Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Beach Wrack
2.2. Beach Wrack Composition and Quantitative Analysis
2.3. Proximate and Ultimate Analysis
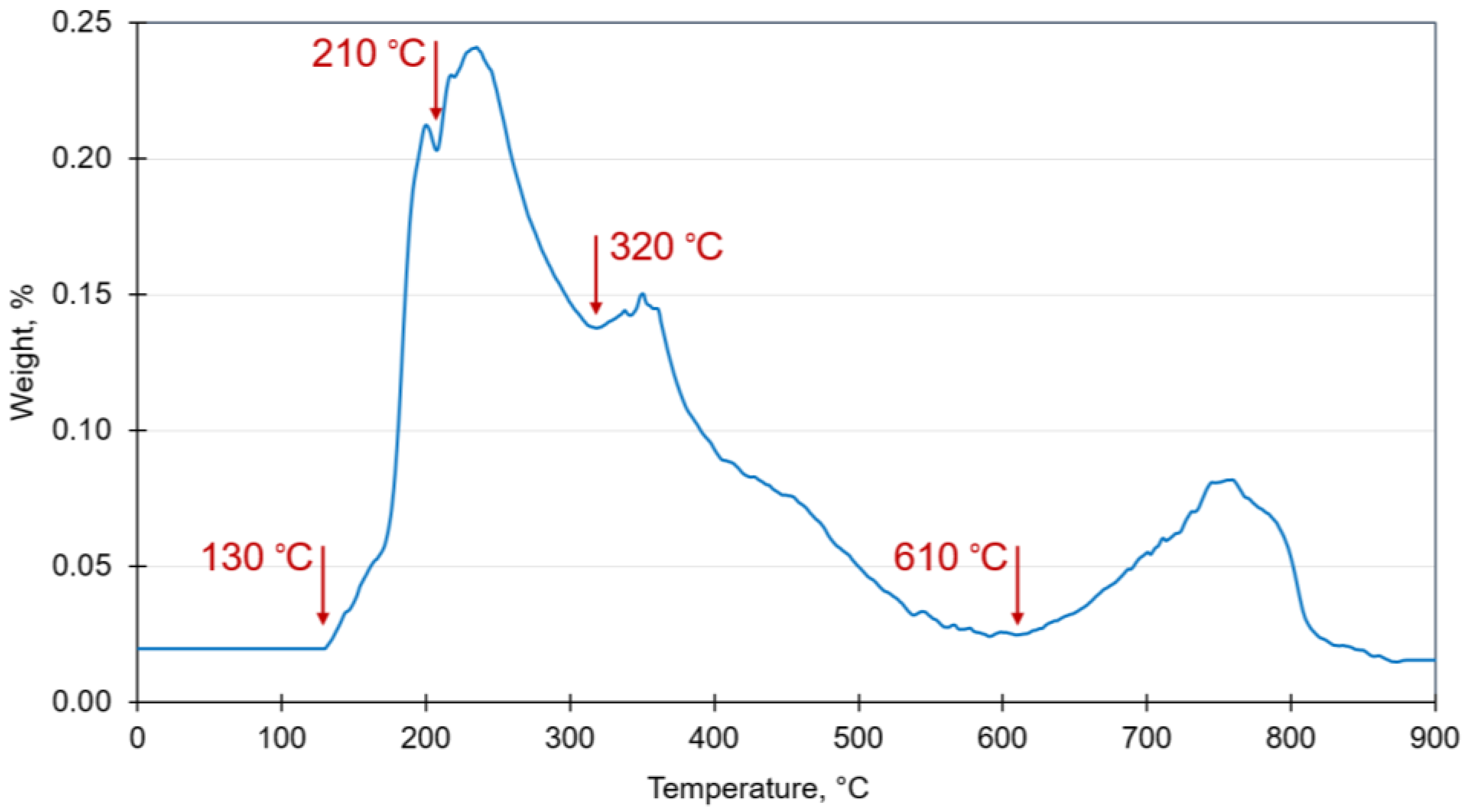
2.4. Calorific Character and Thermogravimetric Analysis
2.5. Experimental Gasification and Syngas Analysis
3. Results and Discussion
3.1. Beach Wrack Mass Composition
3.2. Characterization of Beach Wrack
3.2.1. Quantification of Major and Minor Elements
3.2.2. Proximate and Ultimate Analysis Outcomes
3.3. Estimated Calorific Values and Thermogravimetric Analysis
3.4. Characterization of Gasification and Its Outcomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; US EIA: Washington, DC, USA, 2016; 276p. [CrossRef] [Green Version]
- Van Asselt, H. Governing fossil fuel production in the age of climate disruption: Towards an international law of ‘leaving it in the ground’. Earth Syst. Gov. 2021, 9, 100118. [Google Scholar] [CrossRef]
- Barbot, Y.N.; Al-Ghaili, H.; Benz, R. A review on the valorization of macroalgal wastes for biomethane production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Ahmed, A.; Lam, S.S.; Lee, J.; Show, P.L.; Park, Y.-K. Sustainable valorization of algae biomass via thermochemical processing route: An overview. Bioresour. Technol. 2022, 344, 126399. [Google Scholar] [CrossRef] [PubMed]
- Felix, C.B.; Chen, W.-H.; Ubando, A.T.; Park, Y.-K.; Lin, K.-Y.A.; Pugazhendhi, A.; Nguyen, T.-B.; Dong, C.-D. A comprehensive review of thermogravimetric analysis in lignocellulosic and algal biomass gasification. Chem. Eng. J. 2022, 445, 136730. [Google Scholar] [CrossRef]
- Lymperatou, A.; Engelsen, T.K.; Skiadas, I.V.; Gavala, H.N. Different pretreatments of beach-cast seaweed for biogas production. J. Clean. Prod. 2022, 362, 132277. [Google Scholar] [CrossRef]
- Milledge, J.; Smith, B.; Dyer, P.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef] [Green Version]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Proced. 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Nandhini, R.; Berslin, D.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Thermochemical conversion of municipal solid waste into energy and hydrogen: A review. Environ. Chem. Lett. 2022, 20, 1645–1669. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Anaerobic digestion and gasification of seaweed. In Grand Challenges in Biology and Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 237–258. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.B.H.J.M.; Chew, K.W.; Munawaroh, H.S.H.; Shamsuddin, A.H.; Show, P.L. Algae as potential feedstock for various bioenergy production. Chemosphere 2022, 287, 131944. