Physical and Chemical Characteristics of Agricultural-Plastic Wastes for Feasibility of Solid Fuel Briquette Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Pre-Treatment
2.2. Proximate Analysis
2.3. Determination of Moisture Content
- w1 = weight of the empty crucible (g)
- w2 = weight of empty crucible + sample (g)
- w3 = weight of the crucible + sample after heating (g)
2.4. Determination of Volatile Matter
- w4 = weight of the empty crucible (g)
- w5 = weight of empty crucible + sample (g)
- w6 = weight of the crucible + ash (g)
2.5. Determination of Ash Content
- w7 = weight of the empty crucible (g)
- w8 = weight of empty crucible + sample (g)
- w9 = weight of the crucible + ash (g)
2.6. Fixed Carbon Determination
2.7. Ultimate Analysis
2.8. Calorific Value
- W = weight of water in calorimeter (kg)
- w = weight equivalent of apparatus
- T1 = initial temperature of water (°C)
- T2 = final temperature of water (°C)
- x = weight of fuel sample taken (kg)
2.9. Carbon and Hydrogen
2.10. Nitrogen
2.11. Sulfur
2.12. Fourier Transform-Infrared Spectrophotometry (FTIR)
3. Results and Discussion
3.1. Characteristics of Mango Peel
3.2. Proximate Analysis
3.3. Ultimate Analysis
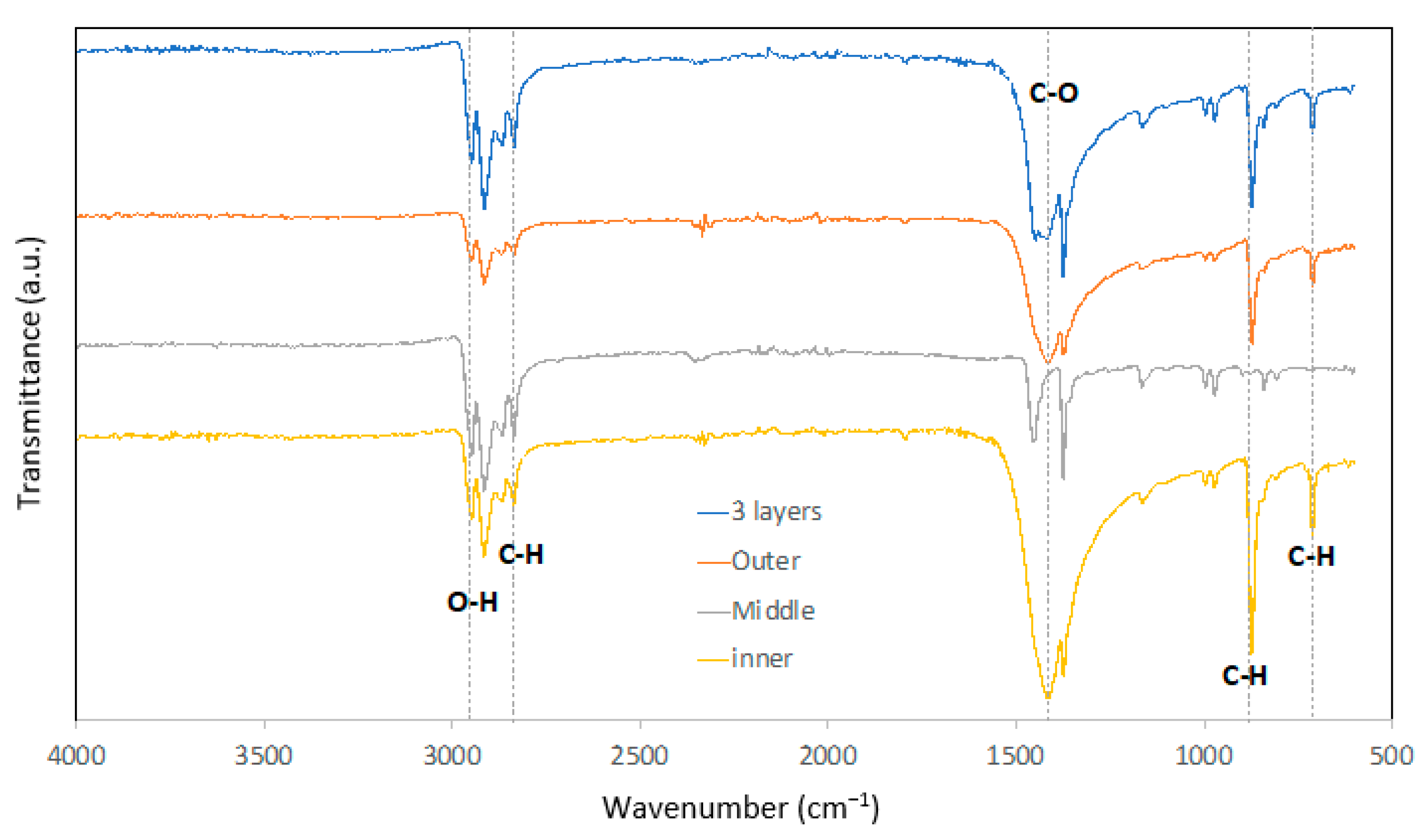
3.4. Fourier Transform-Infrared (FTIR) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamaroddin, M.; Nyakuma, B. Characterisation of the fuel properties of Imperata cylindrica grass for thermal applications. In Proceedings of the 4th International Graduate Conference on Engineering, Science and Humanities (IGCESH), Johor Bahru, Malaysia, 16–17 April 2013; pp. 590–593. [Google Scholar]
