The Nutrient Composition of Three Mosquito (Diptera: Culicidae) Species, Aedes caspius, Anopheles hyrcanus, and Culex pipiens, Harvested from Rice Fields for Their Potential Utilization as Poultry Feed Ingredients
Abstract
:1. Introduction
2. Material and Methods
2.1. Mosquito Sampling and Identification
2.2. Proximate Analysis
2.2.1. Crude Protein Content
2.2.2. Crude Fat Content
2.2.3. Moisture and Ash Content
2.3. Fatty Acid Profile
2.4. Mineral Content
2.5. Antioxidant Activity
2.6. Microbiological Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Mosquito Collections
3.2. Proximate Composition
3.3. Fatty Acid (FA) Profile
3.4. Mineral Profile
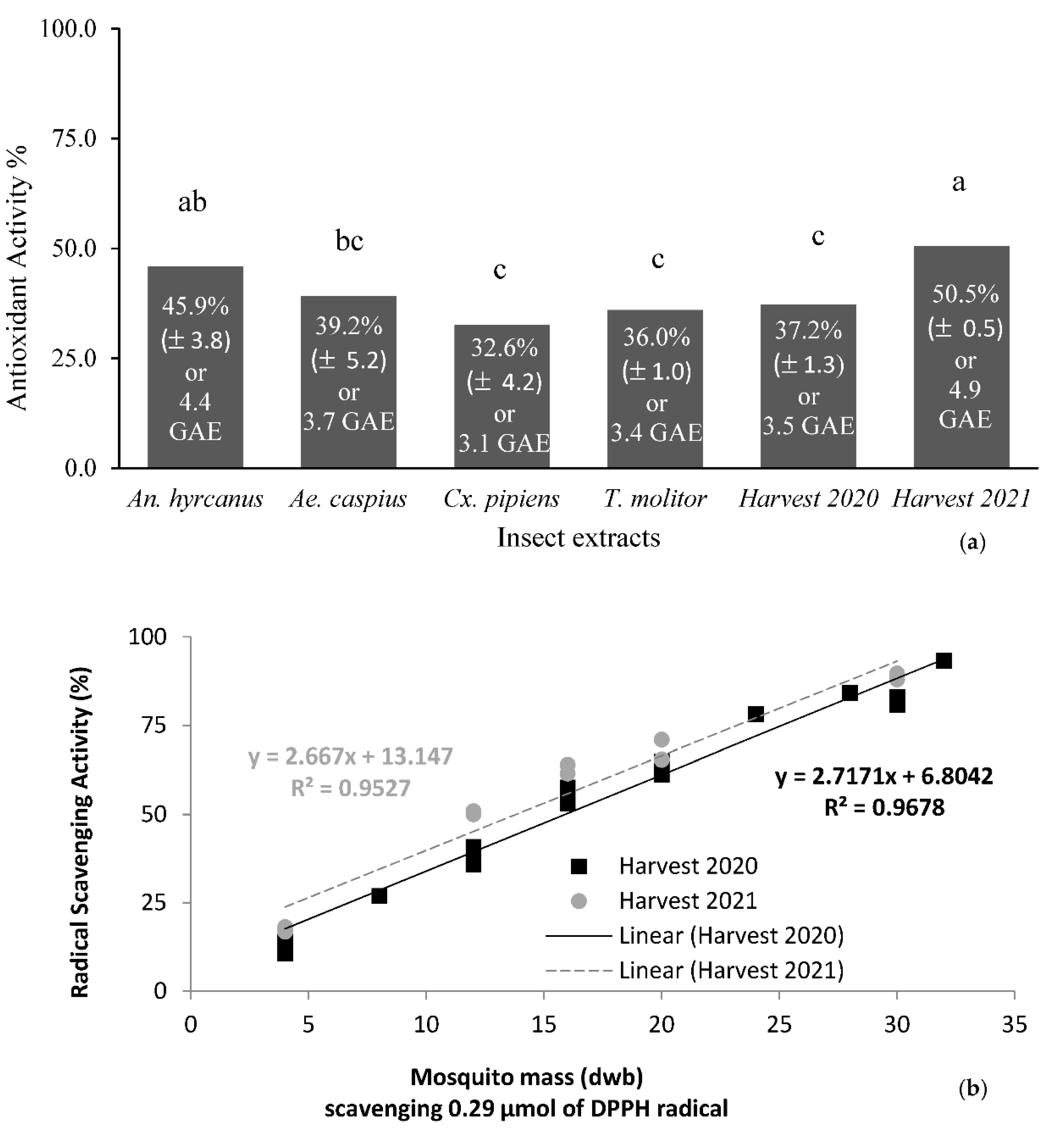
3.5. Antioxidant Properties
3.6. Microbiological Screening
4. Conclusions and Moving Forward
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Agriculture: Towards 2015/2030; Summary Report; Food & Agriculture Organization: Rome, Italy, 2002. [Google Scholar]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Cheseto, X.; Ekesi, S.; Dicke, M.; van Loon, J.J.A. Nutritional composition of black soldier fly larvae feeding on agro-industrial by-products. Entomol. Exp. Appl. 2020, 168, 472–481. [Google Scholar] [CrossRef]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting global feed protein demand: Challenge, opportunity, and strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef] [PubMed]
- HPLE. The Impacts on Global Food Security and Nutrition of the Military Conflict in Ukraine. Available online: https://knowledge4policy.ec.europa.eu/publication/impacts-global-food-security-nutrition-military-conflict-ukraine_en (accessed on 11 July 2022).
- van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A. Prospects of insects as food and feed. Org. Agric. 2021, 11, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and Challenges; Food & Agriculture Organization: Rome, Italy, 2017. [Google Scholar]