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Potential process ‘hurdles’ in the use of macroalgae as feedstock for biofuel production in the British Isles. J. Chem. Technol. Biotechnol. 2016, 91, 2221–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.A.; Begum, R.A.; Abdolrasol, M.G.; Hossain Lipu, M.S.; Mohamed, A.; Rashid, M.M. Review of baseline studies on energy policies and indicators in Malaysia for future sustainable energy development. Renew. Sust. Energ. Rev. 2018, 94, 551–564. [Google Scholar] [CrossRef]
- Suursaar, Ü.; Torn, K.; Martin, G.; Herkül, K.; Tiit, K. Formation and species composition of stormcast beach wrack in the Gulf of Riga, Baltic Sea. Oceanologia 2014, 56, 673–695. [Google Scholar] [CrossRef]
- Chubarenko, B.; Woelfel, J.; Hofmann, J.; Aldag, S.; Beldowski, J.; Burlakovs, J.; Garrels, T.; Gorbunova, J.; Guizani, S.; Kupczyk, A.; et al. Converting beach wrack into a resource as a challenge for the Baltic Sea (an overview). Ocean Coast. Manag. 2021, 200, 105413. [Google Scholar] [CrossRef]
- Pardilhó, S.; Boaventura, R.; Almeida, M.; Dias, J.M. Marine macroalgae waste: A potential feedstock for biogas production. J. Environ. Manag. 2022, 304, 114309. [Google Scholar] [CrossRef]
- Esiukova, E. Plastic pollution on the Baltic beaches of Kaliningrad region, Russia. Mar. Pollut. Bull. 2017, 114, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Haseler, M.; Weder, C.; Buschbeck, L.; Wesnigk, S.; Schernewski, G. Cost-effective monitoring of large micro- and meso-litter in tidal and flood accumulation zones at south-western Baltic Sea beaches. Mar. Pollut. Bull. 2019, 149, 110544. [Google Scholar] [CrossRef] [PubMed]
- Fetisov, S.; Chubarenko, I. Marine litter stormy wash-outs: Developing the neural network to predict them. Pollutants 2021, 1, 156–168. [Google Scholar] [CrossRef]
- Risén, E.; Nordström, J.; Malmström, M.E.; Gröndahl, F. Non-market values of algae beach-cast management—Study site Trelleborg, Sweden. Ocean Coast. Manag. 2017, 140, 59–67. [Google Scholar] [CrossRef]
- Takolander, A.; Cabeza, M.; Leskinen, E. Climate change can cause complex responses in Baltic Sea macroalgae: A systematic review. J. Sea Res. 2017, 123, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Liiv, J.; Zekker, I.; Tämm, K.; Rikmann, E. Greenhouse Gases Emissions and Climate Change—Beyond Mainstream. MOJ Bioorg. Org. Chem. 2020, 4, 10–16. Available online: https://medcraveonline.com/MOJBOC/MOJBOC-04-00104.pdf (accessed on 3 August 2022).
