pyrolysis-gas chromatography/mass spectrometry and headspace-solid phase microextraction-gas chromatography/mass spectrometry. J. Chromatogr. A 2011, 1218, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.F.; Luo, X.; Li, C.; Michel, F.C.; Li, Y. Biodegradability of crude glycerol-based polyurethane foams during composting, anaerobic digestion and soil incubation. Polym. Degrad. Stab. 2018, 102, 195–203. [Google Scholar] [CrossRef]
- Barratt, S.R.; Ennos, A.R.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungi are the predominant micro-organisms responsible for degradation of soil-buried polyester polyurethane over a range of soil water holding capacities. J. Appl. Microbiol. 2003, 95, 78–85. [Google Scholar] [CrossRef]
- Cosgrove, L.; McGeechan, P.L.; Robson, G.D.; Handley, P.S. Fungal Communities Associated with Degradation of Polyester Polyurethane in Soil. Appl. Environ. Microbiol. 2007, 73, 5817–5824. [Google Scholar] [CrossRef]
- Khan, S.; Nadir, S.; Shah, Z.U.; Shah, A.A.; Karunarathna, S.C.; Xu, J.; Khan, A.; Munir, S.; Hasan, F. Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ. Pollut. 2017, 225, 469–480. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Avérous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs); U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2014.

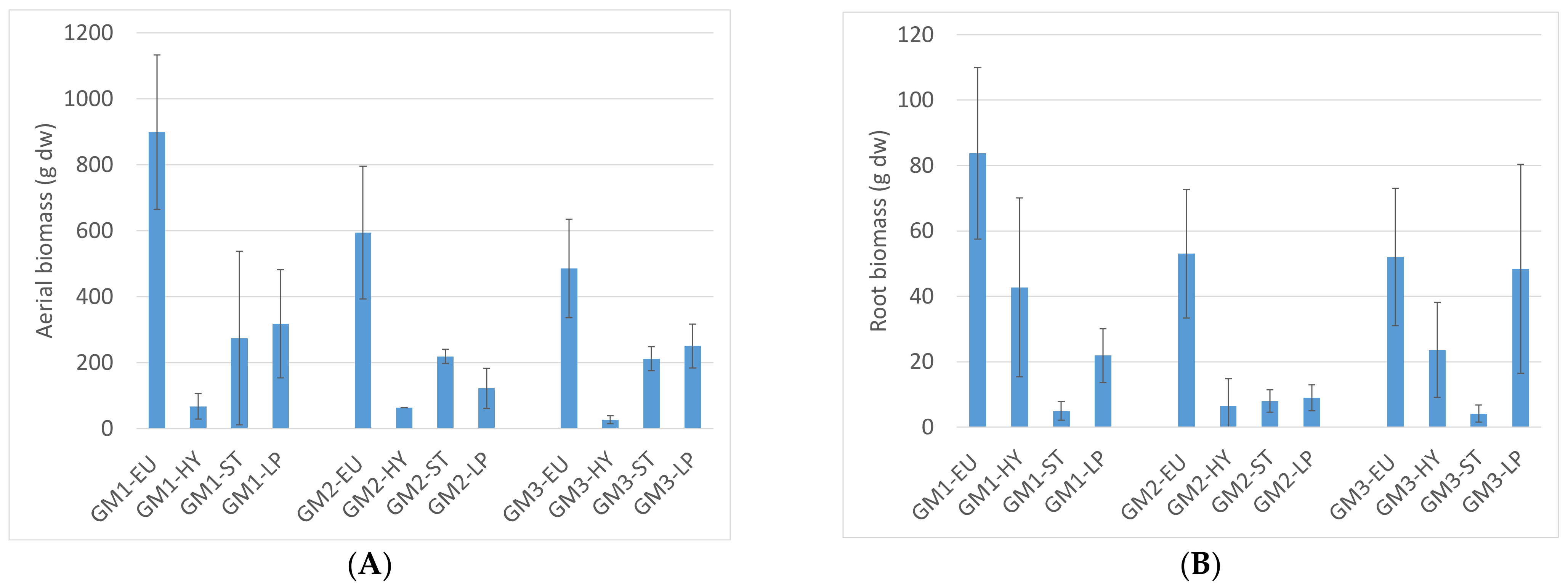
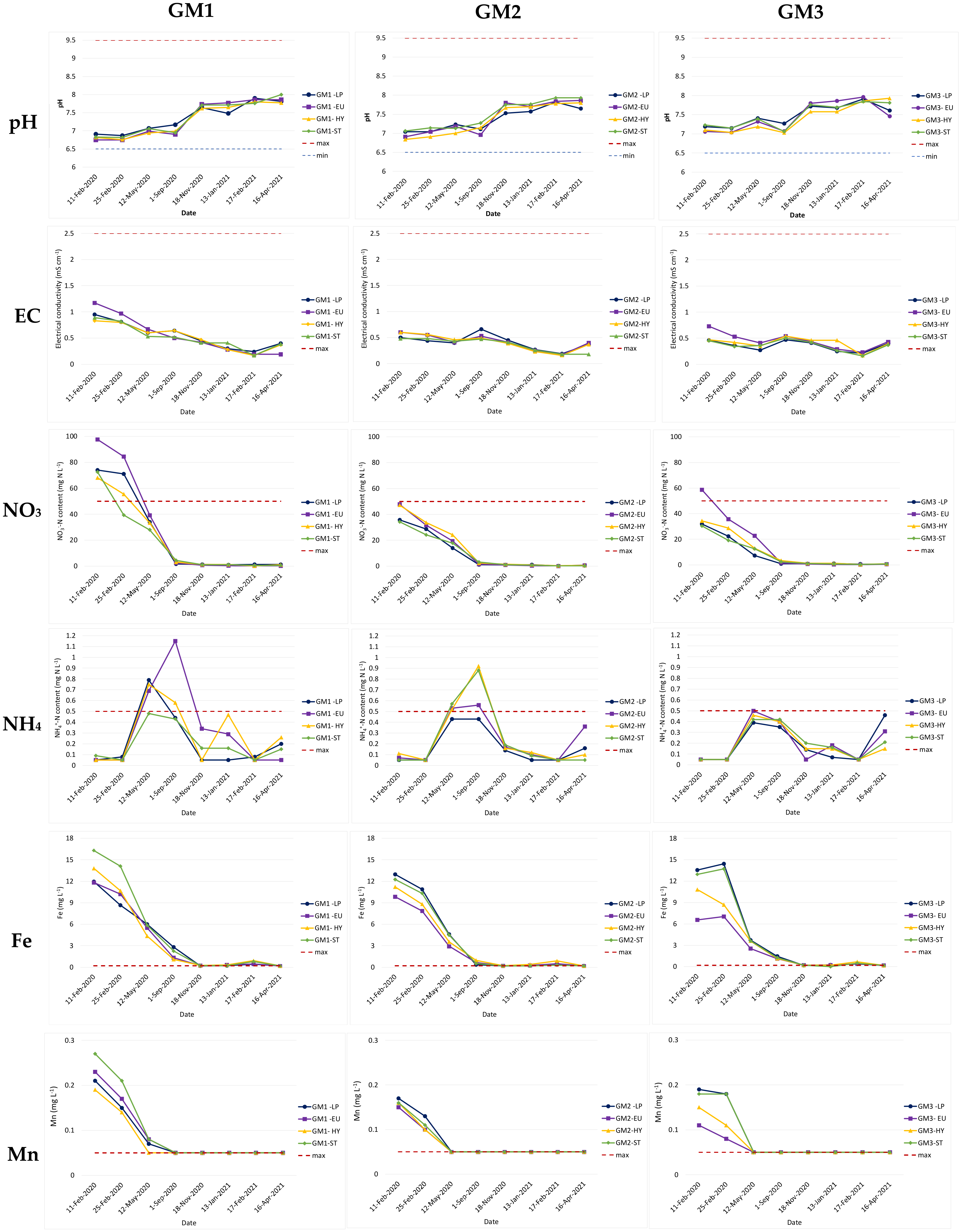

| Growing Media (GM) | Sanitised Compost: Foam Mixes (M) Vol. Ratio | Proportion of M in the GM (% vol.) | Proportion of TS in the GM (% vol.) | Load for 12 cm Thickness (kg·m−2) 1 |
|---|---|---|---|---|
| GM1 | M1 60:40 | 80 | 20 | 129 |
| GM2 | M2 40:60 | 80 | 20 | 111 |
| GM3 | M2 40:60 | 60 | 40 | 179 |
| Compost | M1 | M2 | TS | Unit | |
|---|---|---|---|---|---|
| pH | 9.1 | 6.75 | 7.06 | 7.70 | - |
| Electrical conductivity | 9.1 | 2.15 | 2.6 | 0.10 | dS·cm−1 |
| Organic matter | 44.6 | 55.7 | 63.7 | 5.04 | g·100 g−1 dw |