- Tucho, G. Feasible biomass energy conversion technologies in developing countries. Int. J. Eng. Res. Technol. 2013, 2, 2720–2728. [Google Scholar] [CrossRef]
- Pallav, P.; Atul, K.T.; Kandpal, C.T. Energetics of coal substitution by briquettes of agricultural residues. Energy 2006, 31, 1321–1331. [Google Scholar] [CrossRef]
- Prasityousil, J.; Muenjia, A. Properties of solid fuel briquettes produced from rejected material of municipal waste composting. Procedia Environ. Sci. 2013, 17, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Aragaw, T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef]
- Estiaty, L.M.; Fatimah, D.; Widodo. Bio-coal briquettes using low-grade coal. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012066. [Google Scholar] [CrossRef]
- Sugumaran, P.; Seshadri, S. Biomass Charcoal Briquetting Technology for Alternative Energy Based Income Generation in Rural Areas; Shri AMM Murugappa Chettiar Research Centre: Chennai, India, 2010; pp. 1–20. [Google Scholar]
- Sansaniwal, S.; Pal, K.; Rosen, M.; Tyagi, S. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Afolabi, H.K.; Al-Qadami, E.H.H.; Roslan, F.A.S.; Rahim, S.A.; Ghaleb, A.S. Production and Characterization of Rice Husk Biochar and Kenaf Biochar for Value-Added Biochar Replacement for Potential Materials Adsorption. Ecol. Eng. Environ. Technol. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Fadere, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Forbes. Available online: https://www.forbes.com/sites/victoriaforster/2021/03/07/why-you-should-not-burn-your-covid-19-masks-here-are-the-potential-health-hazards/?sh=29f8990d66f3 (accessed on 7 March 2021).