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV, and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Official Journal of the European Union L138. pp. 92–116. Available online: http://data.europa.eu/eli/reg/2017/893/oj (accessed on 18 January 2022).
- Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than Fur Animals, with Protein Derived from Animals. Official Journal of the European Union L295. pp. 1–17. Available online: http://data.europa.eu/eli/reg/2021/1372/oj (accessed on 18 January 2022).
- Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 Authorising the Placing on the Market of Dried Tenebrio molitor Larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Official Journal of the European Union L 194. pp. 16–20. Available online: http://data.europa.eu/eli/reg_impl/2021/882/oj (accessed on 18 January 2022).
- Commission Implementing Regulation (EU) 2021/1975 of 12 November 2021 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Locusta migratoria as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470. Official Journal of the European Union L 402. pp. 10–16. Available online: http://data.europa.eu/eli/reg_impl/2021/1975/oj (accessed on 18 January 2022).
- Association of American Feed Control Officials (AAFCO). Definition T60.117(C). Ingredient Definitions Committee Report. 2021. Available online: https://www.aafco.org/Portals/0/SiteContent/Meetings/Annual/2021/Committee-Reports/Ingredient_Definitions_Minutes_2021_Midyear.pdf (accessed on 18 January 2022).
- Federal Food, Drug, and Cosmetic Act (FFDCA). Title 21, Chapter 1—Food and Drug Administration, Department of Health and Human Services, Subchapter B—Food for Human Consumption, Part 117—Current Good Manufacturing Practice, Hazard Analysis, and Risk—Based Preventive Controls for Human Food, Subpart A—General Provisions, Sec. 117.3 Definitions. 2022. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=117.3 (accessed on 18 January 2022).