- Vigouroux, G.; Destouni, G. Gap identification in coastal eutrophication research—Scoping review for the Baltic system case. Sci. Total Environ. 2022, 839, 156240. [Google Scholar] [CrossRef] [PubMed]
- Franzén, D.; Infantes, E.; Gröndahl, F. Beach-cast as biofertiliser in the Baltic Sea region-potential limitations due to cadmium-content. Ocean Coast. Manag. 2019, 169, 20–26. [Google Scholar] [CrossRef]
- Domnin, D.; Chubarenko, B.; Grave, A. Baseline assessment of beach cast appearance in the South-Eastern Baltic by video monitoring at a pilot site in the Kaliningrad Oblast (Russia). Mar. Pollut. Bull. 2021, 173, 112994. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, Y.; Sukhikh, S.; Kalashnikova, O.; Chupakhin, E.; Ivanova, I.; Chubarenko, B.; Gorbunova, J.; Babich, O. Assessment of the resource potential of Baltic Sea macroalgae. Appl. Sci. 2022, 12, 3599. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Trevathan-Tackett, S.M.; Baldock, J.A.; Kelleway, J.J. Converting beach-cast sea-grass wrack into biochar: A climate-friendly solution to a coastal problem. Sci. Total Environ. 2017, 574, 90–94. [Google Scholar] [CrossRef]
- Kupczyk, A.; Kołecka, K.; Gajewska, M. Solving the beach wrack problems by on-site treatment with reed beds towards fertilizer amendments. J. Ecol. Eng. 2019, 20, 252–261. [Google Scholar] [CrossRef]
- Liu, S.; Trevathan-Tackett, S.M.; Ewers Lewis, C.J.; Ollivier, Q.R.; Jiang, Z.; Huang, X.; Macreadie, P.I. Beach-cast sea-grass wrack contributes substantially to global greenhouse gas emissions. J. Environ. Manag. 2019, 231, 329–335. [Google Scholar] [CrossRef]
- Malm, T.; Råberg, S.; Fell, S.; Carlsson, P. Effects of beach cast cleaning on beach quality, microbial food web, and littoral macrofaunal biodiversity. Estuar. Coast. Shelf Sci. 2004, 60, 339–347. [Google Scholar] [CrossRef]
- Eklund, B.; Svensson, A.P.; Jonsson, C.; Malm, T. Toxic effects of decomposing red algae on littoral organisms. Estuar. Coast. Shelf Sci. 2005, 62, 621–626. [Google Scholar] [CrossRef]
- Inácio, M.; Karnauskaitė, D.; Baltranaitė, E.; Kalinauskas, M.; Bogdzevič, K.; Gomes, E.; Pereira, P. Ecosystem services of the Baltic Sea: An assessment and mapping perspective. Geogr. Sustain. 2020, 1, 256–265. [Google Scholar] [CrossRef]
- Heckwolf, M.J.; Peterson, A.; Jänes, H.; Horne, P.; Künne, J.; Liversage, K.; Sajeva, M.; Reusch, T.B.H.; Kott, J. From ecosystems to socio-economic benefits: A systematic review of coastal ecosystem services in the Baltic Sea. Sci. Total Environ. 2021, 755, 142565. [Google Scholar] [CrossRef] [PubMed]
- Bucholc, K.; Szymczak-Żyła, M.; Lubecki, L.; Zamojska, A.; Hapter, P.; Tjernström, E.; Kowalewska, G. Nutrient content in macrophyta collected from southern Baltic Sea beaches in relation to eutrophication and biogas production. Sci. Total Environ. 2014, 473–474, 298–307. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sust. Energ. Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Ebadi, A.G.; Hisoriev, H. Bio-Oil Production from Fast Pyrolysis of Cladophora glomerata in a Fluidized Bed Reactor. Bulg. Chem. Commun. 2017, 49, 504–508. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_49_Number_2_2017/49-2-2017-Ebadi-504-508.pdf (accessed on 3 August 2022).