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; He, F.; Gao, B. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total. Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef]
- Bhoumick, M.C.; Sarker, N.C.; Hasan, M.; Roy, B.K. Conversion of Waste Plastic into Solid Briquette in Combination with Biomass: Bangladesh Perspective. Int. Adv. Res. J. Sci. Eng. Technol. 2016, 3, 142–146. [Google Scholar] [CrossRef]
- Kumar, K.P.; Srinivas, S. Catalytic Co-pyrolysis of Biomass and Plastics (Polypropylene and Polystyrene) Using Spent FCC Catalyst. Energy Fuels 2019, 34, 460–473. [Google Scholar] [CrossRef]
- Harussani, M.M.; Rashid, U.; Sapuan, S.M.; Abdan, K. Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers 2021, 13, 3980. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Cooke, W.; Theobald, B.; Hall, P. Producing a high heating value and weather resistant solid fuel via briquetting of bended wood residues and thermoplastics. Fuel 2021, 238, 119263. [Google Scholar] [CrossRef]
- Oginni, O. COVID-19 disposable face masks: A precursor for synthesis of valuable bioproducts. Environ. Sci. Pollut. Res. 2021, 29, 85574–85576. [Google Scholar] [CrossRef] [PubMed]
- Szefer, E.; Majka, T.; Pielichowski, K. Characterization and Combustion Behavior of Single-Use Masks Used during COVID-19 Pandemic. Materials 2021, 14, 3501. [Google Scholar] [CrossRef] [PubMed]
- Burrell, A.M.; Pepper, A.E.; Hodnett, G.; Goolsby, J.A.; Overholt, W.A.; Racelis, A.E.; Diaz, R.; Klein, P.E. Exploring origins, invasion history and genetic diversity of Imperata cylindrica (L.) P. Beauv. (Cogongrass) in the United States using genotyping by sequencing. Mol. Ecol. 2015, 24, 2177–2193. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weed: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977; p. 610. [Google Scholar]
- Syahrinuddin; Denich, M.; Becker, M.; Hartati, W.; Vlek, P.L.G. Biomass and carbon distribution on Imperata cylindrica grasslands. Biodivers. J. Biol. Divers. 2020, 21, 74–79. [Google Scholar] [CrossRef]
- Idris, S.S.; Zailan, M.I.; Azron, N.; Rahman, N.A. Sustainable Green Charcoal Briquette from Food Waste via Microwave Pyrolysis Technique: Influence of Type and Concentration of Binders on Chemical and Physical Characteristics. Int. J. Renew. Energy Dev. 2021, 10, 425–433. [Google Scholar] [CrossRef]
- Jahid, M.; Gupta, A.; Sharma, D.K. Production of Bioethanol from Fruit Wastes (Banana, Papaya, Pineapple and Mango Peels) Under Milder Conditions. J. Bioprocess. Biotech. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Jayalaxmi, B.; Vijayalakshmi, D.; Durgannavar, N.A.; Chandru, R. Mango peel: A potential source of natural bioactive phyto-nutrients in functional food. J. Dairy. Foods Home Sci. 2015, 34, 75–77. [Google Scholar] [CrossRef]
- Hosseinzaei, B.; Hadianfard, M.J.; Aghabarari, B.; García-Rollán, M.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pyrolysis of pistachio shell, orange peel and saffron petals for bioenergy production. Bioresour. Technol. Rep. 2022, 19, 101209. [Google Scholar] [CrossRef]
- Mibulo, T.; Nsubuga, D.; Kabenge, I.; Wydra, K.D. Characterization of briquettes developed from banana peels, pineapple peels and water hyacinth. ResearchSquare 2022. [CrossRef]
- Alvarez, J.; Hooshdaran, B.; Cortazar, M.; Amutio, M.; Lopez, G.; Freire, F.B.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Valorization of citrus wastes by fast pyrolysis in a conical spouted bed reactor. Fuel 2018, 224, 111–120. [Google Scholar] [CrossRef]
- Brunerová, A.; Roubík, H.; Brožek, M.; Herák, D.; Šleger, V.; Mazancová, J. Potential of Tropical Fruit Waste Biomass for Production of Bio-Briquette Fuel: Using Indonesia as an Example. Energies 2017, 10, 2119. [Google Scholar] [CrossRef] [Green Version]
- Rosas, J.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. On the preparation and characterization of chars and activated carbons from orange skin. Fuel Process. Technol. 2010, 91, 1345–1354. [Google Scholar] [CrossRef]
- Jani, S.M. The proximate analysis and mechanical properties of rice husk charcoal briquette. J. Trop. Agric. Food Sci. 2016, 44, 243–251. [Google Scholar]
- Hardianto, T.; Pambudi, F.F.; Irhamna, A.R. A study on lignin characteristic as internal binder in hot briquetting process of organic municipal solid waste. AIP Conf. Proc. 2018, 1984, 030013. [Google Scholar] [CrossRef]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Ultimate and Proximate analysis of Malaysia Pineapple biomass from MD2 cultivar for biofuel application. Chem. Eng. Transcations 2018, 63, 127–132. [Google Scholar] [CrossRef]
- Loison, R.; Foch, P.; Boyer, A. Coke Quality and Production, 2nd ed.; Butterworth: London, UK, 1989; Available online: https://www.worldcat.org/title/coke-quality-and-production/oclc/916793649 (accessed on 19 April 2022).