- Biancarosa, I.; Liland, N.S.; Day, N.; Belghit, I.; Amlund, H.; Lock, E.-J.; Gilburn, A.S. The chemical composition of two seaweed flies (Coelopa frigida and Coelopa pilipes) reared in the laboratory. J. Insects Food Feed 2018, 4, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-Protein Conversion Factors for Edible Insects on the Swiss Market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant activity of predigested protein obtained from a range of farmed edible insects. Int. J. Food Sci. Technol. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- Elemo, B.O.; Elemo, G.N.; Makinde, M.; Erukainure, O.L. Chemical evaluation of African palm weevil, Rhychophorus phoenicis, larvae as a food source. J. Insect Sci. 2011, 11, 146. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of three species of wild caught insects, pallid-winged grasshopper, rhinoceros beetles and white-lined sphinx moth. J. Insects Food Feed 2015, 1, 281–292. [Google Scholar] [CrossRef]
- Shantibala, T.; Lokeshwari, R.K.; Debaraj, H. Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. J. Insect Sci. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Blásquez, J.R.-E.; Moreno, J.M.P.; Camacho, V.H.M. Could Grasshoppers Be a Nutritive Meal? Food Nutr. Sci. 2012, 3, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Lenaerts, S.; Callens, A.; Van Campenhout, L. Effect of blanching followed by refrigerated storage or industrial microwave drying on the microbial load of yellow mealworm larvae (Tenebrio molitor). Food Control 2017, 71, 311–314. [Google Scholar] [CrossRef]
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient composition of selected whole invertebrates. Zoo Biol. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Sci. Technol. Int. 2016, 23, 17–23. [Google Scholar] [CrossRef]
- Banjo, A.D.; Lawal, O.A.; Songonuga, E.A. The nutritional value of fourteen species of edible insects in Southwestern Nigeria. Afr. J. Biotechnol. 2006, 5, 298–301. [Google Scholar]
- Ekpo, K.E.; Onigbinde, A.O.; Asia, I.O. Pharmaceutical potentials of the oils of some popular insects consumed in southern Nigeria. Afr. J. Pharm. Pharmacol. 2009, 3, 51–57. [Google Scholar]
- Mullen, G.; Durden, L. Medical and Veterinary Entomology; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Eldridge, B.F.; Edman, J.D. Medical Entomology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Chaskopoulou, A.; Koehler, P.G. Greece and the mosquito crisis. Pest Control News 2012, 93, 24–25. [Google Scholar]
- Chaskopoulou, A.; Latham, M.D.; Pereira, R.M.; Connelly, R.; Bonds, J.A.S.; Koehler, P.G. Efficacy of aerial ULV applications of two novel water-based formulations of unsynergized pyrethroids against riceland mosquitoes in Greece. J. Am. Mosq. Control Assoc. 2011, 27, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, E.A.; Chaskopoulou, A.; Grigoraki, L.; Tsiamantas, A.; Kounadi, S.; Georgiou, L.; Vontas, J. Analysis of population structure and insecticide resistance in mosquitoes of the genus Culex, Anopheles and Aedes from different environments of Greece with a history of mosquito borne disease transmission. Acta Trop. 2017, 174, 29–37. [Google Scholar] [CrossRef]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Springer: Heidelberg, Germany, 2010; pp. 91–111. [Google Scholar]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous versus organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- O'Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Psathas, D.; Lioupi, A.; Rebholz, A.M.; Zinoviadou, K.; Tsaftaris, A.; Theodoridis, G.; Papoti, V.T. Volatile profile and quality characteristics of the Greek “Chondrolia Chalkidikis” virgin olive oils: Effect of ripening stage. Eur. Food Res. Technol. 2022, 248, 1977–1990. [Google Scholar] [CrossRef]
- Mindak, W.R.; Dolan, S.P. Elemental Analysis Manual for Food and Related Products; U.S. Food and Drug Administration: Silver Spring, MA, USA, 2010. [Google Scholar]
- Nenadis, N.; Lazaridou, O.; Tsimidou, M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007, 55, 5452–5460. [Google Scholar] [CrossRef]
- INRAE-CIRAD-AFZ. Feed Tables: Composition and Nutritive Values of Feeds for Cattle, Sheep, Goats, Pigs, Poultry, Rabbits, Horses, and Salmonids. Available online: https://www.feedtables.com (accessed on 10 January 2022).