| Dry matter | 58.9 | 57.1 | 60.7 | 92.5 | g·100 g−1 dw |
| Total nitrogen | 11.8 | 15.91 | 18.04 | 2.60 | gN·kg−1 dw |
| NH4+-N | 1.5 | 2.24 | 2.81 | <0.10 | gN·kg−1 dw |
| NO3−-N | 2300 | 495.2 | 338.9 | 52.0 | mg·kg−1 dw |
| B | 16.9 | 19.40 | 19.40 | 0.37 | mg·kg−1 dw |
| Co | 5.5 | 2.80 | 2.65 | 15 | mg·kg−1 dw |
| Fe | 11,594.7 | 9134.8 | 10,788.7 | 86.80 | mg·kg−1 dw |
| Mn | 192.9 | 248.9 | 203.4 | 21.20 | mg·kg−1 dw |
| Mo | 1.2 | 0.90 | 1.20 | 0.50 | mg·kg−1 dw |
| CaO | 121.7 | 24.05 | 20.33 | 7.15 | g·kg−1 dw |
| K2O | 16.2 | 14.93 | 10.60 | 0.52 | g·kg−1 dw |
| MgO | 7.75 | 4.66 | 3.65 | 0.39 | g·kg−1 dw |
| Na2O | 1.28 | 0.098 | 0.070 | 0.085 | g·kg−1 dw |
| CEC | 54.44 | 41.22 | 31.14 | 9.89 | meq·100 g−1 dw |
| P2O5 Olsen | 0.71 | 1.53 | 1.06 | 0.16 | g·kg−1 dw |
| Bulk density | 0.44 | 0.20 | 0.16 | 1.28 | g·cm−3 |
| Total porosity | 79.94 | 88.71 | 90.53 | 49.72 | % vol |
| Macroporosity | 39.04 | 52.01 | 65.73 | 5.30 | % vol |
| Available water | 0.89 | 0.52 | 0.62 | 1.07 | mm·cm−1 |
| Water field capacity (−10 kPa) | 0.50 | 0.367 | 0.248 | 0.444 | % vol |
| Compost | M1 | M2 | Unit | |
|---|---|---|---|---|
| Cd | 0.4 | 0.6 | 0.4 | mg·kg−1 dw |
| Cr | 24.8 | 20 | 21.8 | mg·kg−1 dw |
| Cu | 142 | 145.3 | 112.4 | mg·kg−1 dw |
| Hg | 0.3 | 0.097 | 0.189 | mg·kg−1 dw |
| Ni | 15.4 | 13.5 | 13.2 | mg·kg−1 dw |
| Pb | 23.8 | 23.2 | 22.4 | mg·kg−1 dw |
| Zn | 338 | 342.2 | 382.4 | mg·kg−1 dw |
| VOCs | <DL | <DL | 280.4 | µg·kg−1 dw |
| Sum of 7 PCBs | <DL | <DL | <DL | µg·kg−1 dw |
| Sum of 16 PAHs | <DL | 37 | 49 | µg·kg−1 dw |
| BDE 28 | -- | 4.69 | 4.23 | µg·kg−1 dw |
| BDE 47 | -- | 248.13 | 295.29 | µg·kg−1 dw |
| BDE 99 | -- | 219.96 | 405.70 | µg·kg−1 dw |
| BDE 100 | -- | 44.92 | 84.32 | µg·kg−1 dw |
| BDE 153 | -- | 14.44 | 28.68 | µg·kg−1 dw |
| BDE 154 | -- | 10.46 | 22.68 | µg·kg−1 dw |
| BDE 183 | -- | 2.86 | 3.04 | µg·kg−1 dw |