- Enweremadu, C.C.; Ojediran, J.O. Evaluation of energy potential in husks from soy-bean and cowpea. Sci. Focus 2004, 8, 2828–2866. [Google Scholar]
- Gouveia, S.; Otero, L.A.; Fernández-Costas, C.; Filgueira, D.; Sanromán, Á.; Moldes, D. Green Binder Based on Enzymatically Polymerized Eucalypt Kraft Lignin for Fiberboard Manufacturing: A Preliminary Study. Polymers 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangil, S.; Bhargav, V.K. Influences of binderless briquetting stresses on intrinsic bioconstituents of rice straw based solid biofuel. Renew. Energy 2019, 133, 462–469. [Google Scholar] [CrossRef]
- Wang, Z.; Lei, T.; Yan, X.; Chen, G.; Xin, X.; Yang, M.; Guan, Q.; He, X.; Gupta, A.K. Common characteristics of feedstock stage in life cycle assessments of agricultural residue-based biofuels. Fuel 2019, 253, 1256–1263. [Google Scholar] [CrossRef]
- Yulinah, T.; Denny, L.; Djoko, S. Eko-briket dari sampah plastik campuran dan lignoselulosa. J. Purikasi 2007, 8, 139–144. [Google Scholar]
- Wilaipon, P. The effects of briquetting pressure on banana-peel briquette and the banana waste in northern Thailand. Am. J. Appl. Sci. 2009, 6, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Grover, P.D.; Mishra, S.K. Biomass Briquetting: Technology and Practice; Regional Wood Energy Development Programme in Asia: Bangkok, Thailand, 1996. [Google Scholar]
- Husain, Z.; Zainac, Z.; Abdullah, Z. Briquetting of palm fibre and shell from the processing of palm nuts to palm oil. Biomass Bioenergy 2002, 22, 505–509. [Google Scholar] [CrossRef]
- Aina, O.M.; Adetogun, A.C.; Iyiola, K.A. Heat energy from value-added sawdust briquettes of Albiziazygia. Ethiop. J. Environ. Stud. Manag. 2009, 2, 42–49. [Google Scholar] [CrossRef]
- Raju, C.A.I.; Praveena, U.; Satya, M.; Jyothi, K.R.; Rao, S.S. Studies on development of fuel briquettes using biodegradable waste materials. J. Bioprocess. Chem. Eng. 2014, 1, 1–10. [Google Scholar]
- Ríos, B.I.M.; Luzardo, O.I.; García, T.J.F.; Santos, C.J.; Gutiérrez, A.C. Production and characterization of fuel pellets from rice husk and wheat straw. Renew. Energy 2020, 145, 500–507. [Google Scholar] [CrossRef]
- Sokhansanj, S.; Mani, S.; Bi, X.; Zaini, P.; Tabil, L. Binderless Pelletization of Biomass; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005. [Google Scholar] [CrossRef]
- Bhaskar, T.; Pandey, A. Recent Advances in Thermochemical Conversion of Biomass; Elsevier: Cambridge, MA, USA, 2015. [Google Scholar]
- Pilarska, A.A.; Bula, K.; Myszka, K.; Rozmanoski, T.; Karolina, S.R.; Pilarsiki, K. Functional polypropylene composites filled with ultra-fine magnesium hydroxide. Cent. Eur. J. Chem. 2015, 13, 161–171. [Google Scholar] [CrossRef]
- Haykiri, A.H.; Yaman, S. Effect of co-combustion on the burnout of lignite/biomass blends: A Turkish case study. Waste Manag. 2008, 28, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Neumann, G.; Rager, A.; Fuglein, E.; Blumm, J.; Denner, T. A novel direct coupling of simultaneous thermal analysis (STA) and Fourier transform-infrared (FT-IR) spectroscopy. J. Therm. Anal. Calorim. 2013, 113, 1091–1102. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Yi, C.; Wang, L.; Liu, H.; Xu, W. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J. Therm. Anal. Calorim. 2009, 97, 929–935. [Google Scholar] [CrossRef]