- Ardolino, F.; Arena, U. Biowaste-to-biomethane: An LCA study on biogas and syngas roads. Waste Manag. 2019, 87, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Bolívar Caballero, J.J.; Zaini, I.N.; Yang, W. Reforming processes for syngas production: A mini-review on the current status, challenges, and prospects for biomass conversion to fuels. Appl. Energy Combust. Sci. 2022, 10, 100064. [Google Scholar] [CrossRef]
- Indrawan, N.; Kumar, A.; Moliere, M.; Sallam, K.A.; Huhnke, R.L. Distributed power generation via gasification of biomass and municipal solid waste: A review. J. Energy Inst. 2020, 93, 2293–2313. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- Klavins, M.; Bisters, V.; Burlakovs, J. Small scale gasification application and perspectives in circular economy. Environ. Clim. Technol. 2018, 22, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Katrantsiotis, C.; Sachpazidou, V.; Ibrahim, A.; Bisters, V.; Burlakovs, J.; Hogland, W. Case Study 5: The Baltic Beach Wrack Thermal Recovery and Relevant Analytical Performances (ALREA). In Case Studies for Innovative Solutions of Beach Wrack Use: Report of the Interreg Project CONTRA; Chubarenko, B., Schubert, H., Woelfel, J., Eds.; Interreg Baltic Sea Region Project CONTRA: Rostock, Germany, 2021; pp. 52–62. Available online: https://www.beachwrack-contra.eu/wp-content/uploads/2021/05/CONTRA-Output05-WEB.pdf (accessed on 3 August 2022).
- Sheth, P.N.; Babu, B.V. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresour. Technol. 2009, 100, 3127–3133. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. The trends in research on the effects of biochar on soil. Sustainability 2020, 12, 7810. [Google Scholar] [CrossRef]
- Pathy, A.; Ray, J.; Paramasivan, B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2020, 2, 287–305. [Google Scholar] [CrossRef]
- Bird, M.I.; Wurster, C.M.; de Paula Silva, P.H.; Bass, A.M.; de Nys, R. Algal biochar—Production and properties. Bioresour. Technol. 2011, 102, 1886–1891. [Google Scholar] [CrossRef]
- Galhetas, M.; Lopes, H.; Freire, M.; Abelha, P.; Pinto, F.; Gulyurtlu, I. Characterization, leachability and valorization through combustion of residual chars from gasification of coals with pine. Waste Manag. 2012, 32, 769–779. [Google Scholar] [CrossRef]
- García-García, A.; Gregório, A.; Franco, C.; Pinto, F.; Boavida, D.; Gulyurtlu, I. Unconverted chars obtained during biomass gasification on a pilot-scale gasifier as a source of activated carbon production. Bioresour. Technol. 2003, 88, 27–32. [Google Scholar] [CrossRef]
- Boateng, A.A.; Cooke, P.H.; Hicks, K.B. Microstructure development of chars derived from high-temperature pyrolysis of barley (Hordeum vulgare L.) hulls. Fuel 2007, 86, 735–742. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Sánchez, M.E.; Mora, M.; Barrón, V. Slow pyrolysis of relevant biomasses in the Mediterranean basin. Part 2. Char characterisation for carbon sequestration and agricultural uses. J. Clean. Prod. 2016, 120, 191–197. [Google Scholar] [CrossRef]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar] [CrossRef]
- Hernández, J.J.; Ballesteros, R.; Aranda, G. Characterisation of tars from biomass gasification: Effect of the operating conditions. Energy 2013, 50, 333–342. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Stankevica, K. Impact of micro- and macroelement content on potential use of freshwater sediments (gyttja) derived from lakes of eastern Latvia. Environ. Geochem. Health 2018, 40, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Jagustyn, B.; Kmiec, M.; Smedowski, L.; Sajdak, M. The content and emission factors of heavy metals in biomass used for energy purposes in the context of the requirements of international standards. J. Energy Inst. 2017, 90, 704–714. [Google Scholar] [CrossRef]
- ISO 18134-1:2015; Solid Biofuels. Determination of Moisture Content. Oven Dry Method. Part 1: Total Moisture. ISO: Geneva, Switzerland, 2015; 5p.
- ISO 18122:2015; Solid Biofuels. Determination of Ash Content. ISO: Geneva, Switzerland, 2015; 6p.