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Kim, I.H. Fish meal—nutritive value. J. Anim. Physiol. Anim. Nutr. 2011, 95, 685–692. [Google Scholar] [CrossRef]
- Banaszkiewicz, T. Nutritional value of soybean meal. In Soybean and Nutrition; El-Shemy, H., Ed.; IntechOpen: London, UK, 2011. [Google Scholar]
- Bajželj, B.; Laguzzi, F.; Röös, E. The role of fats in the transition to sustainable diets. Lancet Planet. Health 2021, 5, e644–e653. [Google Scholar] [CrossRef]
- Anankware, J.P.; Roberts, B.J.; Cheseto, X.; Osuga, I.; Savolainen, V.; Collins, C.M. The nutritional profiles of five important edible insect species from West Africa—An analytical and literature synthesis. Front. Nutr. 2021, 8, 792941. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Official Journal of the European Union L 136. pp. 1–40. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32012R0432 (accessed on 13 June 2022).
- Bi, X.; Siow, P.C.; Lim, S.W.; Henry, C.J. Dietary fatty acids analysis and its relevance to human health. SM J. Nutr. Metab. 2015, 1, 1005. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 Fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sushchik, N.N.; Yurchenko, Y.A.; Gladyshev, M.I.; Belevich, O.E.; Kalachova, G.S.; Kolmakova, A.A. Comparison of fatty acid contents and composition in major lipid classes of larvae and adults of mosquitoes (Diptera: Culicidae) from a steppe region. Insect Sci. 2013, 20, 585–600. [Google Scholar] [CrossRef]
- Komínková, D.; Rejmánková, E.; Grieco, J.; Achee, N. Fatty acids in anopheline mosquito larvae and their habitats. J. Vector Ecol. 2012, 37, 382–395. [Google Scholar] [CrossRef]
- National Research Council; Board on Agriculture; Subcommittee on Poultry Nutrition. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio molitor larvae as an alternative food source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, N.F.N.M.; Seok-Kian, A.Y.; Seng, L.L.; Mustafa, S.; Kim, Y.-S.; Shapawi, R. Nutritional value of black soldier fly (Hermetia illucens) larvae processed by different methods. PLoS ONE 2022, 17, e0263924. [Google Scholar] [CrossRef] [PubMed]
- Leiva, A.; Granados-chinchilla, F. Fatty acid profiling in animal feeds and related food matrixes using a fast GC/MS method and in situ derivatization. Int. J. Agric. Environ. Food Sci. 2020, 4, 70–89. [Google Scholar] [CrossRef]
- Mariod, A.A.; Abdel-Wahab, S.I.; Ain, N.M. Proximate amino acid, fatty acid and mineral composition of two Sudanese edible pentatomid insects. Int. J. Trop. Insect Sci. 2011, 31, 145–153. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL 2010, 17, 267–275. [Google Scholar] [CrossRef] [Green Version]