- Mohté, C.; de Castro, B.C.S.; Mothé, M. Characterization by TG/DTG/DSC and FTIR of frying and fish oil residues to obtain biodiesel. J. Therm. Anal. Calorim. 2011, 106, 811–817. [Google Scholar] [CrossRef]
- Nam, S.; Slopek, R.; Wolf, D.; Warnock, M.; Condon, B.D.; Sawhney, P.; Gbur, E.; Reynolds, M.; Allen, C. Comparison of biodegradation of low-weight hydroentangled raw cotton nonwoven fabric and that of commonly used disposable nonwoven fabrics in aerobic Captina silt loam soil. Text. Res. J. 2016, 86, 155–166. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhang, X.; Huang, C.; Jin, X. Design of electret polypropylene melt blown air filtration material containing nucleating agent for effective PM2.5 capture. RSC Adv. 2018, 8, 7932–7941. [Google Scholar] [CrossRef] [PubMed]

| Fruit Waste | Hemicellulose * (%) | Cellulose * (%) | Lignin * (%) |
|---|---|---|---|
| Banana peel | 9.4 | 34.8 | 4.5 |
| Pineapple peel | 11.1 | 22.4 | 6.5 |
| Papaya peel | 24.6 | 20.4 | 2.7 |
| Mango peel | 13.9 | 38.4 | 27.9 |
| Parameter | Mango Peel | Imperata cylindrica | Face Mask Waste |
|---|---|---|---|
| Moisture content (MC), % | 5.2 | <1 | <1 |
| Volatile matter (VM), % | 72.1 | 94.6 | 82.3 |
| Ash (AC), % | 7.5 | 2.3 | 6.75 |
| Fixed carbon (FC), % | 16.1 | 3.1 | 10.95 |
| Calorific value (CV), (MJ/kg) | 18.1 | 17.8 | 26.19 |
| Samples | C (%) | H (%) | N (%) | S (%) |
|---|---|---|---|---|
| Face mask waste | 63.6 ± 1.5 | 10.00 ± 0.5 | 0.11 ± 0.05 | 0.89 ± 0.03 |
| Mango peel | 44.0 ± 0.5 | 6.15 ± 0.2 | 0.52 ± 0.05 | 0.62 ± 0.04 |
| Imperata cylindrica | 40.8 ± 0.7 | 6.95 ± 0.3 | 0.83 ± 0.05 | 0.66 ± 0.01 |
| Functional Group | Wavenumber (cm−1) |
|---|---|
| C–H | 2850–3300 |
| C=O | 1680–1750 |
| C–O | 1000–1300 |
| O–H (alcohols) | 3230–3550 |
| O–H (acids) | 2500–3300 (very broad) |
| Material | Group | Compound | Functional Group |
|---|---|---|---|
| Imperata cylindrica | Oxygen | Alcohol | C–O–H |
| Mango peel | Oxygen | Alcohol | C–O–H |
| Face mask waste | Methyl | Alkene | CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ab Jalil, N.A.; Mokhtaruddin, N.A.; Chia, C.H.; Ahmad, I.K.; Saad, M.J.; Sarif, M. Physical and Chemical Characteristics of Agricultural-Plastic Wastes for Feasibility of Solid Fuel Briquette Production. Sustainability 2022, 14, 15751. https://doi.org/10.3390/su142315751
Ab Jalil NA, Mokhtaruddin NA, Chia CH, Ahmad IK, Saad MJ, Sarif M. Physical and Chemical Characteristics of Agricultural-Plastic Wastes for Feasibility of Solid Fuel Briquette Production. Sustainability. 2022; 14(23):15751. https://doi.org/10.3390/su142315751
Chicago/Turabian StyleAb Jalil, Nurul Ain, Nur Asyikin Mokhtaruddin, Chin Hua Chia, Irfana Kabir Ahmad, Mohamad Jani Saad, and Mahanim Sarif. 2022. "Physical and Chemical Characteristics of Agricultural-Plastic Wastes for Feasibility of Solid Fuel Briquette Production" Sustainability 14, no. 23: 15751. https://doi.org/10.3390/su142315751
APA StyleAb Jalil, N. A., Mokhtaruddin, N. A., Chia, C. H., Ahmad, I. K., Saad, M. J., & Sarif, M. (2022). Physical and Chemical Characteristics of Agricultural-Plastic Wastes for Feasibility of Solid Fuel Briquette Production. Sustainability, 14(23), 15751. https://doi.org/10.3390/su142315751