- ISO 18123:2015; Solid Biofuels. Determination of the Content of Volatile Matter. ISO: Geneva, Switzerland, 2015; 8p.
- ISO 17828:2015; Solid Biofuels. Determination of Bulk Density. ISO: Geneva, Switzerland, 2015; 8p.
- Montero, G.; Coronado, M.A.; Torres, R.; Jaramillo, B.E.; García, C.; Stoytcheva, M.; Valenzuela, E. Higher heating value determination of wheat straw from Baja California, Mexico. Energy 2016, 109, 612–619. [Google Scholar] [CrossRef]
- ISO 16948:2015; Solid Biofuels. Determination of Total Content of Carbon, Hydrogen and Nitrogen. ISO: Geneva, Switzerland, 2015; 20p.
- ISO 16994:2016; Solid Biofuels. Determination of Total Content of Sulfur and Chlorine. ISO: Geneva, Switzerland, 2016; 11p.
- ISO 16993:2016; Solid Biofuels. Conversion of Analytical Results from One Basis to Another. ISO: Geneva, Switzerland, 2016; 10p.
- ISO 18125:2017; Solid Biofuels. Determination of Calorific Value. ISO: Geneva, Switzerland, 2017; 56p.
- Burlakovs, J. Contamination Remediation with Soil Amendments by Immobilization of Heavy Metals. Ph.D. Thesis, University of Latvia, Riga, Latvia, 2015; 146p. [Google Scholar]
- Greger, M.; Malm, T.; Kautsky, L. Heavy metal transfer from composted macroalgae to crops. Eur. J. Agron. 2007, 26, 257–265. [Google Scholar] [CrossRef]
- Magalhaes, I.B.; de Paula Pereira, A.S.A.; Silva, T.A.; dos Santos Renato, N. Predicting the higher heating value of microalgae biomass based on proximate and ultimate analysis. Algal Res. 2022, 64, 102677. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Salam, P.A. Estimation of higher heating value of biomass from proximate analysis: A new approach. Fuel 2012, 99, 55–63. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of higher heating value (HHV) based on the structural composition for biomass. Fuel 2021, 299, 120860. [Google Scholar] [CrossRef]
- Díaz-Rey, M.R.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.A.; Amadeo, N.; Laborde, M.; Alemany, L.J. Hydrogen-rich gas production from algae-biomass by low temperature catalytic gasification. Catal. Today 2015, 257 Pt 2, 177–184. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Larina, O.M.; Sytchev, G.A. Manufacturing gaseous products by pyrolysis of microalgal biomass. Int. J. Hydrogen Energy 2020, 45, 1569–1577. [Google Scholar] [CrossRef]
- Tezer, O.; Karabag, N.; Ongen, A.; Colpan, C.O.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Garrels, T. Case Study 2: Bio-Coal from Beach Wrack (BWC). In Case Studies for Innovative Solutions of Beach Wrack Use: Report of the Interreg Project CONTRA; Chubarenko, B., Schubert, H., Woelfel, J., Eds.; Interreg Baltic Sea Region Project CONTRA: Rostock, Germany, 2021; pp. 16–27. Available online: https://www.beachwrack-contra.eu/wp-content/uploads/2021/05/CONTRA-Output05-WEB.pdf (accessed on 3 August 2022).