- D'Antonio, V.; Serafini, M.; Battista, N. Dietary modulation of oxidative stress from edible insects: A mini-review. Front. Nutr. 2021, 8, 642551. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.M.; Nielsen-LeRoux, C.; Cappellozza, S.; Jensen, A.B. Diseases in insects produced for food and feed. J. Insects Food Feed 2015, 1, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A.; Van Itterbeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agricultural Organisation of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Fang, J. Ecology: A world without mosquitoes. Nature 2010, 466, 432–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO; IAEA. Guidelines for Mass-Rearing of Aedes Mosquitoes; Food and Agriculture Organization of the United Nations International Atomic Energy Agency: Vienna, Austria, 2020. [Google Scholar]
- Hiscox, A.; Takken, W. Mass mosquito trapping for malaria control: Past successes and future directions. In Innovative Strategies for Vector Control—Ecology and Control of Vector-Borne Diseases; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; Volume 6, pp. 143–154. [Google Scholar]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Ayieko, M.A.; Ogola, H.J.; Ayieko, I.A. Introducing rearing crickets (gryllids) at household levels: Adoption, processing and nutritional values. J. Insects Food Feed 2016, 2, 203–211. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]

| An. hyrcanus | Ae. caspius | Cx. pipiens | Harvest 2020 | Harvest 2021 | Fishmeal 1 | SBM 1 | |
|---|---|---|---|---|---|---|---|
| Moisture (%) | 55.5 (±1.0) a | 57.2 (±1.7) a | 37.3 (±1.5) c | 48.9 (±2.7) b | 53.0 (±0.5) ab | 7.7–7.9 | 12.0–12.3 |
| Crude protein (%, dwb) | 61.8 (±0.3) a | 56.8 (±0.7) b | 55.6 (±0.3) bc | 53.9 (±0.7) c | 53.8 (±1.1) c | 67.8–74.8 | 49.5–55.2 |
| Crude fat (%, dwb) | 23.4 (±6.2) a | 21.0 (±4.3) a | 27.6 (±6.0) a | 15.9 (±2.5) a | 15.7 (±1.0) a | 9.8–10.3 | 1.7–1.9 |
| Ash (%, dwb) | 7.7 (±0.1) b | 8.5 (±0.3) a | 6.6 (±0.6) c | 7.7 (±0.3) b | 7.3 (±0.2) b | 15.2–19.2 | 7.1–7.4 |
| % of Total Fatty Acids | |||||
|---|---|---|---|---|---|
| Fatty Acids * | An. hyrcanus | Ae. caspius | Cx. pipiens | Harvest 2020 | Harvest 2021 |
| Lauric acid; C12:0 | 0.23 (±0.06) a,b | 0.15 (±0.06) c | 0.27 (±0.01) a | 0.19 (±0.01) b,c | 0.18 (±0.01) b,c |
| Myristic acid; C14:0 | 0.94 (±0.01) c | 0.82 (±0.01) d | 1.75 (±0.06) a | 0.91 (±0.01) c | 1.00 (±0.01) b |
| Tetradecenoic acid; C14:1 | 0.23 (±0.01) b | 0.32 (±0.11) b | 0.99 (±0.01) a | 0.23 (±0.06) b | 0.30 (±0.01) b |
| Palmitic acid; C16:0 | 25.52 (±0.15) b | 23.78 (±0.12) d | 30.43 (±0.10) a | 25.25 (±0.06) b | 25.82 (±0.17) b |
| Palmitoleic acid; C16:1n7 | 28.54 (±0.06) d | 31.04 (±0.23) b | 37.04 (±0.06) a | 30.59 (±0.12) c | 31.33 (±0.25) b |
| Stearic acid; C18:0 | 2.73 (±0.01) c | 3.11 (±0.10) a | 2.12 (±0.01) d | 3.04 (±0.12) a,b | 2.90 (±0.01) b |
| Oleic acid; C18:1n9 | 26.57 (±0.10) c | 29.10 (±0.10) a | 18.29 (±0.06) d | 28.18 (±0.15) b | 27.42 (±0.26) c |
| Octadecenoic acid; C18:1 | 1.22 (±0.06) d | 1.84 (±0.01) b | 3.19 (±0.01) a | 1.19 (±0.01) d | 1.31 (±0.01) c |
| Linoleic acid; C18:2n6 | 4.60 (±0.10) a | 2.25 (±0.06) c | 1.48 (±0.01) d | 3.01 (±0.10) b | 2.83 (±0.12) b |
| Linolenic acid; C18:3n3 (ALA) | 5.19 (±0.06) a | 2.60 (±0.01) c | 1.35 (±0.06) d | 3.24 (±0.06) b | 3.33 (±0.15) b |
| Eicosanoic acid; C20:0 | 0.14 (±0.06) c | 0.21 (±0.01) b,c | 0.26 (±0.01) a | 0.17 (±0.01) b,c | 0.27 (±0.06) b |