| BDE 209 | -- | 57.10 | 60.70 | µg·kg−1 dw |
| Collection Date | R | PET | Drainage | |||||
|---|---|---|---|---|---|---|---|---|
| GM1 | GM2 | GM3 | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| 11-Feb-20 | 153.3 | 52.5 | 60.5 | 2.5 | 52.9 | 3.6 | 57.2 | 2.8 |
| 25-Feb-20 | 22.4 | 21.1 | 44.4 | 2.5 | 42.5 | 2.4 | 45.7 | 1.6 |
| 12-May-20 | 205.6 | 215.6 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| 1-Sep-20 | 155.4 | 523.7 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| 18-Nov-20 | 189.8 | 160.8 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| 13-Jan-21 | 107.9 | 34.8 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| 17-Feb-21 | 93.0 | 33.8 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| 16-Apr-21 | 40.9 | 125.5 | 37.0 | 0 | 37.0 | 0 | 37.0 | 0 |
| Total | 927.4 | 1042.3 | 326.7 | 317.2 | 324.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavo, P.; Artous, M.; Lemmel, O.; Buord, H.; Vidal-Beaudet, L.; Guénon, R. Agronomic Evaluation of Recycled Polyurethane Foam-Based Growing Media for Green Roofs. Sustainability 2022, 14, 13679. https://doi.org/10.3390/su142013679
Cannavo P, Artous M, Lemmel O, Buord H, Vidal-Beaudet L, Guénon R. Agronomic Evaluation of Recycled Polyurethane Foam-Based Growing Media for Green Roofs. Sustainability. 2022; 14(20):13679. https://doi.org/10.3390/su142013679
Chicago/Turabian StyleCannavo, Patrice, Mathieu Artous, Olivier Lemmel, Hervé Buord, Laure Vidal-Beaudet, and René Guénon. 2022. "Agronomic Evaluation of Recycled Polyurethane Foam-Based Growing Media for Green Roofs" Sustainability 14, no. 20: 13679. https://doi.org/10.3390/su142013679
APA StyleCannavo, P., Artous, M., Lemmel, O., Buord, H., Vidal-Beaudet, L., & Guénon, R. (2022). Agronomic Evaluation of Recycled Polyurethane Foam-Based Growing Media for Green Roofs. Sustainability, 14(20), 13679. https://doi.org/10.3390/su142013679