| Element | Concentration Range (n = 17), mg/kg | No. of Excluded Outliers and Their Origin (Indicating Value, mg/kg) | Total Concentration Range (n = 3) in Biochar (and Mean), mg/kg | |
|---|---|---|---|---|
| Total Relevant | Statistically Relevant (and Mean) | |||
| As | 2.99–16.81 | 4.26–9.20 (6.37) | 0 | 7.55–8.65 (8.10) |
| Cd | 0.48–2.52 | 0.74–1.49 (0.98) | 0 | 1.82–1.98 (1.90) |
| Cr | 1.03–30.57 | 3.19–14.14 (9.31) | 1, SE (52.30) | 20.60–28.64 (24.62) |
| Cu | 1.50–24.44 | 3.22–11.71 (7.76) | 1, SE (26.03) | 7.94–10.00 (8.97) |
| Mn | 39.70–5426.19 | 137.62–2253.05 (328.58) | 1, EE (12,680.39) | 3943.90–3953.54 (3948.72) |
| Ni | 2.13–26.44 | 5.55–13.90 (10.41) | 1, SE (29.99) | 169.24–173.02 (171.13) |
| Pb | 1.01–7.10 | 1.10–3.50 (2.15) | 2, SE (11.84; 20.04) | 2.26–2.62 (2.44) |
| Zn | 16.37–146.69 | 25.75–78.31 (46.13) | 0 | 68.29–80.11 (74.20) |
| Sample Origin | Cd, mg/kg | P, mg/kg | Cd/P Ratio, mg Cd/kg P |
|---|---|---|---|
| SE (Sweden) | 1.41 | 1212.75 | 1161 |
| LV (Latvia) | 0.73 | 1309.13 | 558 |
| EE (Estonia) | 0.84 | 1639.52 | 513 |
| Average | 0.98 | 1390.79 | 705 |
| Parameter | Moisture, % | Ash, % | Volatile Matter, % | Fixed Carbon, % | Bulk Density, kg/m3 |
|---|---|---|---|---|---|
| AR | 62.7–64.1 | 7.7–8.5 | 20.9–21.7 | 5.7–8.7 | 190–230 |
| DB | 3.5–5.1 | 21.7–22.5 | 57.8–58.6 | 13.8–17.0 | n/a |
| Element | C, % | H, % | N, % | O, % | S, % | Cl, % |
|---|---|---|---|---|---|---|
| AR | 12.51–14.73 | 1.56–1.84 | 0.96–1.48 | 9.83–12.65 | 0.25–0.65 | 0.21–0.43 |
| DB | 36.22–38.44 | 4.52–4.80 | 3.07–3.59 | 29.11–31.93 | 1.03–1.43 | 0.76–0.98 |
| Sample Origin | Cl Concentration, mg/g | Theoretical Value of NaCl, mg/g |
|---|---|---|
| SE (Sweden) | 13.73 | 21.19 |
| LV (Latvia) | 8.49 | 13.11 |
| EE (Estonia) | 12.83 | 19.80 |
| Average | 11.68 | 18.03 |
| Component | For Biogas | For Syngas | |
|---|---|---|---|
| Biomass input | Utilized biomass examples | Organic waste, manure, sewage sludge | Lignocellulosic residues, sawdust, wood remains |
| Consistency | Wet | Dry or semi-dry | |
| Total solids, % | <30 | >70 | |
| Processing method | Biological: anaerobic digestion | Thermochemical: gasification | |
| Gas output | CH4, % | 50–70 | 6–12 |
| H2, % | – | 28–40 | |
| CO2, % | 30–50 | 14–25 | |
| CO, % | – | 16–25 | |
| N2, % | <1 | 0–5 | |
| O2, % | <0.5 | – | |
| H2O, % | – | 6–30 | |
| Hydrocarbons, % | – | 1.5–2.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincevica-Gaile, Z.; Sachpazidou, V.; Bisters, V.; Klavins, M.; Anne, O.; Grinfelde, I.; Hanc, E.; Hogland, W.; Ibrahim, M.A.; Jani, Y.; et al. Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion. Sustainability 2022, 14, 13712. https://doi.org/10.3390/su142113712
Vincevica-Gaile Z, Sachpazidou V, Bisters V, Klavins M, Anne O, Grinfelde I, Hanc E, Hogland W, Ibrahim MA, Jani Y, et al. Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion. Sustainability. 2022; 14(21):13712. https://doi.org/10.3390/su142113712
Chicago/Turabian StyleVincevica-Gaile, Zane, Varvara Sachpazidou, Valdis Bisters, Maris Klavins, Olga Anne, Inga Grinfelde, Emil Hanc, William Hogland, Muhammad Asim Ibrahim, Yahya Jani, and et al. 2022. "Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion" Sustainability 14, no. 21: 13712. https://doi.org/10.3390/su142113712
APA StyleVincevica-Gaile, Z., Sachpazidou, V., Bisters, V., Klavins, M., Anne, O., Grinfelde, I., Hanc, E., Hogland, W., Ibrahim, M. A., Jani, Y., Kriipsalu, M., Pal, D., Pehme, K.-M., Shanskiy, M., Saaremäe, E., Pilecka-Ulcugaceva, J., Celms, A., Rudovica, V., Hendroko Setyobudi, R., ... Burlakovs, J. (2022). Applying Macroalgal Biomass as an Energy Source: Utility of the Baltic Sea Beach Wrack for Thermochemical Conversion. Sustainability, 14(21), 13712. https://doi.org/10.3390/su142113712