| Arachidonic acid; C20:4n6 (ARA) | 1.10 (±0.01) a | 0.73 (±0.06) c | 0.73 (±0.01) c | 0.95 (±0.06) b | 0.79 (±0.10) c |
| Eicosapentaenoic acid; C20:5n3 (EPA) | 1.79 (±0.06) c | 2.36 (±0.12) a | 1.49 (±0.01) d | 2.04 (±0.06) b | 1.70 (±0.10) c |
| Mineral (mg/kg, dwb) | An. hyrcanus | Ae. caspius | Cx. pipiens | Harvest 2020 | Harvest 2021 | SBM 1 | Fishmeal 1 |
|---|---|---|---|---|---|---|---|
| Calcium (Ca) | 9773 | 12,113 | 6626 | 11,371 | 11,122 | 3867 | 42,367 |
| Potassium (K) | 9884 | 10,305 | 9079 | 10,216 | 10,941 | 24,200 | 8733 |
| Phosphorus (P) | 18,600 | 18,800 | 13,700 | 17,500 | 17,300 | 7100 * | 27,767 |
| Magnesium (Mg) | 3667 | 4604 | 2753 | 4136 | 4062 | 3200 | 2367 |
| Sodium (Na) | 2593 | 2556 | 2414 | 2625 | 2668 | 127 | 11,067 |
| Iron (Fe) | 131.8 | 146.8 | 146.4 | 111.5 | 118.5 | 249.7 | 367 |
| Manganese (Mn) | 261.9 | 218.6 | 86.9 | 185.2 | 176.7 | 41.7 | 17.3 |
| Copper (Cu) | 23.6 | 19.0 | 19.7 | 20.1 | 20.8 | 17.3 | 11.3 |
| Zinc (Zn) | 188.0 | 158.0 | 166.4 | 151.1 | 159.3 | 53.3 | 105.7 |
| Mosquito Samples | Total Viable Bacteria | Enterobacteriaceae | Lactic Acid Bacteria | Coagulase-Positive Staphylococci |
|---|---|---|---|---|
| An. hyrcanus | 6.47 (±0.06) b | 5.46 (±0.06) a | 5.48 (±0.01) a | 5.41 (±0.3) a |
| Ae. caspius | 6.61 (±0.02) b | 5.47 (±0.05) a | 5.59 (±0.01) a | 5.11 (±0.1) a |
| Cx. pipiens | 6.63 (±0.02) b | 5.61 (±0.02) a | 5.26 (±0.02) a | 5.29 (±0.2) a |
| Harvest 2020 | 7.01 (±0.02) a | 5.91 (±0.04) a | 5.28 (±0.04) a | 4.34 (±0.2) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christaki, A.; Zinoviadou, K.G.; Papoti, V.T.; Miaoulis, M.; Chaskopoulou, A. The Nutrient Composition of Three Mosquito (Diptera: Culicidae) Species, Aedes caspius, Anopheles hyrcanus, and Culex pipiens, Harvested from Rice Fields for Their Potential Utilization as Poultry Feed Ingredients. Sustainability 2022, 14, 13852. https://doi.org/10.3390/su142113852
Christaki A, Zinoviadou KG, Papoti VT, Miaoulis M, Chaskopoulou A. The Nutrient Composition of Three Mosquito (Diptera: Culicidae) Species, Aedes caspius, Anopheles hyrcanus, and Culex pipiens, Harvested from Rice Fields for Their Potential Utilization as Poultry Feed Ingredients. Sustainability. 2022; 14(21):13852. https://doi.org/10.3390/su142113852
Chicago/Turabian StyleChristaki, Androniki, Kyriaki G. Zinoviadou, Vassiliki T. Papoti, Michael Miaoulis, and Alexandra Chaskopoulou. 2022. "The Nutrient Composition of Three Mosquito (Diptera: Culicidae) Species, Aedes caspius, Anopheles hyrcanus, and Culex pipiens, Harvested from Rice Fields for Their Potential Utilization as Poultry Feed Ingredients" Sustainability 14, no. 21: 13852. https://doi.org/10.3390/su142113852
APA StyleChristaki, A., Zinoviadou, K. G., Papoti, V. T., Miaoulis, M., & Chaskopoulou, A. (2022). The Nutrient Composition of Three Mosquito (Diptera: Culicidae) Species, Aedes caspius, Anopheles hyrcanus, and Culex pipiens, Harvested from Rice Fields for Their Potential Utilization as Poultry Feed Ingredients. Sustainability, 14(21), 13852. https://doi.org/10.3390/su142113852







